Introduction
The rapid development of human society and economy
has rendered great changes in people's ways of work and life
(1). Factors such as aging of
social population, obesity, unhealthy living habits and heredity,
have given rise to the gradually increased morbidity of diabetes
mellitus (DM) (2). DM has become
one of the global public health problems severely threatening human
health after cardiovascular disease and tumor (2). According to the International
Diabetes Federal (IDF) statistics, the world has witnessed
approximately 0.246 billion DM patients in 2007 (1). Such fig. will increase to 0.380
billion by 2025 if no comprehensive control measure is carried out.
DM epidemiological investigation in China in 1980 and 1994
suggested that DM morbidity in adult was 0.9 and 2.5%, respectively
(3).
DM is associated with long course of disease, many
complications and high medical expenses, which has greatly affected
the patients and the society (4).
At present, non-infectious diseases dominated by DM, tumor and
cardiovascular disease have become the major causes of global death
(5). Therefore, carrying out
research on the prevention and treatment of DM and its
complications is of great social significance (5). Disorder of sugar, lipid and protein
metabolism will induce systemic pathophysiological damage in
multiple systems, tissues and organs (5). Moreover, it will also lead to
complications in organs like eye, kidney, heart and brain (5). Of them, nervous system complications
are the most common, such as DM neuropathy (5). At present, numerous reports regarding
DM peripheral neuropathy are available from domestic and foreign
medical scientific research (6).
Particularly, DM cognitive impairment induced by DM central nervous
system injury has been extensively studied.
Silent information regulator 1 (SIRT1) is a
nicotinamide adenine dinucleotide (NAD+)-dependent histone
deacetylase (7). It can act on
histone and multiple non-histones, thus being involved in multiple
physiopathological processes like anti-inflammation, anti-oxidative
stress, anti-apoptosis and anti-cell aging (8).
miRNA is a big family of small molecule non-coding
single-strand RNA with the length of approximately 20–25 basic
groups (9). It is formed by a
segment of single-strand RNA precursor with hairpin loop structure,
which is 70–90 basic groups in length, after Dicer digestion
(9). It can regulate gene
expression at transcription level or post-transcription level
(10). miRNA can bind with the
3′untranslated region (UTR) of target message RNA (mRNA), thus
exerting its function (10). In
addition, it can result in transcription inhibition, and mRNA
instability or degradation, and thus affect the pathophysiological
processes of diseases (11).
Plenty of miRNAs have been discovered in brain
tissue of mammals at present (10). They are related to brain tissue
development, neuronal differentiation and senior neurological
functions (such as learning and memory) (6). Moreover, they are also associated
with diseases like neurodegenerative disease, mental disorder and
brain tumor (6). As is found in
recent research, miRNAs are also involved in regulating neuron cell
cycle control and apoptosis in Alzheimer's disease (AD) and
Parkinson's disease (PD) (12).
Besides, they also participate in regulating the pathological
process of post-ischemic brain injury (12). In this report, we investigated the
exact roles and mechanisms of miRNA-23b-3p on cognitive impairment
of diabetic rats.
Materials and methods
Animals
Firstly, male 8-week-old Wistar rats (180–220 g)
were purchased from the Laboratory Animal Center of Jilin
University, and housed in standard laboratory cages at 22–24°C with
a 12-h light/dark cycle and were provided free access to food and
water. The experimental animals were randomly divided into two
groups, the control group (n=6) and the DM group (n=6). DM model
group, Wistar rats were induced via a single injection of 50 mg/kg
streptozotocin (STZ; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany)
into the left lower abdominal cavity. On 8 week after STZ
injection, the rats were anaesthetised with sodium pentobarbital
(35 mg/kg, intraperitoneally; Beijing Propbs Biotechnology, Co.,
Ltd., Beijing, China). All experimental protocols and procedures
were approved by the Animal Care and Ethics Committee of The Second
Hospital of Jilin University (Jilin, China).
Next, 18 rats were randomly divided into three
groups, the control group (n=6), the DM group (n=6) and SIRT1 group
(n=6). SIRT1 group, after injection of STZ for 4 weeks, DM rat was
gavaged with 50 mg/kg of SRT1720 (SIRT1 agonist, MedChemExpress)
for 4 weeks.
Haematoxylin and eosin (H&E)
staining
After treatment with SRT1720, rats was anaesthetised
with 35 mg/kg of sodium pentobarbital and sacrificed by decollation
(13). Then, brain tissue samples
were separated and hippocampus was stripped as reference (14). Hippocampus was fixed by 4%
paraformaldehyde for 24 h, and sample was cleared with xylene and
routinely embedded in paraffin. Tissue was cut into Serial coronal
sections (10 µm), and then deparaffinised, rehydrated and then
stained with H&E at room temperature for 15 min. Samples were
observed via light microscopy (Olympus, Tokyo, Japan).
RNA extraction for microRNA expression
analysis
Total RNA was extracted from PC12 cells or
hippocampus tissue using miRNeasy® Mini kit (Qiagen
GmbH, Hilden, Germany). 500 ng total RNA was changed to cDNA by
miScript II RT kit (Qiagen, Inc., Valencia, CA, USA). QuantiTect
SYBR Green PCR Master (Qiagen, Inc.) was used to Qpcr using a
7900HT thermocycler (Applied Biosystems; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Qpcr were as follows: 50°C for 2 min, 95°C
for 10 min, followed by a third step for denaturation at 95°C for
15 sec and at 60°C for 1 min repeated for 40 cycles. Data were
presented as fold change in expression and were calculated as
2−ΔΔCq.
Morris water maze teat
Cognitive function was evaluated using Morris water
maze test after treatment with SRT1720. Rat was trained twice per
day and the test was performed blindly for five days. Swimming
study was video tracked, and latency, path length and swim speed.
Then, cumulative distance at the platform was recorded. The mean
time spent in the correct quadrant containing the platform after a
probe trial and the mean number of time that mice crossed the
former platform position during 1.5 min were analzyed.
