Introduction
Glioblastoma (GBM) is the most aggressive primary
brain tumor, originating from the glial cells in adults (1,2).
Patients with GBM often present with seizures, which increases the
difficulty of treatment. Previous studies have reported that GBM
accounts for ~75% in all malignant tumors in the brain (1–3).
According to pathological evaluations of GBM malignancy, the World
Health Organization categorized GBM into 4 grades. GBM demonstrates
infiltrative growth, and different malignant grades result in
diverse tumor morphology (3).
Therefore, developing effective therapeutic strategies for the
treatment of patients with glioblastoma is imperative.
Interleukin (IL)-2 is a pleiotropic cytokine which
exerts important effects on cells of the innate and adaptive immune
systems (4). IL-2 was the first
effective immunotherapeutic approved by the US Food and Drug
Administration for metastatic melanoma (5), and is involved in T-cell activation
and effector functions, including T-cell proliferation, interferon
(IFN)-γ production and cytotoxicity (6). IL-2 stimulates the propagation of
lymphocytes and induces cytotoxic T lymphocytes (CTL) and
lymphokine-activated killer cells in response to multiple tumor
cells (7). IL-2 also influences
homeostasis of memory T cells through the regulation of their
numbers, and drives the generation of antigen-specific T cells,
promoting the survival of memory CD8+ T cells (8). Therefore, it has been used for cancer
immunotherapy. It has previously been reported that IL-2 enhances
the therapeutic effects of other anti-cancer agents though
stimulating the immune system to produce tumor-specific immune
cells that attack the tumors (9).
The p53 protein is encoded by the tumor protein p53
gene (TP53) which is a tumor suppressor gene involved in the
regulation of the cell cycle, apoptosis, cell differentiation and
other mechanisms of cell regulation during exposure to DNA-damaging
agents, including ultraviolet radiation and toxins (10). GBM occurrence is closely associated
with p53 mutations (11,12). These mutations appear to be the
most common genetic change observed in human cancer (13–15).
Alteration or inactivation of p53 by mutation, or through its
interactions with oncogenic products of DNA tumor viruses, may
result in cancer (16).
TP53 has become a focus in cancer research because it is
commonly mutated in human cancer, and the spectrum of p53 mutations
in these cancers may enhance understanding of the etiology and
molecular pathogenesis of neoplasia (17,18).
Detection of p53 abnormalities may have diagnostic, prognostic, and
therapeutic implications (19,20).
It has previously been demonstrated that p53 mutations are
important to the classification of gliomas (10). In GBM, mutations of p53 primarily
occur in the DNA-binding domain within 6 mutation hotspot sites
(21). Non-pathogenic mutations of
p53 that protect against neoplastic transformation affect
modulation of the cell cycle, DNA repair, apoptosis, senescence,
angiogenesis and metabolism, resulting in a complex signaling
network (20,22). However, the therapeutic effect of
p53 in GBM remains to be elucidated.
In the present study, murine models of GBM were used
to study the therapeutic effects of p53 combined with the
immunomodulator IL-2. Although the use of p53 as a molecular marker
for GBM remains controversial, with studies failing to demonstrate
an association with prognosis, improvement was observed in patients
with cancer following transfection with a replication-defective
recombinant adenoviral vector encoding p53 (rAd-p53) (23,24).
p53-targeted gene therapy for GBM has reached phase I clinical
trials, while therapeutic drugs remain in preclinical development.
The aim of the present study was to examine the impact of p53 on
GBM cell apoptosis, disease prognosis, and the impact and
immunoregulatory function of IL-2 on lymphocyte infiltration,
toxicity and immunological memory in GBM. The results of the
present study may be a useful reference for GBM treatment,
providing insight into the pathophysiology of the disease, and may
assist in the development of treatments for GBM.
Materials and methods
Ethical statement
The present study was performed in strict accordance
with the recommendations of the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health (Bethesda,
MD, USA) (25). The protocol was
approved by the Chinese Association for Laboratory Animal Sciences,
Animal Health Products, and the Committee on the Ethics of Animal
Experiments Defense Research. All surgery and euthanasia were
performed under sodium pentobarbital (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) anesthesia, and all efforts were made to
minimize suffering.
Animal experiments
Male BALC/c GBM mice (age, 2 months; weight, 30–35
g; n=100) were purchased from the West China Experimental Animal
Center of Sichuan University (Sichuan, China). Mice were housed in
a temperature-controlled facility at 23±1°C and relative humidity
50±5%, with a 12-h light/dark cycle and had free access to food and
water. Mice were randomly assigned to the following 4 groups (n=25
mice/group): rAD-p53 group, rAd-p53 + IL-2 group, IL-2 group and
control group. Each mouse in the treatment groups received an
intratumoral injection of 100 µl rAd-p53 (Gendicine; Shenzhen
SiBiono GeneTech Co., Ltd., Shenzhen, China) and/or an intravenous
injection of 0.2 mg IL-2 once daily, administered continuously in
7-day cycles. The mice in the control groups received normal saline
intravenously, serving as an injection control. Tumor dimensions
were measured every 2 days and 10 times in total. The tumor volumes
were calculated according to the following formula: Length ×
width2 × 0.52. At the final point of measurement, the
tumor diameters were ~10–12 mm. On day 39 following the first
inoculation, the tumors were used for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis.
Cell culture and reagents
U251 and G422GBMcell lines were obtained from the
National Cancer Institute, Frederick Cancer Research Facility,
Division of Cancer Treatment Tumor Repository (Frederick, MD, USA)
and the American Type Culture Collection (Manassas, VA, USA),
respectively. The U251 or G422 cells were cultured in RPMI-1640 or
Eagle's minimum essential medium, respectively, supplemented with
10% heat-inactivated fetal bovine serum (Biowhittaker; Lonza Group,
Basel, Switzerland), 3 mM L-glutamine, 50 µg/ml gentamicin
(Biowhittaker; Lonza Group) and 1% penicillin/streptomycin. Normal
human astrocyte cells were purchased from Clonetics (Biowhittaker;
Lonza Group), and maintained in an astrocyte growth medium bullet
kit from the same supplier.
