Introduction
Influenza viruses are pathogens that cause
respiratory infection, in addition to severe viral pneumonia and
even mortality (1). A number of
pandemic and epidemic outbreaks of influenza have occurred and it
represents a severe threat to human health worldwide.
Influenza viruses are negative single-stranded,
segmented RNA viruses. There are three types of influenza virus; A,
B and C. Influenza A viruses are classified into different subtypes
based on hemagglutinin and neuraminidase antigenicity. Due to
genetic recombination, new influenza A subtypes continually emerge,
including H7N9 and H5N6, which have no suitable vaccines (2,3).
Antiviral drugs have an important role in the
prevention and treatment of influenza. At present, adamantine
derivatives, including amantadine and rimantadine, in addition to
the neuraminidase inhibitors oseltamivir, zanamivir and paramivir,
are widely used and effective in the treatment of clinical
influenza virus infection (4).
However, novel antiviral drugs against resistant strains in
particular are required, due to the development of drug resistance
(3).
Traditional Chinese medicine (TCM) is widely used in
China to treat respiratory disease. TCM is considered to be safe,
effective and multi-targeted (5).
Numerous medicinal plants exhibit antiviral activity through
different mechanisms and these may lead to the development of novel
antiviral drugs (5). Laggera
pterodonta is a medicinal plant used widely in China that is
primarily distributed in the Yunnan province. Certain antiviral
compounds have been isolated from L. pterodonta, including
flavonoids, which have an anti-inflammatory and anti-apoptotic
effect, in addition to three dicaffeoylquinic acids that display
antiviral activity against herpes simplex virus-1, herpes simplex
virus-2 and influenza A in vitro (6–8).
The results from a previous study indicated that a
sesquiterpene fraction isolated from L. pterodonta
demonstrated anti-influenza activity by targeting the nuclear
factor-κB (NF-κB) and p38/mitogen-activated protein kinase (MAPK)
signaling pathways (9). The
present study investigated the C8 fraction, an antiviral component
containing pterodontic acid and pterodondiol isolated from L.
pterodonta. The mechanisms of action of this antiviral
component against influenza A were subsequently investigated in
vitro.
Materials and methods
Plant material, cells and viruses
L. pterodonta (10 kg) was collected manually
during October 2015 in Yunnan (China) and subsequently stored in a
dry, ventilated environment. The herbarium specimen was
authenticated by Professor Rongping Zhang (Kunming Medical
University, Kunming, China) and deposited in the College of
Pharmaceutical Sciences (Kunming Medical University).
Madin-Darby canine kidney (MDCK) and A549 cells were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). The cells were grown in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) with 10% heat-inactivated fetal calf serum (Gibco; Thermo
Fisher Scientific, Inc.). A/PR/8/34 was purchased from ATCC.
A/Guangzhou/GIRD/07/09 (H1N1), A/Guangzhou/GIRD/02/09 (H1N1) and
influenza B virus were isolated from routine clinical throat swab
specimens of infected patients treated in the First Affiliated
Hospital of Guangzhou Medical University (Guangzhou, China).
Several strains of avian influenza virus, including
A/Duck/Guangdong/2009 (H6N2), A/Duck/Guangdong/1994 (H7N3) and
A/Chicken/Guangdong/1996 (H9N2); were provided by Dr Jianxin Chen
(South China Agricultural University, Guangzhou, China) and
subsequently stored in State Key Laboratory of Respiratory Disease,
Guangzhou Medical University. The influenza viruses were grown in
the allantoic cavity of embryonated chicken eggs for 48 h at 35°C,
followed by 12 h at 4°C (9).
Following this, the harvested viruses were preserved at 80°C prior
to further experimentation.
General experimental procedures
Ultra-high-performance liquid
chromatography/quadrupole-time of flight-mass spectrometry
(UHPLC/Q-TOF-MS) was performed using Agilent 1290 UHPLC (Agilent
Technologies, Inc., Santa Clara, CA, USA) and Bruker maXis impact
Q-TOF-MS (Bruker Corporation, Billerica, MA, USA) systems. Column
chromatography (CC) was performed using silica gel (200–300 mesh;
Qingdao Haiyang Chemical Co., Ltd., Qingdao, China), Thin layer
chromatography was performed on pre-coated silica gel
GF254 plates. Spots were visualized under UV light (254
or 356 nm) or using iodine fuming. All solvents used were of
analytical grade and were purchased from Guangzhou Chemical
Reagents Factory. HPLC grade acetonitrile was purchased from Merck
KGaA (Darmstadt, Germany). HPLC grade methanol was purchased from
RCI Labscan, Ltd. (Bangkok, Thailand). HPLC grade formic acid was
purchased from Merck KGaA (Darmstadt, Germany).