In vitro model and transfection
PC12 cells were cultured in DMEM (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) containing 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C in 5%
CO2. 50 nM of miRNA-23b-3p mimics
(CTCAGGTGCTCTGGCTGCTTGGGTTCCTGGCATGCTGATTTGTGACTTAAGATTAAAATCACATTGCCAGGGATTACCACGCAACCACGACCTTGGC)
and negative mimics (CCCCCCCCCCCCCC) were transfected into cell
using with RNAiMAX (Thermo Fisher Scientific, Inc.). After
transfection for 12 h, PC12 cells (1×106 cell/per well)
were treated with 25 mg/ml of glucose for 24, 48 or 72 h.
Cell Proliferation assay
After treatment with glucose for 24, 48 or 72 h,
PC12 cells (1×103 cell/per well) proliferation was
measured using MTT assay (American Type Culture Collection,
Manassas, VA, USA) for 4 h at 37°C. DMSO assay was added into cell
for 20 min at 37°C. Absorbance was measured using ELISA reader (a
Multiskan EX; Thermo Labsystems, Helsinki, Finland) at 490 nm.
Apoptosis assay and Caspase-3/9
activity
After treatment with glucose for 48 h, PC12 cells
(1×106 cell/per well) was harvested and stained with 5
µl Annexin V-PE and 5 µl propidium iodide using an Apoptosis kit
(BD Pharmingen, Franklin Lakes, NJ, USA) for 15 min at darkness.
Apoptosis rate was measured using with a flow cytometer
(FACSCalibur™ system; BD Biosciences, San Jose, CA, USA).
Caspase-3/9 activity was measured using Caspase-3/9
activity kits (Beyotime, Shanghai, China). Absorbance was measured
using ELISA reader (a Multiskan EX; Thermo Labsystems) at 405
nm.
Measurement of oxidatie stress
After treatment with glucose for 48 h or treatment
with SIRT1 for 4 weeks, PC12 cells (1×106 cell/per well)
or hippocampus tissue were collected and protein was splitted using
RIPA assay and protein concentration was determined using BCA kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). 5 µg protein was
used to measure MDA, GSH, GSH-PX, and SOD levels using ELISA kits
and absorbance was measured using ELISA reader (a Multiskan EX;
Thermo Labsystems) at 450 nm.
Western blot analysis
The hippocampus after treatment or PC12 cells
(1×106 cell/per well) after transfection was splitted
using RIPA assay and protein concentration was determined using BCA
kit (Bio-Rad Laboratories, Inc.). Protein samples (50 µg) were
separated via 8–12% (w/v) SDS-PAGE and transferred onto PVDF
membranes. The membranes were blocked in PBST buffer containing 5%
(w/v) skimmed milk for 1 h at 37°C and incubated with Bax (sc-493,
1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), Sirt1
(sc-15404, 1:500; Santa Cruz Biotechnology, Inc.), nuclear factor
erythroid 2-related factor 2 (Nrf2; sc-722, 1:500; Santa Cruz
Biotechnology, Inc.) and GAPDH (sc-25778, 1:500; Santa Cruz
Biotechnology, Inc.) overnight at 4°C. The membranes were then
incubated with the corresponding horseradish peroxidase-conjugated
secondary antibody (7074, 1:5,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA) at 37°C for 1 h. The protein levels were measured
using ECL kit (Bio-Rad Laboratories, Inc.) and quantified using
Quantity One software version 4.62 (Bio-Rad Laboratories,
Inc.).
Statistical analysis
All values are expressed as the mean ± standard
deviation. One-way analysis of variance followed by Dunnett's
t-test was used for comparison between the groups. A two-tailed
value of P<0.05 was considered to indicate a statistically
significant value.
Results
miRNA-23b-3p expression
In order to analyze the change levels of
miRNA-23b-3p in cognitive impairment of diabetic rats, it may be a
importance role for cognitive impairment of diabetic rats or
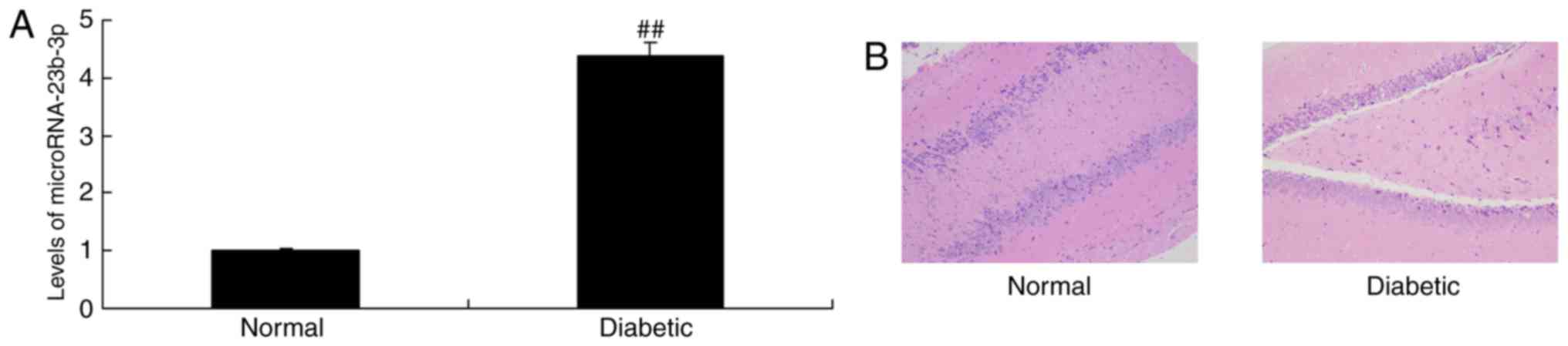
neurocyte cell apoptosis. As shown in Fig. 1, miRNA-23b-3p expression was
up-regulated, and neurocyte appeared dearth in cognitive impairment
of diabetic rats, compared with normal control group.