MTT cytotoxicity assays
The U251 or G422 cells (1×104 cells) were
incubated with 0.1 ml rAd-p53 in 96-well plates for 48, 72 and 96 h
in triplicate for each condition, and 0.1 ml phosphate-buffered
saline (PBS) was added instead of rAd-p53 as a control. Briefly, 20
µl MTT (5 mg/ml; Merck KGaA) in PBS was added to each well and the
plate was further incubated for 4 h at 37°C. Most of the medium was
removed and 100 µl dimethylsulfoxide (Sigma-Aldrich; Merck KGaA)
was added into the wells to solubilize the crystals. The optical
density was measured using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at a wavelength of 450 nm.
The following formula was used: Percentage cell viability =
[(absorbance of untreated cells - absorbance of treated
cells)/absorbance of untreated cells] × 100.
Flow cytometry analysis (FACS)
Tumor samples were minced to obtain single-cell
suspensions. Tumor cell suspensions were then diluted to
10×106 cells/ml, and centrifuged at 1,000 × g for 5 min
at 4°C. Cells were treated with 200 µl freshly prepared cold
fixation buffer (Merck KGaA) for 30 min at 4°C in the dark. Then,
samples were centrifuged at 1,000 × g for 5 min at 4°C and the cell
pellet was suspended in 200 µl freshly prepared pre-warmed (37°C)
permeabilization buffer (Haoran Bioscience, Inc., Shanghai, China).
Following incubation for 30 min at 37°C in the dark, cells were
centrifuged at 1,000 × g for 5 min at 4°C, washed with 200 µl PBS,
centrifuged at 1,000 × g for 5 min at 4°C and the supernatant was
discarded. Then, the tumor cells were labeled with CD3 (ab16669;
1:1,500; Abcam, Cambridge, UK), CD45 (ab10558; 1:1,500; Abcam), CD4
(ab183685; 1:2,000; Abcam) and CD8 (ab4055; 1:2,000; Abcam) at room
temperature in the dark for 30 min to detect the frequency of CD4
and CD8 cell subsets in the total infiltrated immune cells.
Horseradish peroxidase-conjugated anti-rabbit IgG (1706515,
Bio-Rad, Hercules, CA, USA) was used at a 1:5,000 dilution for 1 h
at 37°C. The stained cells were analyzed using a BD FACScan flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and WinMDI
software version 2.9 (The Scripps Institute, La Jolla, CA).
Splenocyte collection and CTL
assays
Spleens were removed from euthanized animals and
splenocytes were isolated by passing the spleens through 100 µm
nylon mesh filters. Cells were stimulated with 50 ng/ml
paramethoxyamphetamine (PMA; Sigma-Aldrich; Merck KGaA) and 1 µg/ml
ionomycin (Sigma-Aldrich; Merck KGaA) in the presence of BD
Golgistop protein transport inhibitor (BD Biosciences) in complete
RPMI-1640 medium (Sigma-Aldrich; Merck KGaA). The cells were then
diluted to 1×105 cells/ml in RPMI-1640, and centrifuged
at 1,000 × g for 5 min and the supernatant was removed. The cells
(1×105/well) were washed with PBS and incubated with
mitomycin-inactivated G422 cells (splenocyte:G422 ratios of 10:1,
20:1 and 40:1). IFN-γ levels were measured in the supernatants on
day 3 using a sandwich ELISA kit (cat. no. ab174443; Abcam). In
addition, T cells (1×106 cells/well) from the
splenocytes were purified as previously described (26) and co-cultured in RPMI-1640 medium
with fresh G422 cells for 4 h at effector:target ratios of 10:1,
20:1 and 40:1. Specific CTL activity to the target cells was
determined by MTT cytotoxicity assays as previously described
(27).
Measurement of relative mRNA
expression levels by RT-qPCR
Total cellular RNA was extracted using an RNeasy
mini kit (Qiagen, Inc., Valencia, CA, USA) and 1 µg RNA was
subjected to a cDNA using reverse transcription kit (1708840;
Bio-Rad Laboratories, Inc.) according to manufacturer's protocol.
The resultant cDNA (10 µl) was subjected to a 25 µl PCR conducted
in an iCycler thermal cycler (Bio-Rad Laboratories, Inc.) using iQ
SYBR-Green Supermix (Bio-Rad Laboratories, Inc.). The thermocycling
conditions were as follows: Initial denaturation at 95°C for 5 min,
followed by 35 cycles at 95°C for 20 sec, at 58°C for 20 sec and at
72°C for 20 sec, with a final extension at 72°C for 5 min. β-actin
was used as the internal reference gene. The relative expression
levels were calculated using the comparative Cq method (28), and gene expression was normalized
to β-actin. The primers used in the present study were: B-cell
lymphoma (Bcl)-2 forward, 5′-CAAAGGTGGATCAGATTCAAG-3′ and reverse,
5′-GGTGAGCATTATCACCCAGAA-3′; Bcl-2 like 2 (Bcl-w) forward,
5′-TGGCAGCAGTGACAGCAGCG-3′ and reverse, 5′-TACGGAGGTGGAGTGGGTGT-3′;
caspase-3 forward, 5′-AAAGTTTTCAATGACCAAGC-3′ and reverse,
5′-TCTGACGAATCTCCTCCAC-3′; caspase-8 forward,
5′-AGTCTATTTTATTATGGGCTCG-3′ and reverse,
5′-TGGATGTTTATGTCACCTTTTC-3′; caspase-9 forward,
5′-ATGGAGAACACTGAAAACTC-3′ and reverse, 5′-TGTGAGCATGGAAACAATAC-3′;
and β-actin forward, 5′-AGCCTTCTCCATGGTCGTGA-3′ and reverse
5′-CGGAGTCAACGGATTTGGTC-3′. Primers were synthesized by Invitrogen;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Statistical analysis
All data were expressed as the mean ± standard error
of 3 independent experiments. Statistical analysis was performed
using SPSS software version 13.0 (SPSS, Inc., Chicago, IL, USA).
