Introduction
Glaucoma is the second-leading cause of blindness
globally, following cataracts. There were 44.7 million people in
the world with open angle glaucoma as of 2010 (1). Glaucoma is a group of eye diseases
that result in damage to the optic nerve and vision loss. Increased
intraocular pressure is the most important risk factor in the
majority of glaucoma cases (2).
Retinal ganglion cells transmit visual information
from the retina in the form of action potentials to the thalamus,
hypothalamus and midbrain. They have a long axon that extends into
the brain, forming the optic nerve, optic chiasm and optic tract.
Loss of retinal ganglion cells has been implicated in a wide range
of glaucoma stages, from preperimetric to advanced (3). An observational cohort study that
examined 116 eyes of 62 glaucoma patients revealed that the rate of
retinal ganglion cell loss resulted in improved detection of
glaucoma progression, compared with either optical coherence
tomography or standard automated perimetry (4). In addition, transplanted stem cells
were reported to migrate into and integrate in different layers of
the retina (5). Therefore, in
order to pave the foundation for retinal ganglion cell therapy for
glaucoma, it is of importance to investigate the factors that may
affect differentiation of retinal progenitor cells into retinal
ganglion cells.
Fibroblast growth factor 2 was revealed to induce
embryonic stem cell-derived neural progenitors to generate retinal
ganglion cell-like cells in vitro (6). The combination of retinal pigment
epithelial cell-conditioned medium and photoreceptor outer segments
stimulated mesenchymal stem cell differentiation toward retinal
pigment epithelial cell phenotype (7). However, the effects of retinal
ganglion cell-conditioned medium on the gene expression and
differentiation of retinal progenitor cells and the effects of
surrounding pressure on the survival and differentiation of retinal
progenitor cells remain unclear.
Nestin is a neuroectodermal stem cell marker, and is
expressed in retinal progenitor cells (8). Upon differentiation, Nestin becomes
down-regulated. Paired box protein (PAX)6 is a key regulatory gene
of eye development (9). Retinal
progenitor cell clones were established by transfection of the
paired box protein 6 (PAX6) gene into mouse induced pluripotent
stem cells (10). Thy1 is a
surface glycoprotein uniquely expressed in retinal ganglion cells
in the retina (11).
Brain-specific homeobox/POU domain protein 3 (Brn3) is involved in
the regulation of differentiation, dendritic stratification and
axonal projection of retinal ganglion cells during development
(12). Therefore, Nestin and PAX6
were utilized to identify retinal progenitor cells, and Thy1 and
Brn3 were used to identify retinal ganglion cells. The retinal
ganglia are a type of neuron near the inner surface of the retina.
They transmit image-forming and non-image forming visual
information from the retina to the thalamus, hypothalamus,
mesencephalon and midbrain in the form of action potentials.
Examining the differentiation of retinal progenitor cells into
retinal ganglion cells may provide insights into vision restoration
following injury in glaucoma. Therefore, the present study aimed to
investigate the effects of retinal ganglion cell-conditioned medium
on gene expression and differentiation in retinal progenitor cells,
and the effects of surrounding pressure on the survival and
differentiation of retinal progenitor cells.
Materials and methods
Reagents and equipment
Dulbecco's modified Eagle's medium (DMEM)/F12, B27,
N2, heparin and glutamine were purchased from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Epithelial growth factor (EGF)
and basic fibroblast growth factor (bFGF) were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Trypsin
(Invitrogen; Thermo Fisher Scientific, Inc.), bicinchoninic acid
assay kit, caspase-3 assay kit (Sigma-Aldrich; Merck KGaA), PBS
(Sigma-Aldrich; Merck KGaA), were used in the present study.
Anti-Nestin antibody, anti-Thy1 antibody and secondary antibody
were purchased from Abcam (Cambridge, UK). Secondary antibodies
included goat anti-rabbit immunoglobulin (Ig)G H&L (Alexa
Fluor® 488; cat. no. ab150077; Abcam, Cambridge, UK),
and donkey anti-rabbit IgG H&L (Alexa Fluor® 555;
cat. no. ab150074; Abcam). Primers and probes, TRIzol reagent,
SuperScript III Reverse Transcriptase, SYBR-Green I and DEPC
H2O were purchased from Invitrogen (Thermo Fisher
Scientific, Inc.). RNase inhibitor was purchased from Fermentas
(Thermo Fisher Scientific, Inc.). Platinum Taq DNA polymerase,
oligo dT/primer and 100 mM dNTPs were purchased from Invitrogen
(Thermo Fisher Scientific, Inc.).
The following equipment was used: Cell incubator
(Thermo Fisher Scientific, Inc.), light microscope (Olympus
Corporation, Tokyo, Japan), CFX96 Touch™ Real-Time
polymerase chain reaction (PCR) Detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), table-type refrigerated
centrifuge, plate reader (Zhengzhou Nanbei Instrument Equipment,
Inc., Hefei, China), LSRII flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA) and FlowJo software 7.6.2 (FlowJo LLC, Ashland, OR,
USA).
Isolation and culture of rat retinal
progenitor cells
A total of 20 Sprague Dawley® (SD) rats
(male; 17 days old; 35–55 g; Shanghai SLAC Laboratory Animal, Inc.,
Shanghai, China) were sacrificed. Animals were raised at 25°C with
65% humidity in normal atmosphere. Animals had free access to food
and water and were housed under 12 h light/dark cycle.
Retinal pigment tissue at the ciliary margin zone of
embryos was isolated under a microscope, and placed into cold PBS.
The tissue was cut into small sections, and digested with 0.1%
trypsin at 37°C for 10 min. DMEM/F12 supplemented with 20% fetal
bovine serum (FBS, Gibco; Thermo Fisher Scientific, Inc.) was added
to stop digestion by trypsin. Trypsin (0.01%; Gibco; Thermo Fisher
Scientific, Inc.) was added at room temperature for 20 min, and a
single cell suspension was prepared by pipetting gently. The cell
suspension was centrifuged at 500 × g for 5 min at room
temperature, and cell pellet was resuspended in DMEM/F12
supplemented with 2% B27, 1% N2, heparin (90 µg/ml), glutamine (2
mmol/l), EGF (20 ng/ml) and basic fibroblast growth factor (bFGF;
10 ng/ml). The cell suspension was filtered (0.22 µm pores) and
cultured in plates (25 cm2) at 37°C in 5% CO2
and 95% air. Cell culture medium was replaced every 3 days. The
current study was approved by the Animal Research Board of Second
People's Hospital and Yan'an Hospital (Kunming, China). All efforts
were made to reduce animal suffering.