Overexpression of miRNA-23b-3p
increased apoptosis in neurocyte cell by high-glucose
We studied the possible implication of miRNA-23b-3p
in neurocyte cell apoptosis by high-glucose. This result suggests
miRNA-23b-3p mimics increased miRNA-23b-3p expression in neurocyte
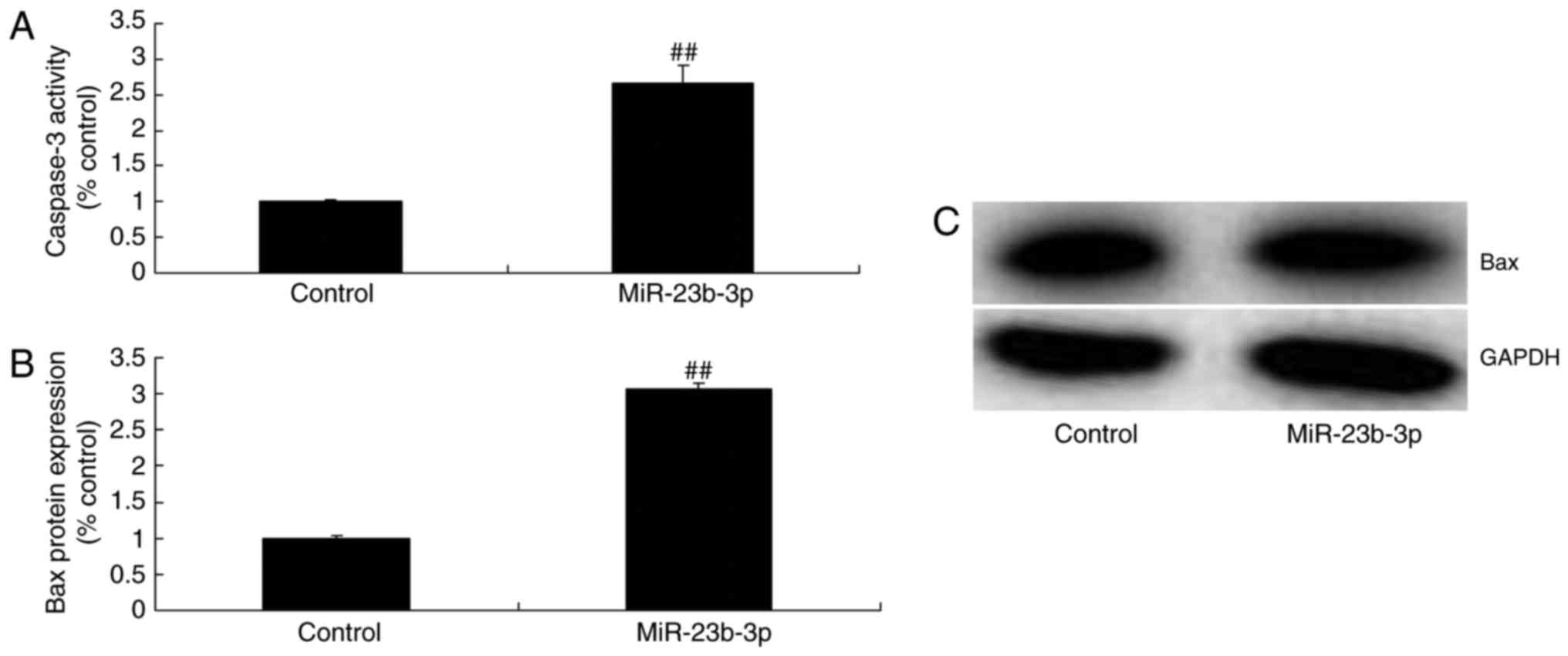
cell by high-glucose, compared with control group (Fig. 2A). Then, overexpression of
miRNA-23b-3p inhibited cell proliferation, and increased apoptosis,
Bax protein expression and caspase-3 activity in neurocyte cell by
high-glucose, compared with control group (Figs. 2B, C and 3).
Overexpression of miRNA-23b-3p
increased oxidative stress in neurocyte cell by high-glucose
Meanwhile, overexpression of miRNA-23b-3p also
increased oxidative stress in neurocyte cell by high-glucose,
compared with control group, which miRNA-23b-3p may mediated
oxidative stress to induce neurocyte cell apoptosis (Fig. 4).
Overexpression of miRNA-23b-3p
suppressed SIRT1 and Nrf2 protein expression in neurocyte cell by
high-glucose
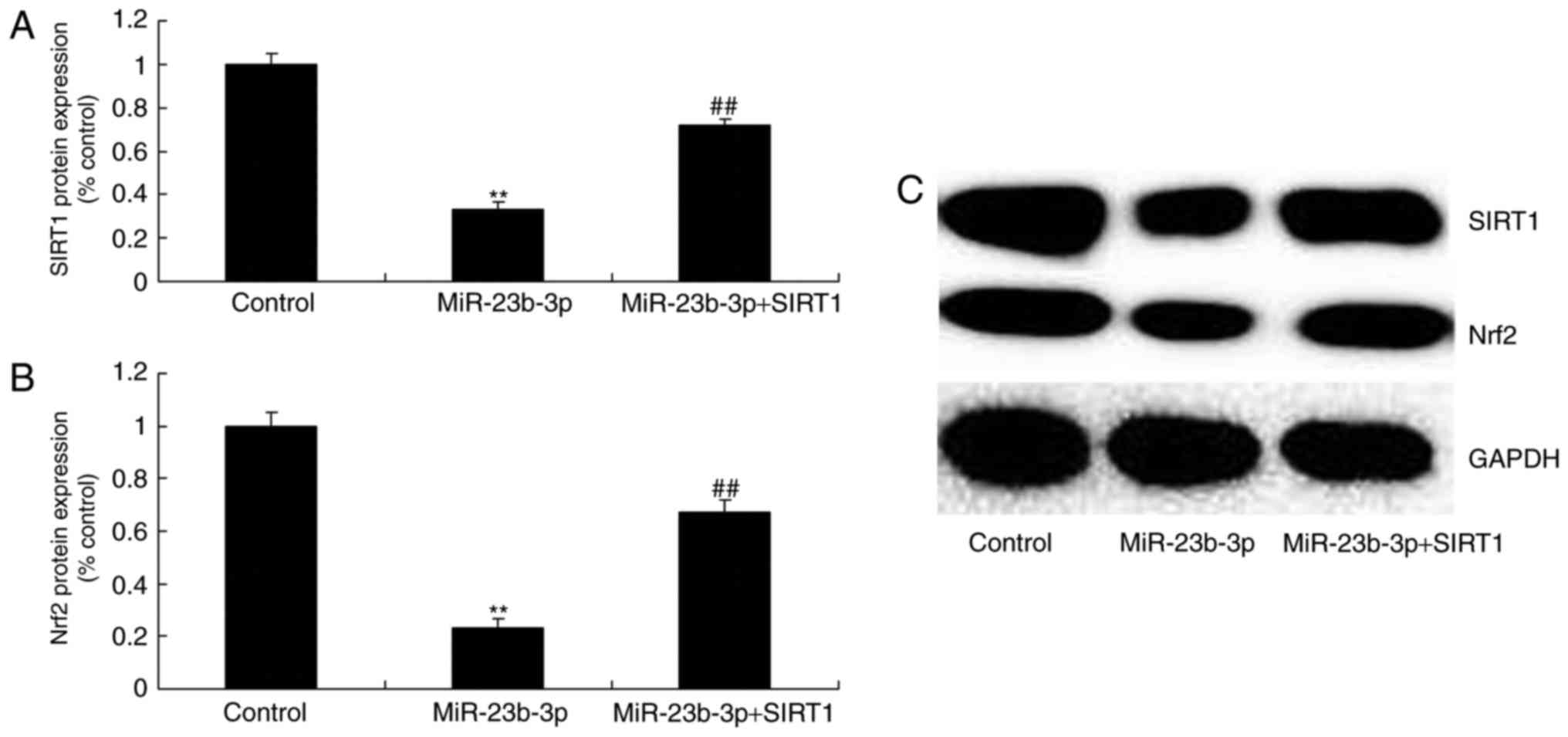
We detected SIRT1 and Nrf2 protein expression to
explore the mechanism of miRNA-23b-3p on oxidative stress, SIRT1
and Nrf2 protein expression were measured. Overexpression of
miRNA-23b-3p suppressed SIRT1 and Nrf2 protein expression in
neurocyte cell by high-glucose (Fig.
5). These results support the theory that miRNA-23b-3p an
oncogene that may contribute to oxidative stress in neurocyte cell
by high-glucose.
In vitro model of neurocyte cell by
high-glucose, the promotion of SIRT1 induced SIRT1 and Nrf2 protein