The statistical significance of differences between groups was
assessed using unpaired Student's t-tests for pair-wise comparisons
or one-way analysis of variance followed by a post hoc
Student-Newman-Keuls test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
rAd-p53 induces apoptosis in GBM in
vitro
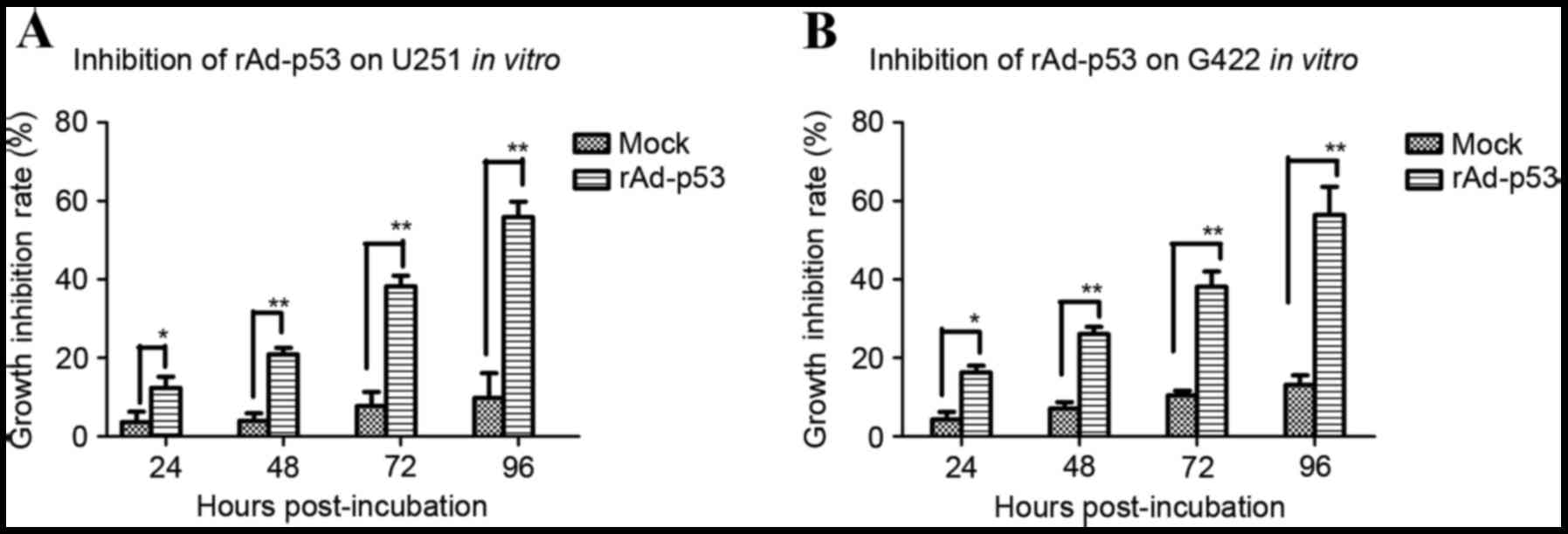
In order to explore whether transfection with
rAd-p53 effectively induces apoptosis in GBM cells in vitro,
the effect of rAd-p53 transfection on human G422 and U251 and
murine GBM cells was measured. The apoptosis rate significantly
increased in U251 cells transfected with rAd-p53 compared with
control cells at 24 (P<0.05; Fig.
1A), 48 (P<0.01; Fig. 1A),
72 (P<0.01; Fig. 1A) and 96 h
(P<0.01; Fig. 1A)
post-incubation. Similar effects were observed in G422 cells, with
significantly increased apoptosis rates in cells transfected with
rAd-p53 compared with control cells at 24 (P<0.05; Fig. 1B), 48 (P<0.01; Fig. 1B), 72 (P<0.01; Fig. 1B) and 96 h (P<0.01; Fig. 1B) post-incubation.
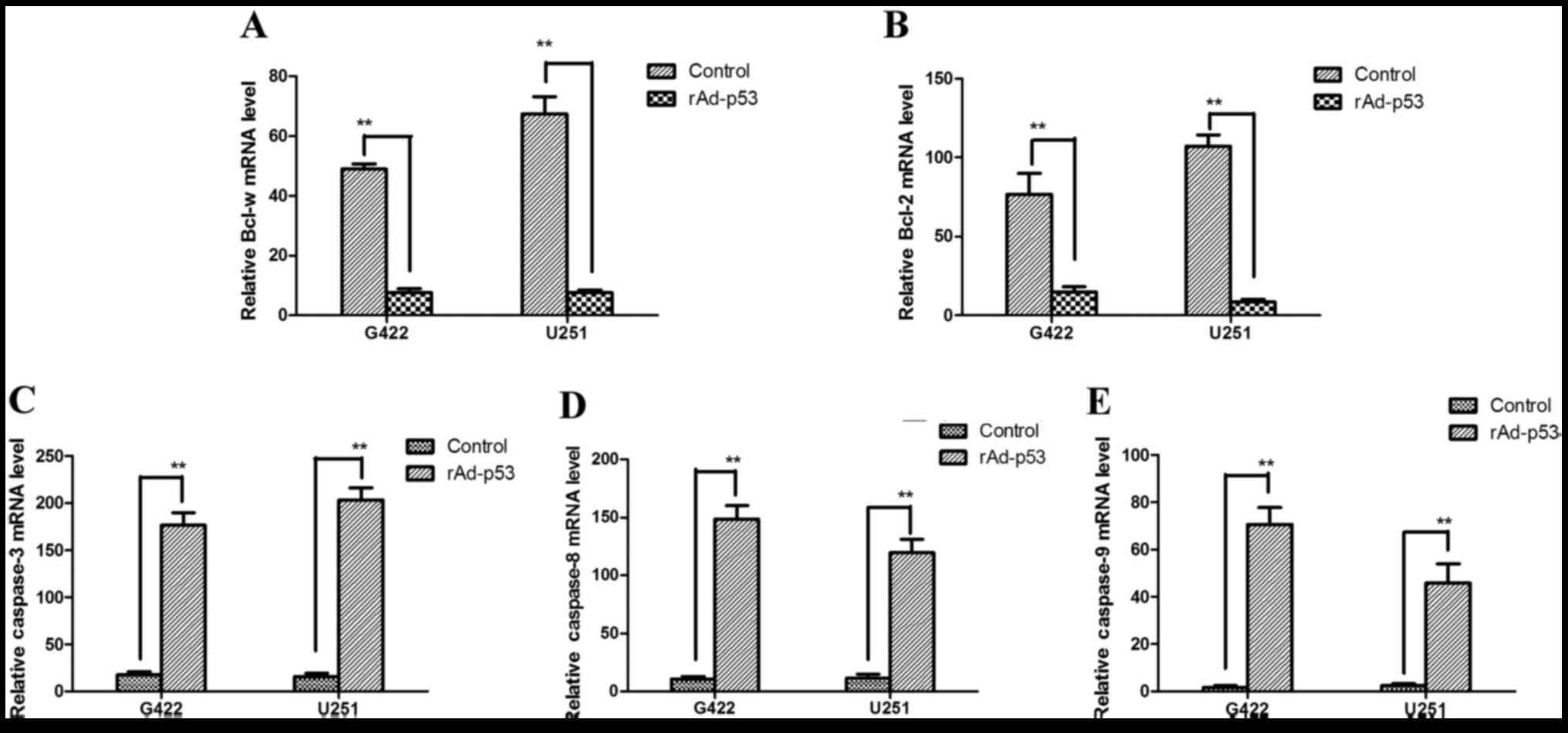
Apoptosis-associated gene expression levels from
cells transfected with rAd-p53 were measured in vivo by
RT-qPCR, including the apoptosis regulator Bcl-2, Bcl-w, caspase-8,
caspase-3, and caspase-9. mRNA expression levels of the
apoptosis-inhibiting genes Bcl-w and Bcl-2 were significantly
decreased in cells transfected with rAd-p53 compared with controls
(G422, P<0.01 and P<0.01, respectively; Fig. 2A and B, respectively; U251,
P<0.01 and P<0.01, respectively; Fig. 2A and B, respectively), and mRNA
expression levels of the pro-apoptotic genes caspase-8, caspase-3,