Isolation and culture of rat retinal
ganglion cells
A total of 20 SD rats (7 days old; male; 15–20 g;
Shanghai SLAC Laboratory Animal, Inc.) were sacrificed. Animals
were raised at 25°C with 65% humidity in normal atmosphere. Animals
had free access to food and water and were housed under 12 h
light/dark cycle. Eyeballs were removed using aseptic technique,
and washed with PBS three times. The cornea was removed followed by
the limbus corneae, lens and vitreous body. The neuronal layer of
the retinal tissue was isolated, washed with PBS supplemented with
1% penicillin-streptomycin three times and digested with 0.05%
trypsin at 37°C for 30 min. The digestion was terminated by adding
DMEM containing 10% FBS. The cell suspension was filtered with a 40
µm filter, and centrifuged at 500 × g at room temperature for 5
min. The cell pellet was resuspended with DMEM and cultured in
plates at 37°C in 5% CO2 and 95% air.
Pressure treatment
Cells (1×105) were plated in a 3.5 cm
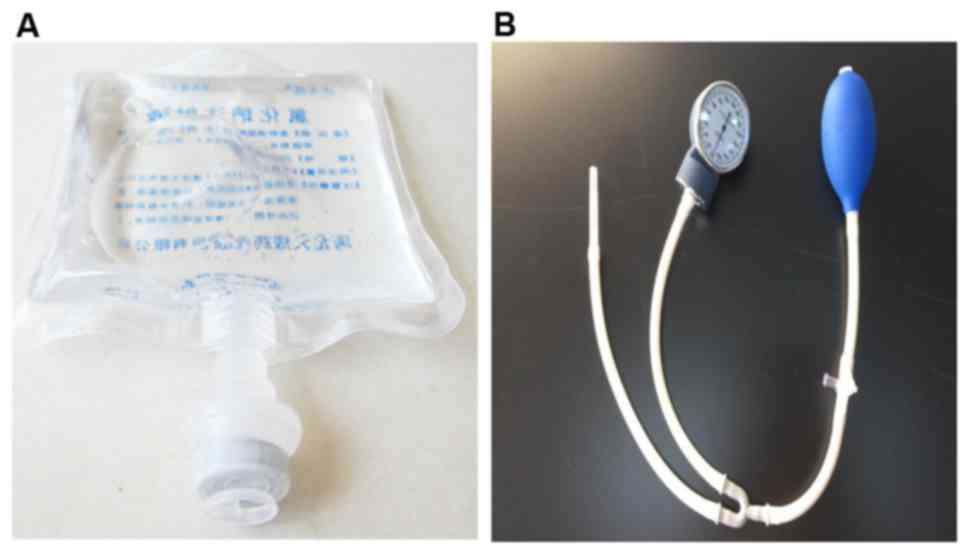
culture dish 1 day prior to the experiments. The bottom of a bag
containing normal saline (Fig. 1A)
was cut open with scissors, and the normal saline was poured out.
The culture dish was placed into the empty bag, and the bottom was
sealed using a plastic envelope machine. Sphygmomanometer (Jiangsu
Yuyue Medical Equipment & Supply Co., Inc., Nanjing, China;
Fig. 1B) was utilized to pump air
into the bag, and the pressure was measured using the instrument.
Cells in the different experimental groups were treated with
various pressures at room temperature for 48 h using this
method.
Immunofluorescence
Rat retinal progenitor cells and ganglion cells were
isolated, and cultured in 24-well plates with cover slips
(3×104 cells/cm2; 50% confluence). Cells
adhered to cover slips were then fixed in 4% paraformaldehyde at
room temperature for 10 min, and blocked with 2% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) for 30 min at room temperature.
Rat retinal progenitor cells were incubated with a primary antibody
against Nestin (1:500; cat. no. ab92391), and retinal ganglion
cells were incubated with a primary antibody against Thy1 (1:500;
cat. no. ab133350) at 4°C overnight. Following overnight
incubation, cover slips were washed with PBS, and incubated in the
dark with fluorescein isothiocyanate-conjugated goat anti-rabbit
secondary antibody (1:1,000) at room temperature for 1 h. Cover
slips were washed with PBS and stained with DAPI at room
temperature for 5 min. Slides were prepared using an anti-quenching
mounting medium. Slides were observed using a fluorescence
microscope (magnification, ×100).
Apoptosis assay
Retinal progenitor cells and ganglion cells were
cultured for 48 h under surrounding pressures of 0, 20, 40, 60 and
80 mmHg, respectively. Cell apoptosis was detected using a
caspase-3 assay kit (BioVision, Inc., Milpitas, CA, USA) according
to the manufacturer's protocol. Proteins from retinal stem cells
and ganglion cells were extracted using cell lysis buffer from the
caspase-3 assay kit, and the protein concentration was measured
using a bicinchoninic acid assay kit. Acetyl-Asp-Glu-Val-Asp
p-nitroanilide (Ac-DEVD-pNA; included in the caspase-3 assay kit),
the substrate that is hydrolyzed by caspase-3, was mixed with cell
proteins at 37°C for 2 h, and the optical density values were
measured at an absorbance of 405 nm using a plate reader.
Experiments were repeated three times.
Induction of retinal progenitor cell
differentiation by retinal ganglion cell-conditioned medium
Rat retinal ganglion cells were cultured to 80%
confluence, and the cell culture medium was replaced with DMEM/F12
medium without serum. Retinal ganglion cells were cultured for a
further 24 h, and the culture supernatant was collected. Retinal
progenitor cells were cultured in retinal ganglion cell-conditioned
medium for 72 h under normal pressure, or under 40 mmHg pressure.
Retinal progenitor cells that were cultured without retinal
ganglion cell-conditioned medium served as control (no treatment).