expression
SRT1720, SIRT1 agonist, 10 µM for 48 h, was added
into cell following miRNA-23b-3p, and SIRT1 and Nrf2 protein
expression were measured. Fig. 6
showed that SIRT1 agonist induced SIRT1 and Nrf2 protein expression
in neurocyte cell by high-glucose following miRNA-23b-3p, compared
with miRNA-23b-3p group.
In vitro model of neurocyte cell by
high-glucose, the promotion of SIRT1 inhibited apoptosis
Then, in vitro model of neurocyte cell by
high-glucose, the promotion of SIRT1 inhibited the suppression of
cell proliferation, and activation of apoptosis rate, Bax and
caspase-3 activity in neurocyte cell by high-glucose following
miRNA-23b-3p, compared with miRNA-23b-3p group (Figs. 7 and 8).
In vitro model of neurocyte cell by
high-glucose, the promotion of SIRT1 inhibited oxidative
stress
Additionally, the promotion of SIRT1 inhibited the
activation of MDA level, and inactivation of GSH, GSH-PX, and SOD
levels in neurocyte cell by high-glucose following miRNA-23b-3p,
compared with miRNA-23b-3p group (Fig.
9). In vitro model of neurocyte cell by high-glucose,
the promotion of SIRT1 inhibited oxidative stress, which may be
regulated neurocyte cell apoptosis to generate cognitive impairment
of diabetic rats.
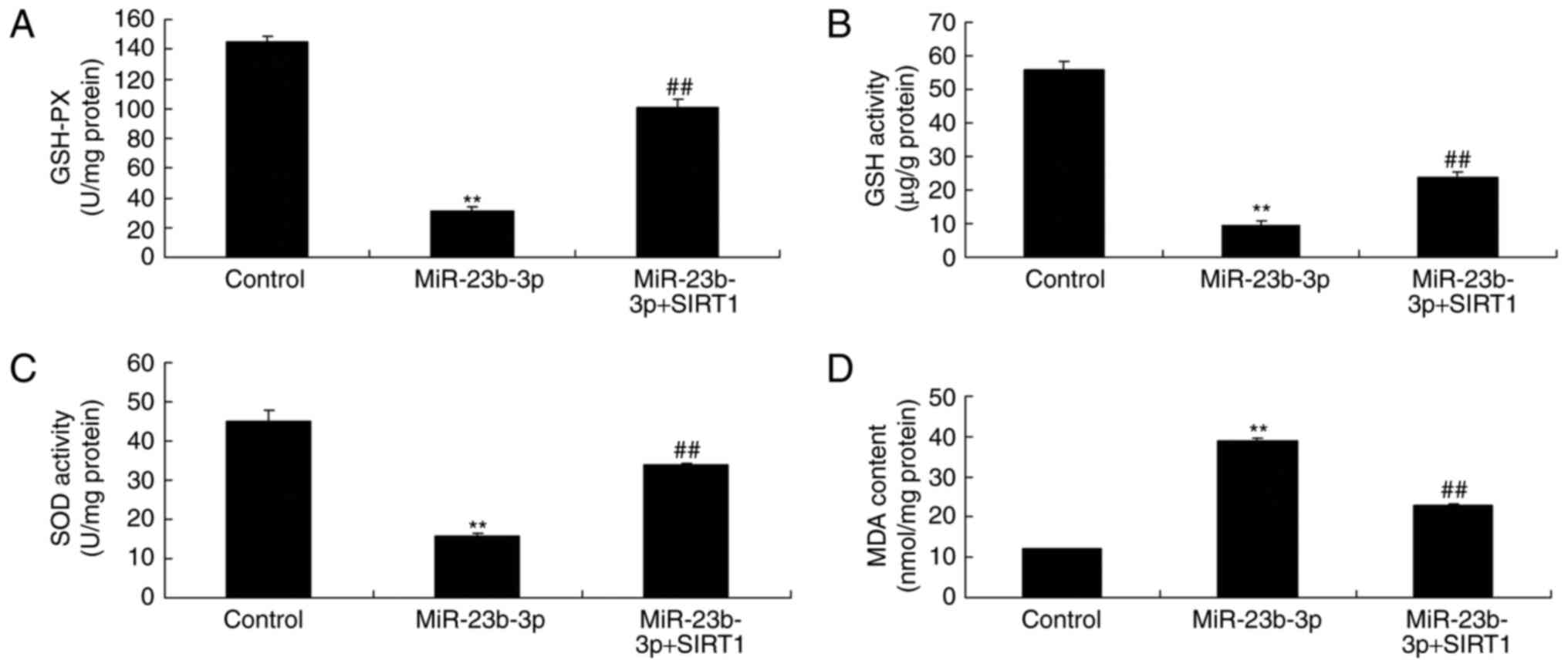 | Figure 9.An in vitro model of
neurocytes with high glucose treatment and the promotion of
SIRT1-induced inhibition of oxidative stress. Levels of (A) GSH-PX,
(B) GSH activity, (C) SOD activity and (D) MDA content. **P<0.01
vs. control group; ##P<0.01 vs. miR-23b-3p group.