and caspase-9 were significantly increased in cells transfected
with rAd-p53 compared with control cells (G422, P<0.01,
P<0.01 and P<0.01, respectively; Fig. 2C-E, respectively; U251, P<0.01,
P<0.01 and P<0.01, respectively; Fig. 2C-E, respectively). These results
suggest that rAd-p53 induces apoptosis by inhibiting the activation
of Bax and Bcl-2, and that GBM is inhibited by the
mitochondrial-dependent apoptosis pathway.
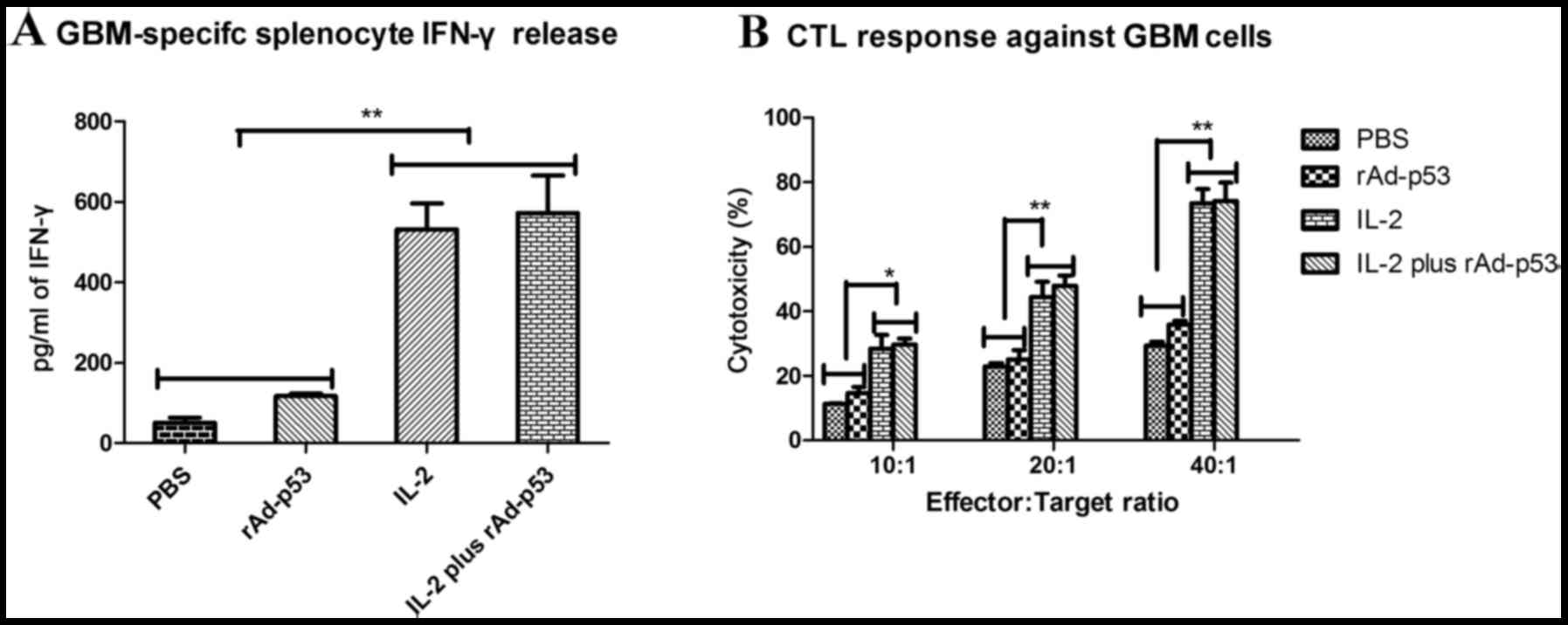
IL-2 invokes cytotoxicity in a murine
GBM model
To confirm that IL-2-treated mice develop an
adaptive immune response to the tumor cells, GBM model mice were
sacrificed on day 39 and assessed for the development of CTL
responses against the tumor cells. GBM-specific CTL activity was
assessed following the purification of T cells co-cultured with
tumor cells. Treatment with IL-2 resulted in increased IFN-γ
release when compared with control groups (Fig. 3A). CTL activity was significantly
increased in cells treated with IL-2 only compared with cells
treated with PBS or rAd-p53 only at all three effector:target
ratios investigated (10:1, P<0.05 and P<0.05, respectively;
20:1, P<0.01 and P<0.01, respectively; 40:1, P<0.01 and
P<0.01, respectively; Fig. 3B),
and a similar effect was observed in cells treated with IL-2 and
rAd-p53, with CTL activity significantly increased compared with
cells treated with PBS or rAd-p53 only (10:1, P<0.05 and
P<0.05, respectively; 20:1, P<0.01 and P<0.01,
respectively; 40:1, P<0.01 and P<0.01, respectively; Fig. 3B). These results suggested that the
treatment of tumors with IL-2 may result in the generation of
tumor-specific CTL responses.