Preparation of standards
Pterodontic acid and pterodondiol were isolated from
L. pterodonta in the laboratory, and were identified by MS
and nuclear magnetic resonance spectroscopy analysis (purity,
>98%). Pterodontic acid (1.25 mg) and pterodondiol (0.99 mg)
were accurately weighed and dissolved in 1 ml methanol to give
individual stock solutions. Pterodontic acid stock solution was
subsequently diluted to 6.25 µg/ml and pterodondiol stock solution
4.95 µg/ml. All solutions were stored at 4°C prior to
UHPLC-Q-TOF-MS analysis.
Preparation of the C8 fraction
Powdered plant material (1 kg) was extracted with
methanol by percolation, followed by the collection and vacuum
concentration of 40 l eluate to yield 135 g methanol extract. The
extract was suspended in H2O (800 ml) and subjected to
liquid-liquid partition by the addition of petroleum ether. The
residue (48 g) of the petroleum ether layer was subjected to silica
gel CC (petroleum ether-ethyl acetate, 10:1) to obtain the C8
fraction (38 g) (10).
Sample preparation
C8 (0.01 g) was accurately weighed into a 5-ml
volumetric flask and dissolved in methanol, and additional methanol
was subsequently added to give a final volume of 5 ml. The sample
solution was filtered through a 0.22-µm polytetrafluoroethylene
filter and diluted 20 times for UHPLC/Q-TOF-MS analysis.
UHPLC/Q-TOF-MS system
UHPLC was performed with the Agilent 1290 ultra-high
performance liquid chromatography system (Agilent Technologies,
Inc.). The chromatography was performed on an Agilent Poroshell 120
EC-C18 column (150×3.0 mm, 2.7 µm; Agilent Technologies, Inc.). The
mobile phase consisted of solvent A (water with 0.1% formic acid)
and solvent B (acetonitrile with 0.1% formic acid). The following
gradient elution procedure was used: 0–8 min, 30–70% B; 8–13 min,
70–100% B; 13–16 min, 100% B. The flow rate was 0.35 ml/min, the
injection volume was 2 µl, and the column temperature was
maintained at 30°C. Eluted compounds were detected with an Agilent
diode array detector (Agilent Technologies, Inc.) over a wavelength
range of 200–400 nm.
Mass spectrometry was performed on a Q-TOF-MS with
an electrospray ionization interface (Bruker Corporation) operating
in the positive mode. The Q-TOF-MS source parameters were as
follows: End plate offset, −500 V; capillary voltage, 4,000 V;
collision energy, 7 eV; nebulizing gas (N2) pressure,
2.0 bar; drying gas (N2) flow rate, 8.0 l/min; drying
gas temperature, 200°C; and mass range, m/z 100–1,300.
Cytotoxicity assay (MTT assay)
The 50% toxic concentration (TC50) of C8
was determined. MDCK cells (2×104 cells/well) were
seeded into 96-well plate for 24 h at 37°C and subsequently washed
with PBS. The cells were treated with the indicated amounts of C8
(0, 12.5, 25, 50, 100 and 200 µg/ml) and cultured at 37°C for 48 h.
The cytotoxicity of the C8 was measured with an MTT assay, as
previously described (10). The
TC50 was calculated using the Reed-Muench method
(11).
Cytopathic effect (CPE) inhibition
assay
MDCK cells (1.0×104 cells/well) were
seeded in 96-well plates and grown to 90% confluence at 37°C for 24
h. To clearly observe the anti-influenza activity of C8, MDCK cells
were washed with PBS and infected with 100 median tissue culture
infective dose (TCID50) of A/PR/8/34 (H1N1) at 37°C for
2 h. Following medium removal, different concentrations of C8
(two-fold dilution) in serum-free Minimum Essential Medium (MEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 2 µg/ml tosyl phenylalanyl chloromethyl
ketone-trypsin. Following incubation for 48 h at 37°C, the
cytopathogenic efficiency (CPE) of the influenza virus was measured
microscopically using a MI12 inverted phase contrast microscope
(Micro-shot Technology Limited, Guangzhou, China; magnification,
×200). The concentration required for 50% inhibition of the
virus-induced CPE (half-maximal inhibitory concentration;
IC50) was calculated by the Reed-Muench method (11). The selection index was calculated
by the ratio of TC50/IC50 (12).