Control, negative control group; miR-23b-3p, overexpression of
microRNA-23b-3p group; miR-23b-3p+SIRT1, miRNA-23b-3p
overexpression and SRT1720 (SIRT1 agonist) group; SIRT1, silent
information regulator 1; GSH-PX, glutathione peroxidase; GSH,
glutathione; SOD, superoxide dismutase; MDA, malondialdehyde. |
In vivo of diabetic rats, the
promotion of SIRT1 induced SIRT1 and Nrf2 protein expression
So, we used vivo of diabetic rats to determine the
function of SIRT1 on cognitive impairment of diabetic rats.
SRT1720, SIRT1 agonist, 50 mg/kg, 4 weeks, induced SIRT1 and Nrf2
protein expression in diabetic rats, compared with DM rat (Fig. 10).
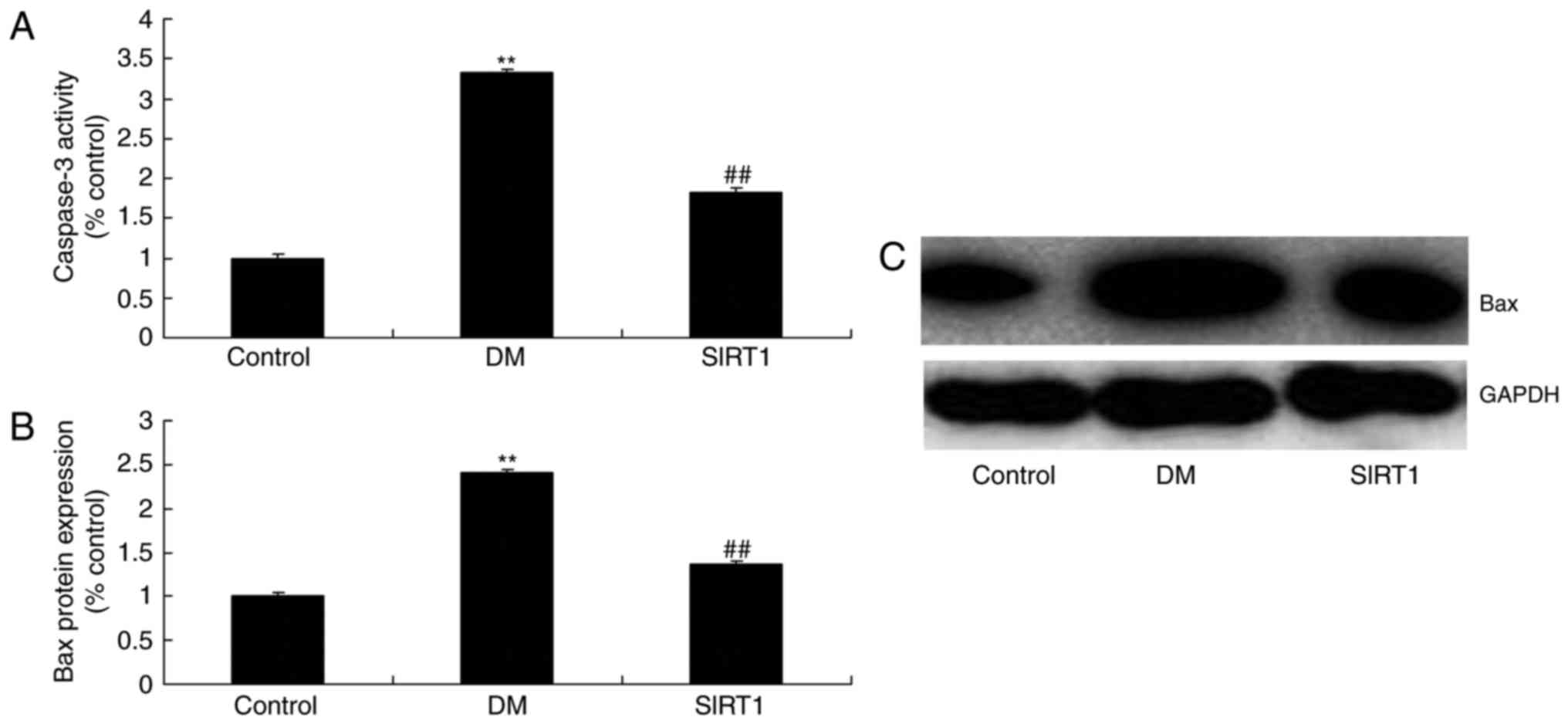
In vivo of diabetic rats, the
promotion of SIRT1 inhibited apoptosis and oxidative stress
The promotion of SIRT1 inhibited the activation of
apoptosis rate, Bax and caspase-3/9 activity, and the induction of
MDA level, and inhibition of GSH, GSH-PX, and SOD levels in
diabetic rats, compared with DM rat (Figs. 11 and 12).