Retinal progenitor cells were collected. Gene expression levels of
Nestin, PAX6, Thy1 and Brn-3 were detected by reverse
transcription-quantitative PCR (RT-qPCR), and flow cytometry was
utilized to evaluate the effects of pressure on the differentiation
of retinal progenitor cells into retinal ganglion cells.
RT-qPCR
Following induction by retinal ganglion
cell-conditioned medium, retinal progenitor cells under normal
pressure were collected. Total RNA was extracted using TRIzol
reagent, following the manufacturer's protocol. A universal cDNA
synthesis kit (Invitrogen; Thermo Fisher Scientific, Inc.) was
utilized for reverse transcription. Each reaction contained 0.5 µl
random primers (0.2 µg/µl) and 1 µl SuperScript III reverse
transcriptase (200 U/µl). The specific primer for Nestin was
forward, CTGGAAGGTGGGCAGCAACT and reverse, TCTCAAGGGTATTAGGCAAGGG;
the primer for PAX6 was forward, CTGGAGTGTCAGTTCCCGTC and reverse,
ATACCGTGCCTTCTGTACGC; the primer for Thy1 was forward,
CAAAACGCGGGGAGAAATGG and reverse, CTGGTGTTCCATCGGGTCTC; and the
primer for Brn-3 was forward, TTTCCCCCTTTGTTCCGCTT and reverse,
GCCTAATGACGCCTAGCCAA. PCR was performed by utilizing a SYBR qPCR
mix kit (Invitrogen; Thermo Fisher Scientific, Inc.). PCR
conditions were as follows: Predenaturation at 95°C for 2 min, 40
cycles of denaturation at 95°C for 10 sec and annealing and
polymerization at 60°C for 30 sec and 70°C for 45 sec. PCR was
performed using a CFX96 Touch™ Real-Time PCR Detection
system (Bio-Rad Laboratories, Inc.). Gene expression was determined
and normalized to β-actin. The following rat β-actin primers were
used: Forward, 5′AGGGAAATCGTGCGTGAC3′ and reverse,
5′CGCTCATTGCCGATAGTG3′. The 2−ΔΔCq method was utilized
to measure PCR results (13).
Flow cytometry
Following induction with retinal ganglion
cell-conditioned medium, retinal progenitor cells were cultured
under surrounding pressure of 0 and 40 mmHg (50% confluence) at
37°C for 48 h. Cells were washed with PBS twice, and incubated with
trypsin at 37°C for 1 min. Following digestion, the cell suspension
was centrifuged at 400 × g at room temperature for 5 min. The cell
pellet was resuspended with PBS and the centrifugation and
resuspension steps were repeated a further two times. Cells were
fixed in 4% paraformaldehyde at room temperature for 10 min, and
blocked with 2% bovine serum albumin (Sigma-Aldrich; Merck KGaA)
for 30 min at room temperature. Anti-Thy1 antibody (10 µl; 1:200)
was added to 100 µl cell suspension, and incubated at 4°C for 30
min. Cells were centrifuged at 400 × g at room temperature for 5
min, and resuspended with PBS three times. Secondary green
fluorescent protein-labeled goat anti-rabbit IgG H&L antibody
(Alexa Fluor® 488; cat. no. ab150077; 1:2,000; Abcam)
was added into the cell suspension, and incubated at 4°C for 30
min. Cells were washed with PBS three times. Cells were resuspended
in 500 µl PBS, and detected by flow cytometry. Data was acquired on
an LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo
software. Experiments were repeated three times.
Statistical analysis
Statistical data was analyzed by GraphPad Prism
version 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
The results are presented as mean ± standard error. Differences
among ≥3 groups were compared by one-way analysis of variance
followed by the Bonferroni post hoc test. Differences between 2
groups were compared by Student's t-test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Derivation and identification of rat
retinal progenitor cells and retinal ganglion cells
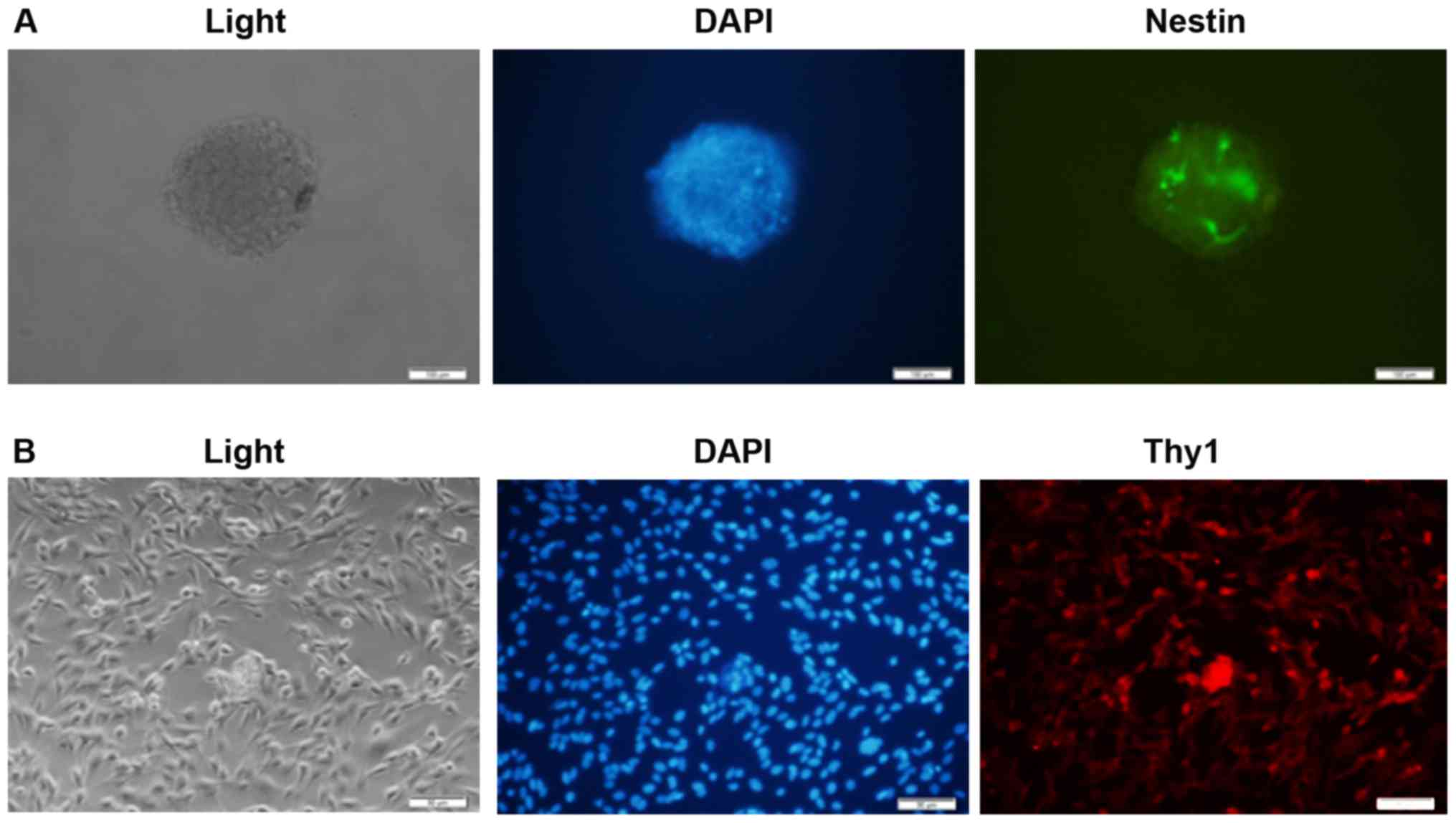
Retinal progenitor cells and ganglion cells were
isolated from rats as described in the methods. Immunofluorescence
was utilized to identify these cells. Rat retinal progenitor cells
were stained with a primary antibody against Nestin, and retinal
ganglion cells were stained with a primary antibody against Thy1.