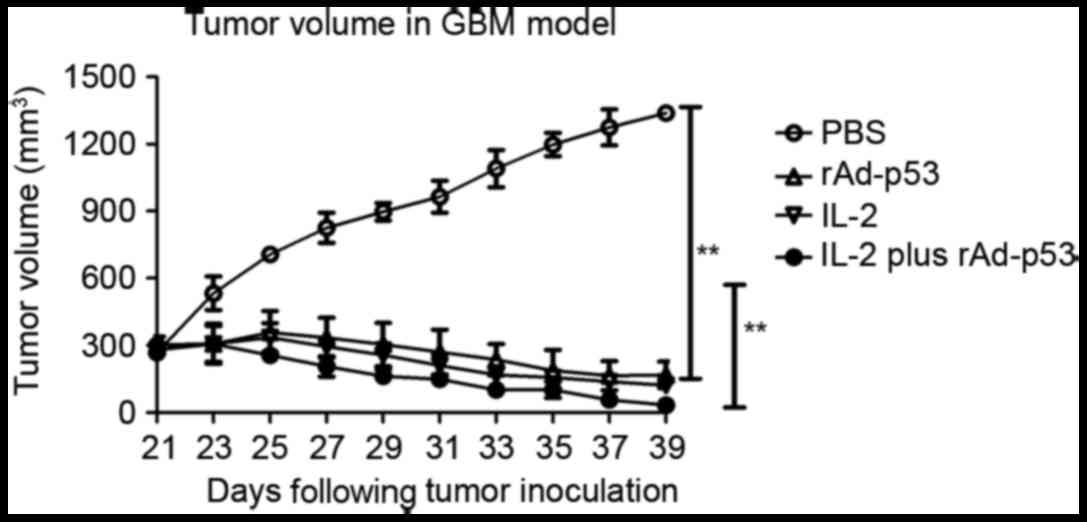
In vivo rAd-p53 and IL-2 enhanced
treatment of GBM
To explore rAd-p53 and IL-2 function as effective
anti-cancer agents in vivo, the anti-tumor activity of
rAd-p53 and IL-2 was investigated in the syngeneic murine GBM
model. Mice were randomly selected from each group (6/10) to
measure the tumor size. Mice (n=60) were sacrificed for further
analysis on day 39 following tumor implantation, while the
remaining animals (n=40) continued to be monitored for tumor growth
and survival until day 180. Tumor size in the animals treated with
rAd-p53 and IL-2 was significantly smaller than that in the mice
treated with PBS (P<0.0001; Fig.
4) or mice treated with the single agents IL-2 (P=0.0084;
Fig. 4) and rAd-p53 (P<0.0038;
Fig. 4).
Treatment of IL-2 results in immune
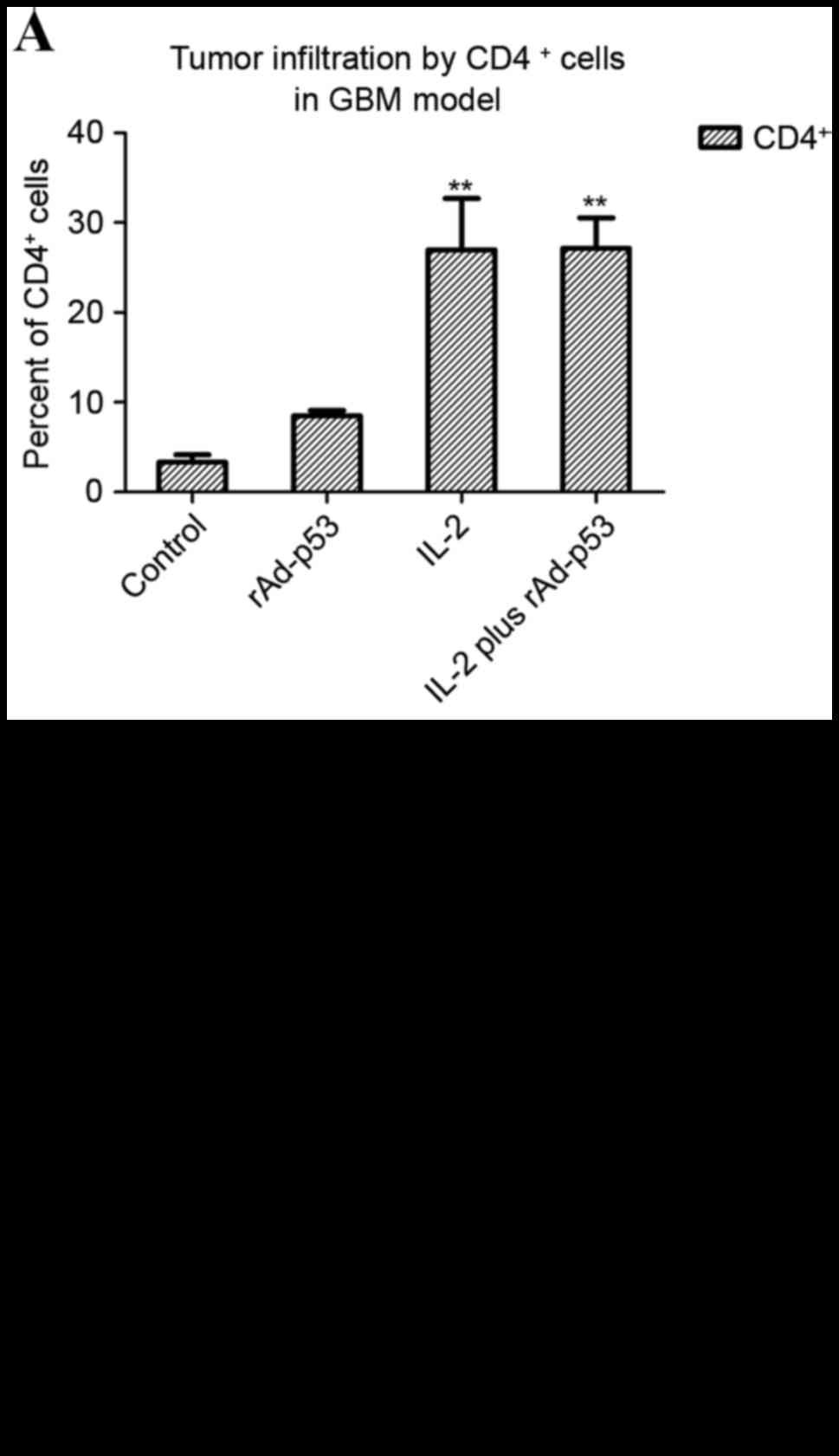
cell accumulation in GBM tumors
Tumors from the sacrificed mice from the late
treatment group described above were collected on day 25,
dissected, filtered, and stained for CD4+ and
CD8+ expression. Tumors from the animals treated with
rAd-p53 and IL-2 or IL-2 exhibited a high degree of both
CD4+ (Fig. 5A) and
CD8+ (Fig. 5B) cell
infiltration in both tumor models, as determined by Student's
paired t-tests. These observations suggested that the IL-2 or
rAd-p53 and IL-2-treated animals developed a stronger immune
response to the tumor.
Treatment of rAd-p53 and IL-2 results
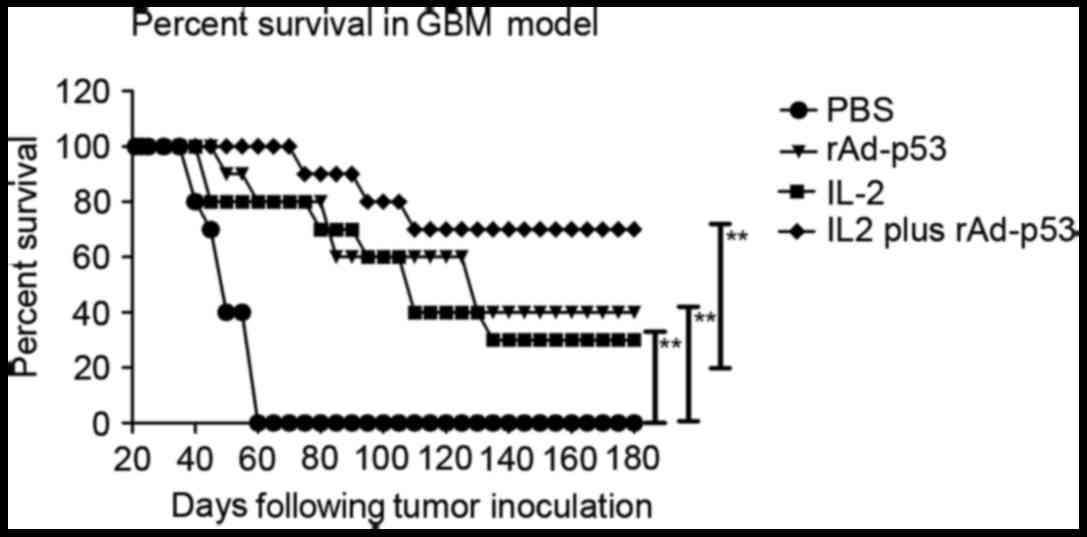
in survival prolongation in GBM mice
rAd-p53 and IL-2-treated mice maintained the highest
survival rate, suggesting that IL-2 has good therapeutic effects
for GBM. Furthermore, long-term survival was monitored for 180 days
following treatment with TNF-α and lenvatinib. rAd-p53 and IL-2
(n=10 in each group) prolonged the survival of mice compared with