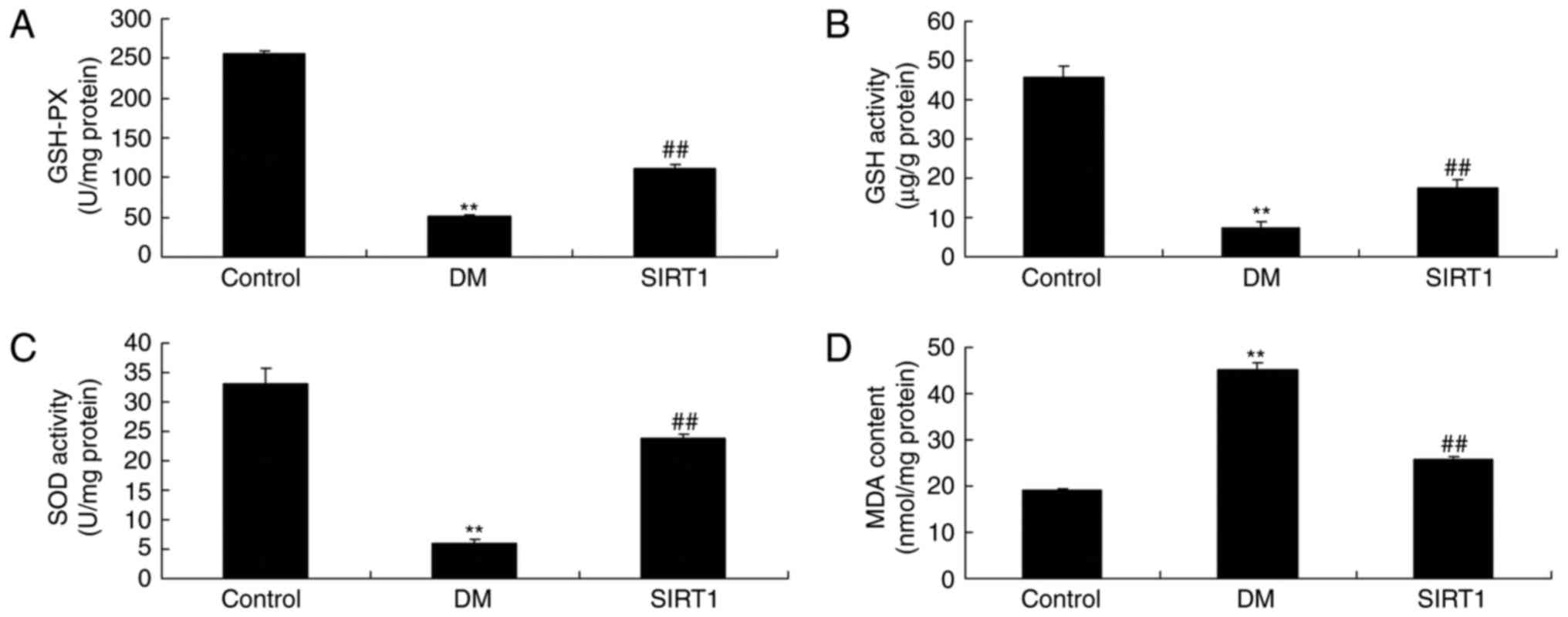 | Figure 12.An in vivo model of diabetes
in rats and the promotion of SIRT1-induced inhibition of oxidative
stress. Levels of (A) GSH-PX, (B) GSH activity, (C) SOD activity
and (D) MDA content. **P<0.01 vs. control group;
##P<0.01 vs. DM group. Control, negative control
group; DM, diabetic rat group; SIRT1, silent information regulator
1, agonist SRT1720 treatment group; GSH-PX, glutathione peroxidase;
GSH, glutathione; SOD, superoxide dismutase; MDA,
malondialdehyde. |
In vivo of diabetic rats, the
promotion of SIRT1 prevents cognitive impairment
In order to assess the effect of SIRT1 on cognitive
impairment of diabetic rats, Morris water maze teat was used to
measure. As showed in Fig. 13A and
B, the promotion of SIRT1 reduced shorter escape latency and
the mean path length in diabetic rats, compared with DM rat.
Meanwhile, DM rats spent less time in the target quadrant and the
number of times the animals crossed the former platform location
were increased by the promotion of SIRT1, compared with DM rat
(Fig. 13C and D).
Discussion
DM is a chronic and lifelong disease. Patients
should master DM monitoring knowledge and detect all indexes
regularly (15). This has played a
vital role in reducing the incidence of complications in DM
patients, as well as DM disability and fatality rates (15). DM cognitive impairment is a
complication characterized by DM-induced cognitive impairment,
accompanying with intracerebral structural and pathophysiological
changes (16). The study suggested
that miRNA-23b-3p expression was up-regulated, and neurocyte
appeared dearth in cognitive impairment of diabetic rats. In this
study, we only used Morris water maze to analyze cognitive
impairment, which is a limitation of the current study, and we will
execute more tests to measure the changes of cognitive impairment
in further study.
Some scholars have suggested renaming it as DM
encephalopathy and DM-related cognitive decline (17). But no consensus has been reached so
far. Its pathogenesis and pathophysiological changes remain
unclear. Previous research on glucose toxicity, insulin signal
disorder, unbalanced homeostasis, inflammation, oxidative stress
injury, vascular disease, and hypothalamus-pituitary-adrenal axis
abnormality, has attained certain progress (17). We found that overexpression of
miRNA-23b-3p inhibited cell proliferation, and increased apoptosis,
Bax protein expression and caspase-3 activity in neurocyte cell by
high-glucose. Zhan et al indicate that that miR-23b-3p
supplement may be a potential anabolic in kainic acid-induced
seizure (18).