Slides were observed with a fluorescence microscope. It was
demonstrated that the isolated retinal progenitor cells were
Nestin-positive, and retinal ganglion cells were Thy1-positive,
suggesting the success of the isolation (Fig. 2A and B). From the
immunofluorescence data, ~40 to 50% isolated cells were
Nestin+ and Thy1+, respectively. Some
non-specific positive staining may be observed.
Surrounding pressure induces apoptosis
in retinal progenitor cells in a pressure-dependent manner
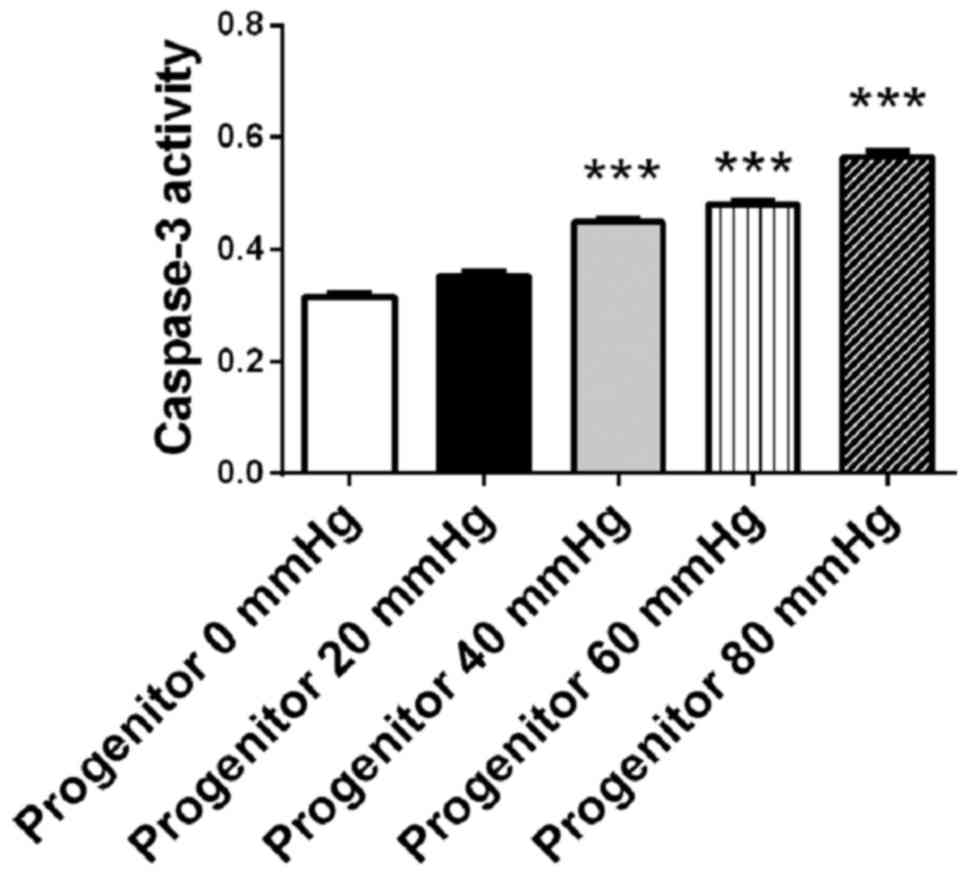
The retinal progenitor cell mixture was cultured for
48 h under surrounding pressures of 0, 20, 40, 60 and 80 mmHg.
Cellular apoptosis was detected using a caspase-3 assay kit. The
activity of caspase-3 increased in the retinal progenitor cell
mixture in a pressure-dependent manner. When the surrounding
pressure reached 40, 60 and 80 mmHg, the activity of caspase-3 in
the retinal progenitor cell mixture increased significantly
compared with cells that were not under pressure (0 mmHg;
P<0.001; Fig. 3). As 40–50% of
the cell mixture constituted retinal progenitor cells, and retinal
progenitor cells are more susceptible to increased pressure
compared with connective tissue cells or the epithelium, the
increase in apoptosis in the cell mixture suggested that this was
due to the presence of retinal progenitor cells. These results
suggested that surrounding pressure induced apoptosis in retinal
progenitor cells in a pressure-dependent manner.