control groups (Fig. 6). These
results indicated that the therapeutic agents against GBM in the
rAd-p53 plus IL-2 group were strong enough to partially protect the
animals and partially eliminate the tumor cells, which translated
into long-term and tumor-free survival.
Discussion
Immunotherapy has demonstrated marked antitumor
activity when associated with other therapeutic methods in animal
models of several types of human cancer (29–31).
Antineoplastic agents used in combination with immunotherapy
effectively target tumor cell-specific recognition domains
(antigens or receptors) (32–35).
At present, multiple immunotherapy agents for cancer are being
tested clinically. Immunotherapy agents, in which an antibody or
interleukin is inserted into a vector, have demonstrated
encouraging results in the treatment of certain advanced tumors in
patients (36). Recombinant
adenovirus with inserted p53 protein (rAd-p53) additionally
demonstrates marked antitumor activity in patients with T-cell
lymphoma and melanoma (37). For
cervical cancer, 1 simian and 10 human adenovirus serotypes were
administered to 30 patients, resulting in necrosis and transient
tumor regression in certain patients (34). Yoshida et al (37) reported that generation of
fiber-mutant recombinant adenovirus for gene therapy to treat
malignant GBM demonstrates impressive antitumor activity by
intratumor administration of anti-cancer agents targeted to tumor
cells. Furthermore, Chen et al (23) investigated the potential antitumor
effects of rAd-p53 by enhancing the sensitivity of gastric cancer
cells to chemotherapy, suggesting that rAd-p53 is an ideal
anti-cancer agent for cancer therapy.
IL-2 has been demonstrated to possess antitumor
activity in human cancer therapy in previous studies (38,39).