Oxidative stress refers to dysfunction of
antioxidant defense system, which leads to excessive production of
reactive oxygen species and reactive nitrogen species, thus leading
to tissue and cell injury (17).
Oxidative stress is indicated in research to participate in DM
genesis and development. It can also induce reduced nerve
conduction velocity and promote neuronal apoptosis in DM mice
(4). This suggests that oxidative
stress is involved in DM-induced neuronal injury (4). In the present study, overexpression
of miRNA-23b-3p also increased oxidative stress in neurocyte cell
by high-glucose. Jiang et al reported that microRNA-23b-3p
up-regulate reactive oxygen species (ROS) and oxidative stress in
acute myeloid leukemia (19).
Oxidative stress is a pathological state caused by
excessive production of oxygen free radical (17). This has given rise to excessive
accumulation of oxygen free radical and related metabolites, thus
producing all kinds of cytotoxicities (20). The occurrence of oxidative stress
can also induce cell aging. SIRT1 has been verified to exert
antioxidation through regulating the transcription activity of some
key enzymes (21).
SIRT1 can protect pancreas islet β cell, regulate
insulin release, improve insulin resistance, reduce inflammatory
response and regulate lipid metabolism (22). Thus, it can participate in
regulating glucose homeostasis and thereby inhibit DM and
DM-induced organ dysfunction (7).
SIRT1 is indicated in research to alleviate DM genesis and
DM-induced complications (23).
Therefore, SIRT1 may become a target for treating insulin
resistance and type 2 DM (23).
The results showed that Overexpression of miRNA-23b-3p suppressed
SIRT1 and Nrf2 protein expression in neurocyte cell by
high-glucose. Zhao et al identified that miR-23b-3p induces
the cellular metabolic memory of high glucose via SIRT1 pathway in
diabetic retinopathy (24).
Nrf2 is the most important transcription factor for
cell to regulate antioxidant stress response (24). Nrf2 activation is the precondition
to exert its function (25). In
contrast, Nrf2 dissociation with Keap1 is the first step for it to
regulate target gene transcription (26). Under physiological status, binding
of Nrf2 with cytoplasmic partner Keap1 is relatively inhibited. Two
cysteine sites on Keapl, C273 and C288, are simultaneously modified
under the action of oxidative stressor. This has resulted in
coupling of Keapl with Nrf2 (26).
Meanwhile, the ubiquitination of ubiquitinating enzyme on Nrf2 is
weakened or eliminated (27). This
leads to uncoupling of Nrf2 with Keapl, followed by nuclear
translocation. Nrf2 then binds with the antioxidant response
element ARE (27). Subsequently,
it initiates the downstream antioxidant gene and expression of
phase-II detoxifying enzyme (27).
Thus, it enhances cell resistance to oxidative damage (28). Downstream antioxidant enzyme
expression is reduced in the presence of disordered or deleted Nrf2
activation (29). Thus, it cannot
act against toxicity of antioxidant stressor on cell. Consequently,
it will lead to cell dysfunction, apoptosis or necrosis (29). We also showed that the promotion of
SIRT1 inhibited apoptosis and oxidative stress, decreased the
miRNA-23b-3p on cognitive impairment of diabetic rats via Nrf2
expression. Zhao et al showed that miR-23b-3p induces the
cellular metabolic memory through a SIRT1-dependent signalling
pathway in diabetic retinopathy (24). However, we only used Western blot
to analyze the protein expression of SIRT1 by miR-23b-3p, which is
a limitation of the current study, and we will execute more tests
to measure the changes of SIRT1 and Nrf2 in further study.
In conclusion, the above histopathological and
molecular biological results suggested that the Sirt1/Nrf2
signalling pathway is involved in oxidative stress-induced
apoptosis and caused cognitive impairment in of diabetic rats
through miR-23b-3p. Enhancing Sirt1/Nrf2 signalling pathway
activity or the inhibition of miR-23b-3p may serve as a potential
therapeutic strategy for cognitive impairment in diabetic.
References
|
1
|
Liu J, Guo B, Chen Z, Wang N, Iacovino M,
Cheng J, Roden C, Pan W, Khan S, Chen S, et al: miR-125b promotes
MLL-AF9-driven murine acute myeloid leukemia involving a
VEGFA-mediated non-cell-intrinsic mechanism. Blood. 129:1491–1502.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buo AM, Tomlinson RE, Eidelman ER, Chason
M and Stains JP: Connexin43 and Runx2 interact to affect cortical
bone geometry, skeletal development and osteoblast and osteoclast
function. J Bone Miner Res. 32:1727–1738. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bu Q, You F, Pan G, Yuan Q, Cui T, Hao L
and Zhang J: miR-125b inhibits anaplastic thyroid cancer cell
migration and invasion by targeting PIK3CD. Biomed Pharmacother.
88:443–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shrivats AR, Hsu E, Averick S, Klimak M,
Watt AC, DeMaio M, Matyjaszewski K and Hollinger JO: Cationic
Nanogel-mediated Runx2 and Osterix siRNA delivery decreases
mineralization in MC3T3 Cells. Clin Orthop Relat Res.
473:2139–2149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang Y, Gao F, Hao J and Liu Z:
microRNA-1246 mediates lipopolysaccharide-induced pulmonary
endothelial cell apoptosis and acute lung injury by targeting
angiotensin-converting enzyme 2. Am J Transl Res. 9:1287–1296.