Surrounding pressure induces apoptosis
in retinal ganglion cells in a pressure-dependent manner
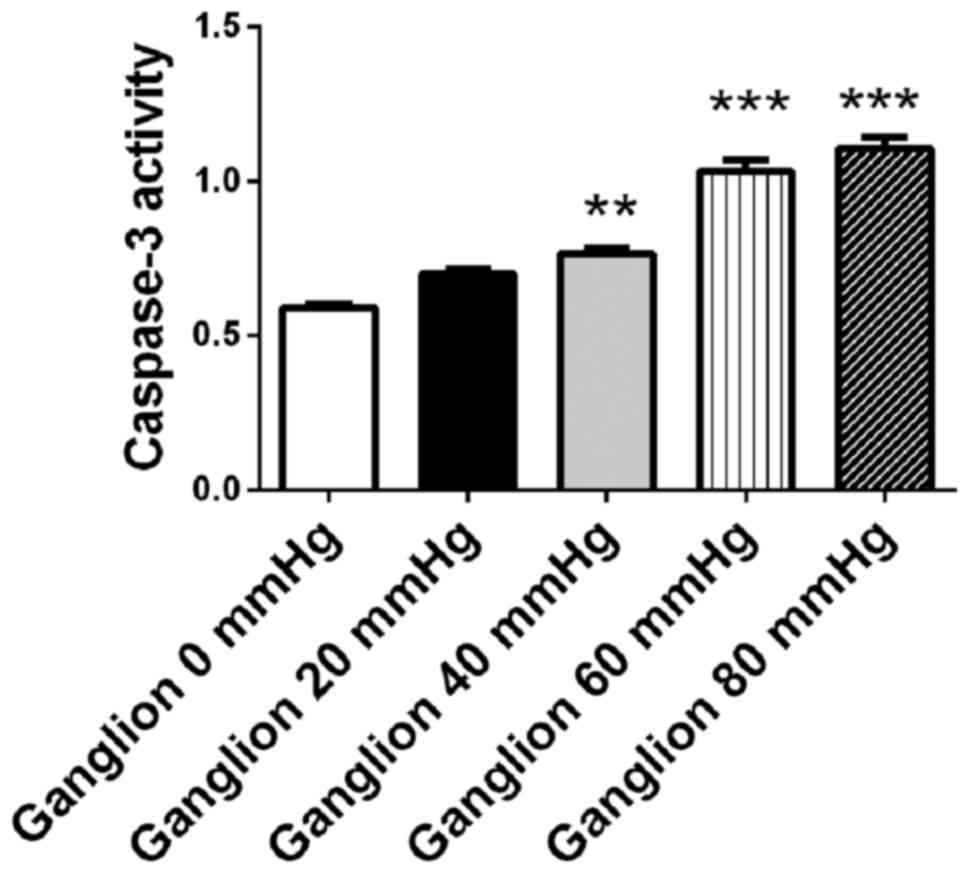
Retinal ganglion cells were cultured for 48 h under
surrounding pressure of 0, 20, 40, 60 and 80 mmHg. Cellular
apoptosis was detected using a caspase-3 assay kit. The activity of
caspase-3 increased in retinal ganglion cells in a
pressure-dependent manner. When the surrounding pressure reached
40, 60 and 80 mmHg, the activity of caspase-3 in retinal progenitor
cells increased compared with cells that were not under pressure
(P<0.01 at 40 mmHg; P<0.001 at 60 and 80 mmHg; Fig. 4). The results demonstrated that
surrounding pressure may induce apoptosis in retinal ganglion cells
in a pressure-dependent manner.
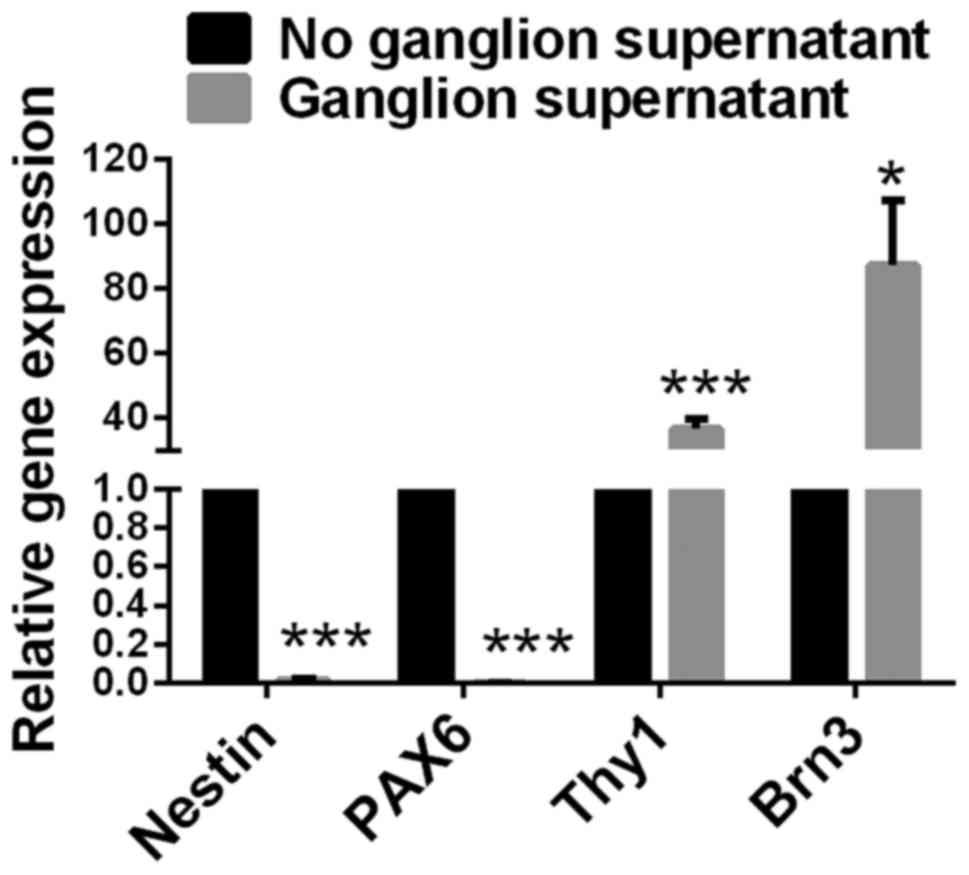
Expression of Nestin and PAX6
significantly decreases, and expression of Thy1 and Brn3 increases
in retinal progenitor cells cultured with retinal ganglion
cell-conditioned medium
The culture supernatant of rat retinal ganglion
cells was collected. Retinal progenitor cells were cultured in
retinal ganglion cell-conditioned medium for 72 h under normal
pressure. Retinal progenitor cells that were cultured without
retinal ganglion cell-conditioned medium served as a control. Gene
expression levels of Nestin, PAX6, Thy1 and Brn-3 in retinal
progenitor cells were detected by RT-qPCR. Compared with retinal
progenitor cells cultured without ganglion cell-conditioned medium,
cells cultured with ganglion cell-conditioned medium had
significantly decreased expression levels of Nestin and PAX6
(P<0.001), and significantly increased expression levels of Thy1
(P<0.001; Fig. 5) and Brn3
(P<0.05; Fig. 5).