IL-2 was well tolerated without apparent indication of
drug-associated toxicity. As targeted therapy provides the
advantage of tumor specificity, it is conceivable that effectively
invoking the toxicity of immune cells for tumor cells may be useful
for GBM tumor therapy. The application of IL-2 would be either
peritumoral application or intravenous injection. These forms of
application would aim at producing tumor-specific killer cells with
improved immunogenicity to stimulate adaptive T cell mediated
anti-tumor immunity (40). This
concept is corroborated by the importance IL-2 signals have for
priming and secondary expansion of memory T cells (41). The best long-term anti-tumor
effects would be expected from T cells, which have specificity for
tumor-associated antigens including memory T cells, CD4 helper T
cells and CD8 cytotoxic T cells. Furthermore, CD8 T cells are an
essential part of the adaptive immune system against intracellular
pathogens and cancerous growths.
In the present study, GBM tumors treated with IL-2
and rAd-p53 stimulated T cells from the immune system in addition
to T cells from patients with cancer in vitro, however, they
also induced apoptosis of GBM cells via the caspase signaling
pathway. In the first case, GBM mice stimulated by injection of
IL-2 demonstrated significantly increased numbers of CD4 and CD8 T
cells expressing the early activation marker CD69 and producing
IFN-γ. In the latter case, IL-2 was used to stimulate T cells
isolated from patient-derived lymph nodes. T cells from cancer
patients activated by IL-2 demonstrated positive therapeutic
effects. For example, the response was tumor-specific by the same
tumor cells, suggesting that autologous tumor antigens had to be
present in the assay to generate a memory response (42). Finally, GBM tumor cells were
significantly inhibited by apoptosis of the mitochondrial signaling
pathway. The abilities of IL-2 and rAd-p53 may have been based on
tumor-specific memory T cells, apoptosis was induced by the
mitochondrial signaling pathway, and was augmented when IL-2
provided further signals.
In conclusion, GBM mice treated with IL-2 and
rAd-p53 were studied and demonstrated potent antitumor activity
against GBM and GBM-initiating cells in vitro. They induced
the regression of established GBM xenografts in vivo,
indicating that they may be of value for the treatment of GBM.
References
|
1
|
Lu M, Zhang X, Zhang M, Chen H, Dou W, Li
S and Dai J: Non-model segmentation of brain glioma tissues with
the combination of DWI and fMRI signals. Biomed Mater Eng. 26 Suppl
1:S1315, 2015–S1324. 2015.
|
|
2
|
Chow KK, Naik S, Kakarla S, Brawley VS,
Shaffer DR, Yi Z, Rainusso N, Wu MF, Liu H, Kew Y, et al: T cells
redirected to EphA2 for the immunotherapy of glioblastoma. Mol
Ther. 21:629–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koekkoek JA, Postma TJ, Heimans JJ,
Reijneveld JC and Taphoorn MJ: Antiepileptic drug treatment in the
end-of-life phase of glioma patients: A feasibility study. Support
Care Cancer. 24:1633–1638. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bai FL, Tian H, Yu QZ, Renl GP and Li DS:
Expressing foreign genes by Newcastle disease virus for cancer
therapy. Mol Biol (Mosk). 49:195–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Snyder A, Zamarin D and Wolchok JD:
Immunotherapy of Melanoma. Prog Tumor Res. 42:22–29.
2015.PubMed/NCBI
|
|
6
|
Bai FL, Yu YH, Tian H, Ren GP, Wang H,
Zhou B, Han XH, Yu QZ and Li DS: Genetically engineered Newcastle
disease virus expressing interleukin-2 and TNF-related
apoptosis-inducing ligand for cancer therapy. Cancer Biol Ther.
15:1226–1238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi L, Zhou Q, Wu J, Ji M, Li G, Jiang J
and Wu C: Efficacy of adjuvant immunotherapy with cytokine-induced
killer cells in patients with locally advanced gastric cancer.
Cancer Immunol Immunother. 61:2251–2259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y, Guo ZQ, Shi CM, Zhou ZF, Ye YB and
Chen Q: Efficacy of adjuvant chemotherapy combined with
immunotherapy with cytokine-induced killer cells for gastric cancer
after d2 gastrectomy. Int J Clin Exp Med. 8:7728–7736.
2015.PubMed/NCBI
|
|
9
|
Lippitz BE: Cytokine patterns in patients
with cancer: A systematic review. Lancet Oncol. 14:e218–e228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karsy M, Neil JA, Guan J, Mahan MA, Colman
H and Jensen RL: A practical review of prognostic correlations of
molecular biomarkers in glioblastoma. Neurosurg Focus. 38:E42015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamanishi Y, Boyle DL, Green DR, Keystone
EC, Connor A, Zollman S and Firestein GS: p53 tumor suppressor gene
mutations in fibroblast-like synoviocytes from erosion synovium and
non-erosion synovium in rheumatoid arthritis. Arthritis Res Ther.
7:R12–R18. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohgaki H, Eibl RH, Schwab M, Reichel MB,
Mariani L, Gehring M, Petersen I, Höll T, Wiestler OD and Kleihues
P: Mutations of the p53 tumor suppressor gene in neoplasms of the
human nervous system. Mol Carcinog. 8:74–80. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sakai E, Rikimaru K, Ueda M, Matsumoto Y,
Ishii N, Enomoto S, Yamamoto H and Tsuchida N: The p53
tumor-suppressor gene and ras oncogene mutations in oral
squamous-cell carcinoma. Int J Cancer. 52:867–872. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Greenblatt MS, Bennett WP, Hollstein M and
Harris CC: Mutations in the p53 tumor suppressor gene: Clues to
cancer etiology and molecular pathogenesis. Cancer Res.
54:4855–4878. 1994.PubMed/NCBI
|
|
15
|
Rivlin N, Brosh R, Oren M and Rotter V:
Mutations in the p53 tumor suppressor gene: Important milestones at
the various steps of tumorigenesis. Genes Cancer. 2:466–474. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yue Q, Yulong G, Liting Q, Shuai Y, Delong
L, Yubao L, Lili J, Sidang L and Xiaomei W: Mutations in and
expression of the tumor suppressor gene p53 in egg-type chickens
infected with subgroup J avian leukosis virus. Vet Pathol.