Western blot analysis
A549 cells (2×104 cells/well) were
cultured at 37°C under 5% CO2 for 24 h. Following this,
cells were washed with PBS and subsequently incubated with
A/PR/8/34 virus [multiplicity of infection (MOI)=0.1], diluted in
PBS, for 30 min at 37°C. Following this, the inoculums were
discarded and cells were incubated with MEM in the absence and
presence of different concentrations (100 and 150 µg/ml) of C8 for
24 h at 37°C. Cell lysis and western blot analysis was performed as
previously described (13). Cells
were lysed on ice for 10 min with radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China)
supplemented with a phosphatase inhibitor cocktail (Beyotime
Institute of Biotechnology). Protein concentration was determined
with the bicinchoninic protein assay kit. Proteins (30 ng/lane)
were separated using 10% SDS-PAGE and subsequently
electrotransferred onto polyvinylidene fluoride (PVDF) membranes.
The PVDF membrane was blocked with 5% bovine serum albumin (BSA;
9048-46-8; GBCBIO Technologies Inc., Guangzhou, China)/TBS-Tween 20
for 1 h at room temperature prior to incubation at 4°C overnight
with antibodies against Toll-like receptor 7 (TLR7; cat. no. 2633),
myeloid differentiation primary response protein 88 (MyD88; cat.
no. 4283), tumor necrosis factor (TNF) receptor associated factor 6
(TRAF6; cat. no. 8028), phosphorylated-p65 (cat. no. 3033), p65
(cat. no. 8242) and GAPDH (cat. no. 2118); all at a dilution of
1:1,000 and purchased from CST Biological Reagents Co., Ltd.
(Shanghai, China). Following this, membranes were incubated with a
horseradish peroxidase-conjugated secondary antibody (cat. no.
7074; 1:5,000; CST Biological Reagents Co., Ltd.) for 60 min at
room temperature. The complexes were detected using a western
lighting chemiluminescence system (Thermo Fisher Scientific,
Inc.).
Indirect immunofluorescence assay
A549 cells were seeded into 48-well plates at 37°C
with 5% CO2. When the cell culture reached 50–70%
confluence, cell were incubated with /PR/8/34 (H1N1; MOI=5) virus
or TNF-α (20 ng/ml; 300-01A; PeproTech China, Suzhou, China) for 2
h at 37°C. The supernatant was subsequently aspirated, cells were
washed twice with PBS and C8 (75, 100 and 150 µg/ml) was added to
wells. After 9 h, cells were washed three times with PBS and fixed
with 4% paraformaldehyde in PBS for 15 min at 4°C. Cells were
permeabilized with 0.5% Triton X-100 in PBS for 15 min at room
temperature and blocked with 3% BSA in PBS for 30 min at 37°C,
followed by incubation with anti-p65 antibody (1:50; cat. no. 8242;
CST Biological Reagents Co., Ltd.) overnight at 4°C. Following a
further wash, cells were incubated with fluorescein
isothiocyanate-conjugated secondary antibody (1:100; cat. no.
SA00003; ProteinTech Group, Inc., Chicago, IL, USA) at 37°C for 1
h. The nuclei were stained with DAPI (5 µg/ml; cat. no.
10236276001; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 30
sec at room temperature, and fluorescence was visualized using a
Zeiss Axiovert 135 fluorescence microscope (Zeiss AG, Oberkochen,
Germany; magnification, ×400) (14).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
A549 cells were cultured in 96-well plates at 37°C
with 5% CO2 for 24 h, and subsequently infected with
A/PR/8/34 virus (MOI=0.1) for 2 h at 37°C. The inoculums were
discarded and the cells were treated with various concentrations of
C8 (100 and 200 µg/ml) for 24 h at 37°C. Total RNA was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Total RNA (1 µg) was reverse transcribed into cDNA using the
Prime-Script RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian,
China) at 50°C for 30 min. qPCR was performed using an ABI7500
Real-time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with the following thermocycling conditions: 95°C for 30 sec,
followed by 35 cycles of 95°C for 5 sec and 60°C for 40 sec
(15). Relative gene expression
levels of C-C motif chemokine ligand 2 (MCP-1), interleukin
(IL)-1β, IL-6, IL-8 and GAPDH were calculated using the
2−∆∆Cq method (16).
The RT-qPCR primers and probes for analyses are listed in Table I.
 | Table I.Primers and probes used in the
reverse transcription-quantitative polymerase chain reaction
analysis. |
Table I.