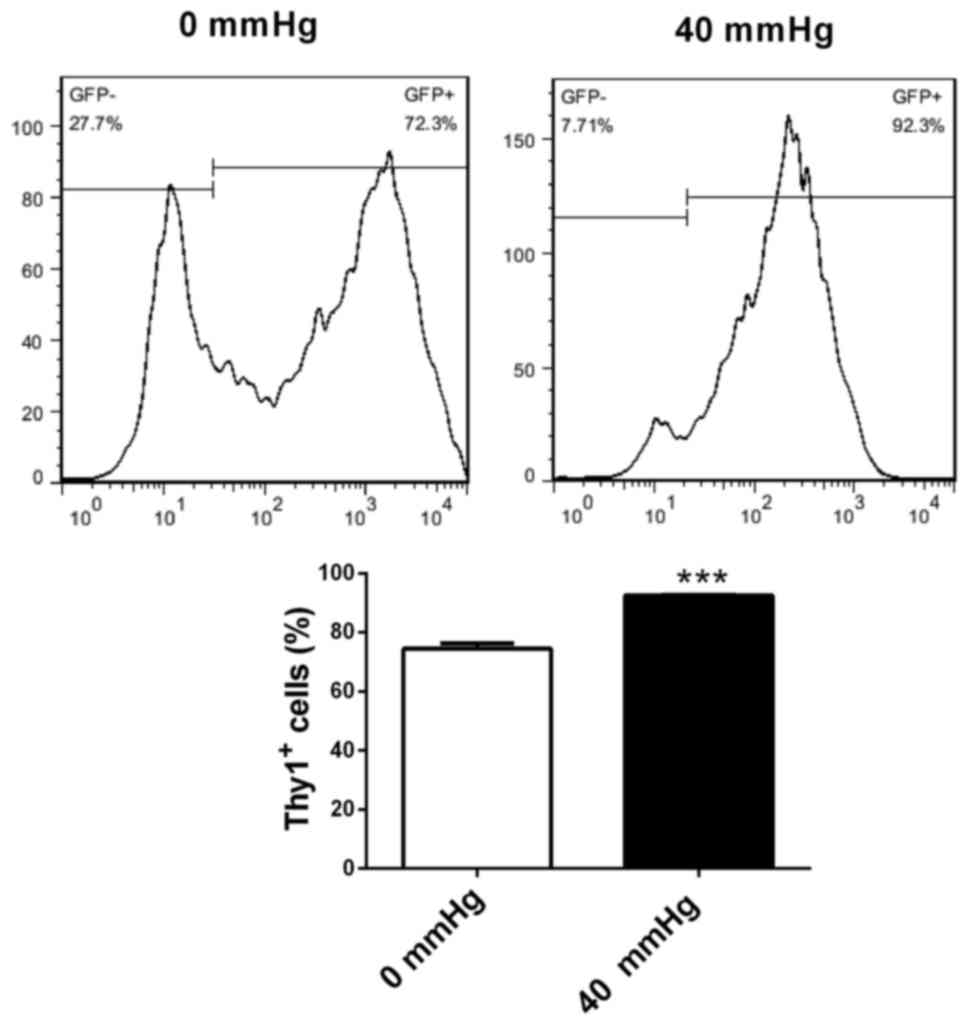
Increased surrounding pressure
stimulates differentiation of retinal progenitor cells into retinal
ganglion-like cells
Retinal progenitor cells were cultured in retinal
ganglion cell-conditioned medium for 72 h under surrounding
pressures of 0 and 40 mmHg. Flow cytometry was utilized to evaluate
the effects of pressure on the differentiation of retinal
progenitor cells into retinal ganglion cells. Compared with 0 mmHg
pressure, retinal progenitor cells cultured in ganglion
cell-conditioned medium under 40 mmHg pressure had increased
percentages of Thy1-positive cells (P<0.001; Fig. 6). This suggested that increased
surrounding pressure stimulated the differentiation of retinal
progenitor cells into retinal ganglion-like cells.
Discussion
The present study demonstrated that apoptosis in rat
retinal progenitor cells and retinal ganglion cells was
pressure-dependent. Retinal ganglion cell-conditioned medium
increased the differentiation of retinal progenitor cells into
retinal ganglion-like cells, and the differentiation increased as
the surrounding pressure increased.
Various animal models of different species have been
used to study glaucoma, including monkeys, dogs, cats, pigs and
rodents (14–18). Glaucoma in these animals was either
spontaneous or induced. These models have provided valuable
information about glaucoma. However, as the molecular mechanism of
glaucoma differs among animal species, data obtained from a
particular model may not be generalized to all species. Previously,
in vitro and ex vivo glaucoma models have been
developed to improve the accuracy and repeatability of experimental
conditions (19,20). Hydrostatic pressure was applied to
cells cultured in vitro and ex vivo. In addition,
transgenic mouse glaucoma models that were modified by the
introduction of a foreign DNA sequence into a mouse egg, have
emerged (21). In the present
study, an in vitro glaucoma model was utilized to ensure
accuracy and repeatability.
It was revealed that apoptosis in rat ganglion cells
was pressure-dependent. In a rat model of glaucoma, elevation of
phosphorylated N-methyl-D-aspartate receptor 2A by cyclin dependent
kinase (cdk)5/p35 was revealed to cause apoptosis in retinal
ganglion cells. Apoptosis was ameliorated by inhibiting cdk5/p35
(22). Reactivated Muller cells
were demonstrated to release excessive adenosine triphosphate,
causing apoptosis in retinal ganglion cells via activation of
purinergic receptor P2X 7 receptors (23). The proliferation and apoptosis of
retinal ganglion cells was additionally reported to be mediated by
the microRNA-187/mothers against decapentaplegic homolog 7 axis. A
decrease in miR-187 induced apoptosis and inhibited proliferation
in retinal ganglion cells (24).