52:1052–1066. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hollstein M, Sidransky D, Vogelstein B and
Harris CC: p53 mutations in human cancers. Science. 253:49–53.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hamzehloie T, Mojarrad M, Hasanzadeh
Nazarabadi M and Shekouhi S: The role of tumor protein 53 mutations
in common human cancers and targeting the murine double minute
2-p53 interaction for cancer therapy. Iran J Med Sci. 37:3–8.
2012.PubMed/NCBI
|
|
19
|
Fagin JA: Tumor suppressor genes in human
thyroid neoplasms: p53 mutations are associated undifferentiated
thyroid cancers. J Endocrinol Invest. 18:140–142. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harris CC and Hollstein M: Clinical
implications of the p53 tumor-suppressor gene. N Engl J Med.
329:1318–1327. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Casson AG, Evans SC, Gillis A, Porter GA,
Veugelers P, Darnton SJ, Guernsey DL and Hainaut P: Clinical
implications of p53 tumor suppressor gene mutation and protein
expression in esophageal adenocarcinomas: Results of a ten-year
prospective study. J Thorac Cardiovasc Surg. 125:1121–1131. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuczyk MA, Serth J, Hervatin C, Arndt H,
Derendorf L, Thon WF and Jonas U: Detection of p53
tumor-suppressor-gene protein in bladder tumors and prostate
cancer: Possible clinical implications. World J Urol. 12:345–351.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen GX, Zheng LH, Liu SY and He XH:
rAd-p53 enhances the sensitivity of human gastric cancer cells to
chemotherapy. World J Gastroenterol. 17:4289–4297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie Q, Liang BL, Wu YH, Zhang J, Chen MW,
Liu HY, Gu XF and Xu J: Synergistic anticancer effect of rAd/P53
combined with 5-fluorouracil or iodized oil in the early
therapeutic response of human colon cancer in vivo. Gene.
499:303–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th.
National Academies Press (US); Washington, DC: 2011
|
|
26
|
Greaves MF and Brown G: Purification of
human T and B lymphocytes. J Immunol. 112:420–423. 1974.PubMed/NCBI
|
|
27
|
Zamarin D, Vigil A, Kelly K, Garcia-Sastre
A and Fong Y: Genetically engineered Newcastle disease virus for
malignant melanoma therapy. Gene Ther. 16:796–804. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thomas AA, Ernstoff MS and Fadul CE:
Immunotherapy for the treatment of glioblastoma. Cancer J.
18:59–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Larsen CJ: Cellular immunotherapy and
glioblastoma: A hopeful treatment? Bull Cancer.
98:4572011.PubMed/NCBI
|
|
31
|
Varghese S, Rabkin SD, Nielsen GP,
MacGarvey U, Liu R and Martuza RL: Systemic therapy of spontaneous
prostate cancer in transgenic mice with oncolytic herpes simplex
viruses. Cancer Res. 67:9371–9379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Husain SR, Behari N, Kreitman RJ, Pastan I
and Puri RK: Complete regression of established human glioblastoma
tumor xenograft by interleukin-4 toxin therapy. Cancer Res.
58:3649–3653. 1998.PubMed/NCBI
|
|
33
|
Debinski W, Gibo DM, Obiri NI, Kealiher A
and Puri RK: Novel anti-brain tumor cytotoxins specific for cancer
cells. Nat Biotechnol. 16:449–453. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bera TK, Viner J, Brinkmann E and Pastan
I: Pharmacokinetics and antitumor activity of a bivalent
disulfide-stabilized Fv immunotoxin with improved antigen binding
to erbB2. Cancer Res. 59:4018–4022. 1999.PubMed/NCBI
|
|
35
|
Ghetie MA, Richardson J, Tucker T, Jones
D, Uhr JW and Vitetta ES: Antitumor activity of Fab' and
IgG-anti-CD22 immunotoxins in disseminated human B lymphoma grown
in mice with severe combined immunodeficiency disease: Effect on
tumor cells in extranodal sites. Cancer Res. 51:5876–5880.
1991.PubMed/NCBI
|
|
36
|
Sinkovics JG and Horvath JC: Natural and
genetically engineered viral agents for oncolysis and gene therapy
of human cancers. Arch Immunol Ther Exp (Warsz). 56 Suppl 1:3S–59S.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoshida Y, Sadata A, Zhang W, Saito K,
Shinoura N and Hamada H: Generation of fiber-mutant recombinant
adenoviruses for gene therapy of malignant glioma. Hum Gene Ther.
9:2503–2515. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kusnierczyk H, Pajtasz-Piasecka E, Koten
JW, Bijleveld C, Krawczyk K and Den Otter W: Further development of
local IL-2 therapy of cancer: Multiple versus single IL-2 treatment
of transplanted murine colon carcinoma. Cancer Immunol Immunother.
53:445–452. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pantuck AJ and Belldegrun AS: Phase I
clinical trial of interleukin 2 (IL-2) gene therapy for prostate
cancer. Curr Urol Rep. 2:332001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Baek S, Kim YM, Kim SB, Kim CS, Kwon SW,
Kim Y, Kim H and Lee H: Therapeutic DC vaccination with IL-2 as a
consolidation therapy for ovarian cancer patients: A phase I/II
trial. Cell Mol Immunol. 12:87–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tan Y, Xu M, Wang W, Zhang F, Li D, Xu X,
Gu J and Hoffman RM: IL-2 gene therapy of advanced lung cancer
patients. Anticancer Res. 16:1993–1998. 1996.PubMed/NCBI
|
|
42
|
Gilly FN, Beaujard A, Bienvenu J, Trillet
Lenoir V, Glehen O, Thouvenot D, Malcus C, Favrot M, Dumontet C,
Lombard-Bohas C, et al: Gene therapy with Adv-IL-2 in unresectable
digestive cancer: Phase I–II study, intermediate report.
Hepatogastroenterology. 46 Suppl 1:S1268–S1273. 1999.
|