2017.PubMed/NCBI
|
|
6
|
Chen S, Feng B, Thomas AA and Chakrabarti
S: miR-146a regulates glucose induced upregulation of inflammatory
cytokines extracellular matrix proteins in the retina and kidney in
diabetes. PLoS One. 12:e01739182017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng J, Lin Z, Dong P, Lu Z, Gao S, Chen
X, Wu C and Yu F: Activation of hepatic stellate cells is
suppressed by microRNA-150. Int J Mol Med. 32:17–24. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu B, Lv X, Su L, Li J, Yu Y, Gu Q, Yan M,
Zhu Z and Liu B: miR-148a functions as a tumor suppressor by
targeting CCK-BR via inactivating STAT3 and Akt in human gastric
cancer. PLoS One. 11:e01589612016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sato T, Liu X, Nelson A, Nakanishi M,
Kanaji N, Wang X, Kim M, Li Y, Sun J, Michalski J, et al: Reduced
miR-146a increases prostaglandin E2 in chronic
obstructive pulmonary disease fibroblasts. Am J Respir Crit Care
Med. 182:1020–1029. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li D, Duan M, Feng Y, Geng L, Li X and
Zhang W: miR-146a modulates macrophage polarization in systemic
juvenile idiopathic arthritis by targeting INHBA. Mol Immunol.
77:205–212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li HP, Huang HY, Lai YR, Huang JX, Chang
KP, Hsueh C and Chang YS: Silencing of miRNA-148a by
hypermethylation activates the integrin-mediated signaling pathway
in nasopharyngeal carcinoma. Oncotarget. 5:7610–7624. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ye EA and Steinle JJ: miR-146a attenuates
inflammatory pathways mediated by TLR4/NF-κB and TNFα to Protect
primary human retinal microvascular endothelial cells grown in high
glucose. Mediators Inflamm. 2016:39584532016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu X, Long L, Liu J, Zhang J, Wu T, Chen
X, Zhou B and Lv TZ: Gambogic acid suppresses inflammation in
rheumatoid arthritis rats via PI3K/Akt/mTOR signaling pathway. Mol
Med Rep. 16:7112–7118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu B, Li F, Shi J, Yang D, Deng Y and
Gong Q: Gastrodin ameliorates subacute phase cerebral
ischemia-reperfusion injury by inhibiting inflammation and
apoptosis in rats. Mol Med Rep. 14:4144–4152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee M, Arikawa K and Nagahama F:
Micromolar levels of sodium fluoride promote osteoblast
differentiation through Runx2 signaling. Biol Trace Elem Res.
178:283–291. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park OJ, Kim J, Yang J, Yun CH and Han SH:
Muramyl dipeptide, a shared structural motif of peptidoglycans, is
a novel inducer of bone formation through induction of Runx2. J
Bone Miner Res. 32:1455–1468. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi YH, Kim GS, Choi JH SW, Kim HG, Han
Y, Lee DY, Choi SI, Kim SY, Ahn YS, et al: Ethanol extract of
Lithospermum erythrorhizon Sieb. et Zucc. promotes
osteoblastogenesis through the regulation of Runx2 and Osterix. Int
J Mol Med. 38:610–618. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhan L, Yao Y, Fu H, et al: Protective
role of miR-23b-3p in kainic acid-induced seizure. Neuroreport.
27:764–768. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang W, Min J, Sui X, et al:
MicroRNA-26a-5p and microRNA-23b-3p up-regulate peroxiredoxin III
in acute myeloid leukemia. Leuk Lymphoma. 56:460–471. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun Z, Cao X, Hu Z, Zhang L, Wang H, Zhou
H, Li D, Zhang S and Xie M: miR-103 inhibits osteoblast
proliferation mainly through suppressing Cav1.2 expression in
simulated microgravity. Bone. 76:121–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ozeki N, Hase N, Hiyama T, Yamaguchi H,
Kawai-Asano R, Nakata K and Mogi M: MicroRNA-211 and
autophagy-related gene 14 signaling regulate osteoblast-like cell
differentiation of human induced pluripotent stem cells. Exp Cell
Res. 352:63–74. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li P, Sun N, Zeng J, Zeng Y, Fan Y, Feng W
and Li J: Differential expression of miR-672-5p and miR-146a-5p in
osteoblasts in rats after steroid intervention. Gene. 591:69–73.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hata A and Kang H: Functions of the bone
morphogenetic protein signaling pathway through microRNAs (Review).
Int J Mol Med. 35:563–568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao S, Li T, Li J, Lu Q, Han C, Wang N,
Qiu Q, Cao H, Xu X, Chen H and Zheng Z: miR-23b-3p induces the
cellular metabolic memory of high glucose in diabetic retinopathy
through a SIRT1-dependent signalling pathway. Diabetologia.
59:644–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan W, Miao L, Lin Y, Huang X, Ge X, Moosa
SL, Liu B, Ren M, Zhou Q, Liang H, et al: Regulation mechanism of
oxidative stress induced by high glucose through PI3K/Akt/Nrf2
pathway in juvenile blunt snout bream (Megalobrama
amblycephala). Fish Shellfish Immunol. 70:66–75. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang XR, Shi GX, Yang JW, Yan CQ, Lin LT,
Du SQ, Zhu W, He T, Zeng XH, Xu Q and Liu CZ: Acupuncture
ameliorates cognitive impairment and hippocampus neuronal loss in
experimental vascular dementia through Nrf2-mediated antioxidant
response. Free Radic Biol Med. 89:1077–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Z, Ji C, Wu L, Qiu J, Li Q, Shao Z
and Chen G: Tert-butylhydroquinone alleviates early brain injury
and cognitive dysfunction after experimental subarachnoid
hemorrhage: role of Keap1/Nrf2/ARE pathway. PLoS One. 9:e976852014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wan P, Su W, Zhang Y, Li Z, Deng C and
Zhuo Y: Trimetazidine protects retinal ganglion cells from acute
glaucoma via the Nrf2/Ho-1 pathway. Clin Sci (Lond). 131:2363–2375.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo Y, Sun J, Li T, Zhang Q, Bu S, Wang Q
and Lai D: Melatonin ameliorates restraint stress-induced oxidative
stress and apoptosis in testicular cells via NF-κB/iNOS and
Nrf2/HO-1 signaling pathway. Sci Rep. 7:95992017. View Article : Google Scholar : PubMed/NCBI
|