In addition, the apoptosis of retinal ganglion cells has been
observed in other retinal diseases. In a rat model of light-induced
retinal damage, transcription factor FOS-related antigen 1 was
observed to be associated with apoptosis in retinal ganglion cells
following light exposure, regulated by p38 mitogen-activated
protein kinase (MAPK) through a cell cycle re-entry mechanism
(25). Palmitic acid induced
apoptosis in retinal ganglion cells through the protein kinase
B/forkhead box protein O1 signaling pathway (26). The gene expression levels and
signaling pathways in retinal ganglion cells that are directly
affected by increased surrounding pressure, which may trigger
cellular apoptosis, require further investigation.
It was additionally demonstrated in the present
study that apoptosis in rat retinal progenitor cells was
pressure-dependent. Various factors may impact on the proliferation
of retinal progenitor cells. Activation of the type 5 metabotropic
glutamate receptor promoted the proliferation of rat retinal
progenitor cells through activation of the phosphatidylinositol
3-kinase and MAPK signaling pathways (27). Mutual antagonism of the paired-type
homeobox genes, visual system homeobox 2 and
diencephalon/mesencephalon homeobox 1, was demonstrated to regulate
retinal progenitor cell cycle exit upstream of cyclin D1 expression
(28). Toll-like receptor and
MyD88-dependent and -independent pathways were revealed to be
negative regulators of proliferation in retinal progenitor cells
(29). SUMOylation controlled
retinal progenitor proliferation by repressing cell cycle exit in
Xenopus laevis (30).
Tropomyosin receptor kinase C signaling was additionally required
for retinal progenitor cell proliferation (31). The present study revealed for the
first time that increased surrounding pressure induced apoptosis in
retinal progenitor cells in a pressure-dependent manner. Increased
research efforts are required to elucidate the possible underlying
molecular mechanisms.
In addition, the present study revealed that retinal
ganglion cell-conditioned medium increased the differentiation of
retinal progenitor cells into retinal ganglion-like cells, and
differentiation increased as surrounding pressure increased. The
differentiation of retinal progenitor cells may be affected by many
factors. The yes-associated protein gene was revealed to be
essential for the cell cycle progression of retinal progenitor
cells, and differentiation towards retinal pigment epithelium in
the developing mouse eye (32).
Perturbations during the early proliferative stages of retinal
progenitor cells fated to be rods and bipolar cells altered the
coordinated time-dependent progression of differentiation, and
synaptic development (33).
Activin/nodal signaling was reported to support the differentiation
of retinal progenitor cells in a narrow time window during
pluripotent stem cell neutralization (34). The Hippo signaling pathway
controlled a switch between retinal progenitor cell proliferation
and photoreceptor cell differentiation in zebrafish (35). Vascular endothelial growth factor
was reported to activate divergent intracellular signaling
components to regulate retinal progenitor cell proliferation and
neuronal differentiation (36). In
the present study, it was demonstrated that retinal ganglion
cell-conditioned medium induced the differentiation of retinal
progenitor cells into ganglion-like cells. It is likely that
various growth factors were secreted by retinal ganglion cells into
the culture medium, which stimulated the differentiation of retinal
progenitor cells towards a ganglion direction. Increased
surrounding pressure, as a stress factor, may activate the
differentiation signaling of retinal progenitor cells towards
ganglion regeneration. The specific growth factors or mediators
involved, and the differentiation signaling pathway that is
switched on in retinal progenitor cells, require further
investigation.
In conclusion, the present study demonstrated that
apoptosis in rat retinal progenitor cells and retinal ganglion
cells was pressure-dependent, and retinal ganglion cell-conditioned
medium increased the differentiation of retinal progenitor cells
into ganglion-like cells. In addition, differentiation increased as
surrounding pressure increased. Although further investigation is
required, these results pave the foundation for possible cell
therapy for glaucoma.
Acknowledgements
The present study was supported by the Joint
Specialized Research Fund from Yunnan Provincial Department of
Science and Technology and Kunming Medical University (grant no.
2014FB075), and Yunnan Provincial Department of Education Research
Fund (grant no. 2014C046Y).
References
|
1
|
Quigley HA and Broman AT: The number of
people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol.
90:262–267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sommer A, Tielsch JM, Katz J, Quigley HA,
Gottsch JD, Javitt J and Singh K: Relationship between intraocular
pressure and primary open angle glaucoma among white and black
Americans. The Baltimore Eye Survey. Arch Ophthalmol.
109:1090–1095. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamazaki M, Omodaka K, Takahashi H and
Nakazawa T: Estimated retinal ganglion cell counts for assessing a
wide range of glaucoma stages, from preperimetric to advanced. Clin
Exp Ophthalmol. 45:310–313. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirooka K, Izumibata S, Ukegawa K, Nitta E
and Tsujikawa A: Estimating the rate of retinal ganglion cell loss
to detect glaucoma progression: An observational cohort study.
Medicine (Baltimore). 95:e42092016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salehi H, Amirpour N, Razavi S, Esfandiari
E and Zavar R: Overview of retinal differentiation potential of
mesenchymal stem cells: A promising approach for retinal cell
therapy. Ann Anat. 210:52–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jagatha B, Divya MS, Sanalkumar R,
Indulekha CL, Vidyanand S, Divya TS, Das AV and James J: In vitro
differentiation of retinal ganglion-like cells from embryonic stem
cell derived neural progenitors. Biochem Biophys Res Commun.
380:230–235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang C, Zhang J, Ao M, Li Y, Zhang C, Xu
Y, Li X and Wang W: Combination of retinal pigment epithelium
cell-conditioned medium and photoreceptor outer segments stimulate
mesenchymal stem cell differentiation toward a functional retinal
pigment epithelium cell phenotype. J Cell Biochem. 113:590–598.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiu G, Seiler MJ, Thomas BB, Wu K,
Radosevich M and Sadda SR: Revisiting nestin expression in retinal
progenitor cells in vitro and after transplantation in vivo. Exp
Eye Res. 84:1047–1059. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Davis LK, Meyer KJ, Rudd DS, Librant AL,
Epping EA, Sheffield VC and Wassink TH: Pax6 3′ deletion results in
aniridia, autism and mental retardation. Hum Genet. 123:371–378.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki N, Shimizu J, Takai K, Arimitsu N,
Ueda Y, Takada E, Hirotsu C, Suzuki T, Fujiwara N and Tadokoro M:
Establishment of retinal progenitor cell clones by transfection
with Pax6 gene of mouse induced pluripotent stem (iPS) cells.
Neurosci Lett. 509:116–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang W, Fileta J, Guo Y and Grosskreutz
CL: Downregulation of Thy1 in retinal ganglion cells in
experimental glaucoma. Curr Eye Res. 31:265–271. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jain V, Ravindran E and Dhingra NK:
Differential expression of Brn3 transcription factors in
intrinsically photosensitive retinal ganglion cells in mouse. J
Comp Neurol. 520:742–755. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vecino E: Animal models in the study of
the glaucoma: Past, present and future. Arch Soc Esp Oftalmol.
83:517–519. 2008.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rasmussen CA and Kaufman PL: Primate
glaucoma models. J Glaucoma. 14:311–314. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brooks DE: Glaucoma in the dog and cat.
Vet Clin North Am Small Anim Pract. 20:775–797. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ruiz-Ederra J, García M, Hernández M,
Urcola H, Hernández-Barbáchano E, Araiz J and Vecino E: The pig eye
as a novel model of glaucoma. Exp Eye Res. 81:561–569. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pang IH and Clark AF: Rodent models for
glaucoma retinopathy and optic neuropathy. J Glaucoma. 16:483–505.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wax MB, Tezel G, Kobayashi S and Hernandez
MR: Responses of different cell lines from ocular tissues to
elevated hydrostatic pressure. Br J Ophthalmol. 84:423–428. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishikawa M, Yoshitomi T, Zorumski CF and
Izumi Y: Effects of acutely elevated hydrostatic pressure in a rat
ex vivo retinal preparation. Invest Ophthalmol Vis Sci.
51:6414–6423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harada T, Harada C, Nakamura K, Quah HM,
Okumura A, Namekata K, Saeki T, Aihara M, Yoshida H, Mitani A and
Tanaka K: The potential role of glutamate transporters in the
pathogenesis of normal tension glaucoma. J Clin Invest.
117:1763–1770. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen J, Miao Y, Wang XH and Wang Z:
Elevation of p-NR2A(S1232) by Cdk5/p35 contributes to retinal
ganglion cell apoptosis in a rat experimental glaucoma model.
Neurobiol Dis. 43:455–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xue B, Xie Y, Xue Y, Hu N, Zhang G, Guan H
and Ji M: Involvement of P2X7 receptors in retinal ganglion cell
apoptosis induced by activated Müller cells. Exp Eye Res.
153:42–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang QL, Wang W, Li J, Tian SY and Zhang
TZ: Decreased miR-187 induces retinal ganglion cell apoptosis
through upregulating SMAD7 in glaucoma. Biomed Pharmacother.
75:19–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Yang X, Zhu R, Dai M, Zhu M, Shen
Y, Fang H, Sang A and Chen H: Involvement of Fra-1 in retinal
ganglion cell apoptosis in rat light-induced retina damage model.
Cell Mol Neurobiol. 37:83–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yan P, Tang S, Zhang H, Guo Y, Zeng Z and
Wen Q: Palmitic acid triggers cell apoptosis in RGC-5 retinal
ganglion cells through the Akt/FoxO1 signaling pathway. Metab Brain
Dis. 32:453–460. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Z, Hu F, Liu Y, Ma B, Chen X, Zhu K,
Shi Y, Wei T, Xing Y, Gao Y, et al: Activation of type 5
metabotropic glutamate receptor promotes the proliferation of rat
retinal progenitor cell via activation of the PI-3-K and MAPK
signaling pathways. Neuroscience. 322:138–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong L, Power N, Miles A and Tropepe V:
Mutual antagonism of the paired-type homeobox genes, vsx2 and
dmbx1, regulates retinal progenitor cell cycle exit upstream of
ccnd1 expression. Dev Biol. 402:216–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shechter R, Ronen A, Rolls A, London A,
Bakalash S, Young MJ and Schwartz M: Toll-like receptor 4 restricts
retinal progenitor cell proliferation. J Cell Biol. 183:393–400.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Terada K and Furukawa T: Sumoylation
controls retinal progenitor proliferation by repressing cell cycle
exit in Xenopus laevis. Dev Biol. 347:180–194. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Das I, Sparrow JR, Lin MI, Shih E, Mikawa
T and Hempstead BL: Trk C signaling is required for retinal
progenitor cell proliferation. J Neurosci. 20:2887–2895. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim JY, Park R, Lee JH, Shin J, Nickas J,
Kim S and Cho SH: Yap is essential for retinal progenitor cell
cycle progression and RPE cell fate acquisition in the developing
mouse eye. Dev Biol. 419:336–347. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chaney SY, Mukherjee S, Giddabasappa A,
Rueda EM, Hamilton WR, Johnson JE Jr and Fox DA: Increased
proliferation of late-born retinal progenitor cells by gestational
lead exposure delays rod and bipolar cell differentiation. Mol Vis.
22:1468–1489. 2016.PubMed/NCBI
|
|
34
|
Bertacchi M, Lupo G, Pandolfini L,
Casarosa S, D'Onofrio M, Pedersen RA, Harris WA and Cremisi F:
Activin/nodal signaling supports retinal progenitor specification
in a narrow time window during pluripotent stem cell neuralization.
Stem Cell Reports. 5:532–545. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Asaoka Y, Hata S, Namae M, Furutani-Seiki
M and Nishina H: The Hippo pathway controls a switch between
retinal progenitor cell proliferation and photoreceptor cell
differentiation in zebrafish. PLoS One. 9:e973652014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hashimoto T, Zhang XM, Chen BY and Yang
XJ: VEGF activates divergent intracellular signaling components to
regulate retinal progenitor cell proliferation and neuronal
differentiation. Development. 133:2201–2210. 2006. View Article : Google Scholar : PubMed/NCBI
|