Primers and probes used in the
reverse transcription-quantitative polymerase chain reaction
analysis.
| Gene | Type | Sequence
(5′→3′) |
|---|
| IL-1β | Forward |
GCACGATGCACCTGTACGAT |
|
| Reverse |
AGACATCACCAAGCTTTTTTGCT |
|
| Probe |
ACTGAACTGCACGCTCCGGGACTC |
| IL-6 | Forward |
CGGGAACGAAAGAGAAGCTCTA |
|
| Reverse |
CGCTTGTGGAGAAGGAGTTCA |
|
| Probe |
TCCCCTCCAGGAGCCCAGCT |
| IL-8 | Forward |
TTGGCAGCCTTCCTGATTTC |
|
| Reverse |
TATGCACTGACATCTAAGTTCTTTAGCA |
|
| Probe |
CCTTGGCAAAACTGCACCTTCACACA |
| MCP-1 | Forward |
CAAGCAGAAGTGGGTTCAGGAT |
|
| Reverse |
AGTGAGTGTTCAAGTCTTCGGAGTT |
|
| Probe |
CATGGACCACCTGGACAAGCAAACC |
| GAPDH | Forward |
GAAGGTGAAGGTCGGAGTC |
|
| Reverse |
GAAGATGGTGATGGGATTTC |
|
| Probe |
CAAGCTTCCCGTTCTCAGCC |
Bio-Plex assay
A549 cells (1×105 cells/well) were grown
in 6-well plates at 37°C with 5% CO2 for 24 h and
subsequently washed with PBS twice. A/PR/8/34 virus (MOI=0.1) was
incubated with the cells for 2 h, followed by treatment with
various concentrations of C8 (100 and 150 µg/ml) at 37°C for 24 h.
The supernatants were collected after 24 h treatment and
centrifuged at 16,000 × g at 4°C to remove cell debris. IL-6, IL-8,
TNF-α, C-X-C motif chemokine 10 (IP-10), MCP-1 and C-C motif
chemokine 5 (RANTES) were detected using the Bio-Plex liquid phase
chips kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) with the
Bio-Plex 200 system (Bio-Rad Laboratories, Inc.) (17).
Statistical analysis
Statistical analyses were performed using SPSS 18.0
(SPSS, Inc., Chicago, IL, USA). One-way analysis of variance
followed by Fisher's Least Significant Difference post-hoc test was
used to calculate statistical significance. Data are presented as
the mean ± standard deviation. Experiments were performed in
triplicate. P<0.05 was considered to indicate a statistically
significant difference.
Results
Quantification of sesquiterpenes in
C8
The presence of pterodontic acid and pterodondiol in
C8 was determined by UHPLC/Q-TOF-MS in the positive ion mode. Base
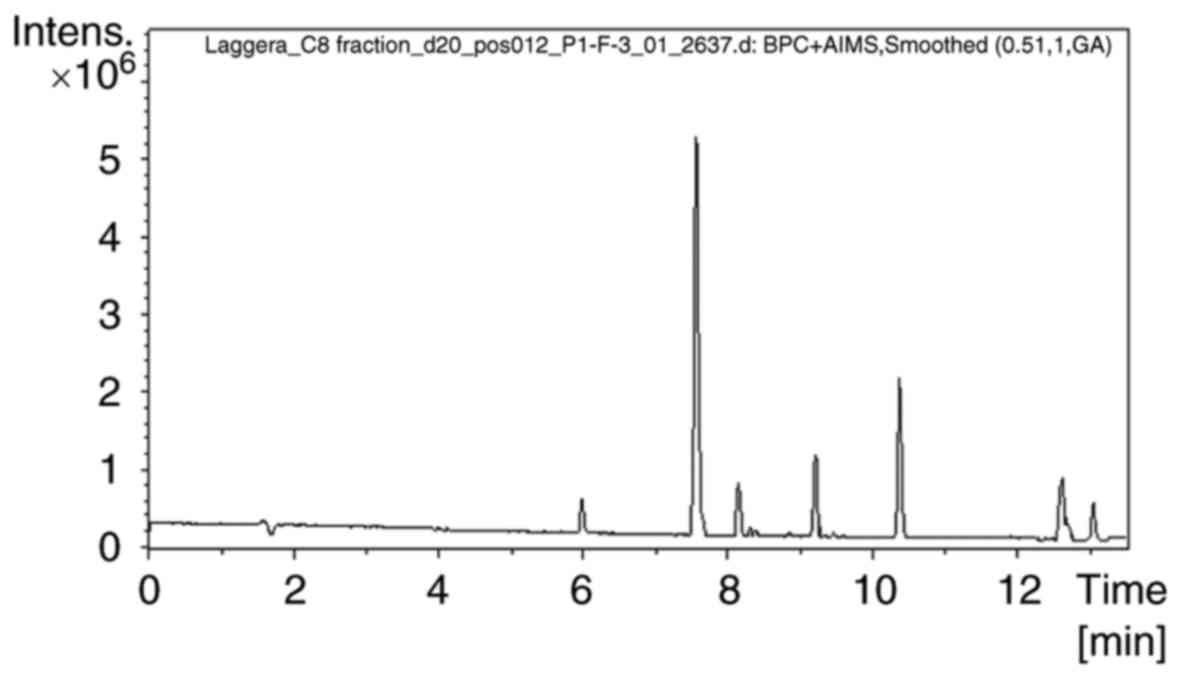
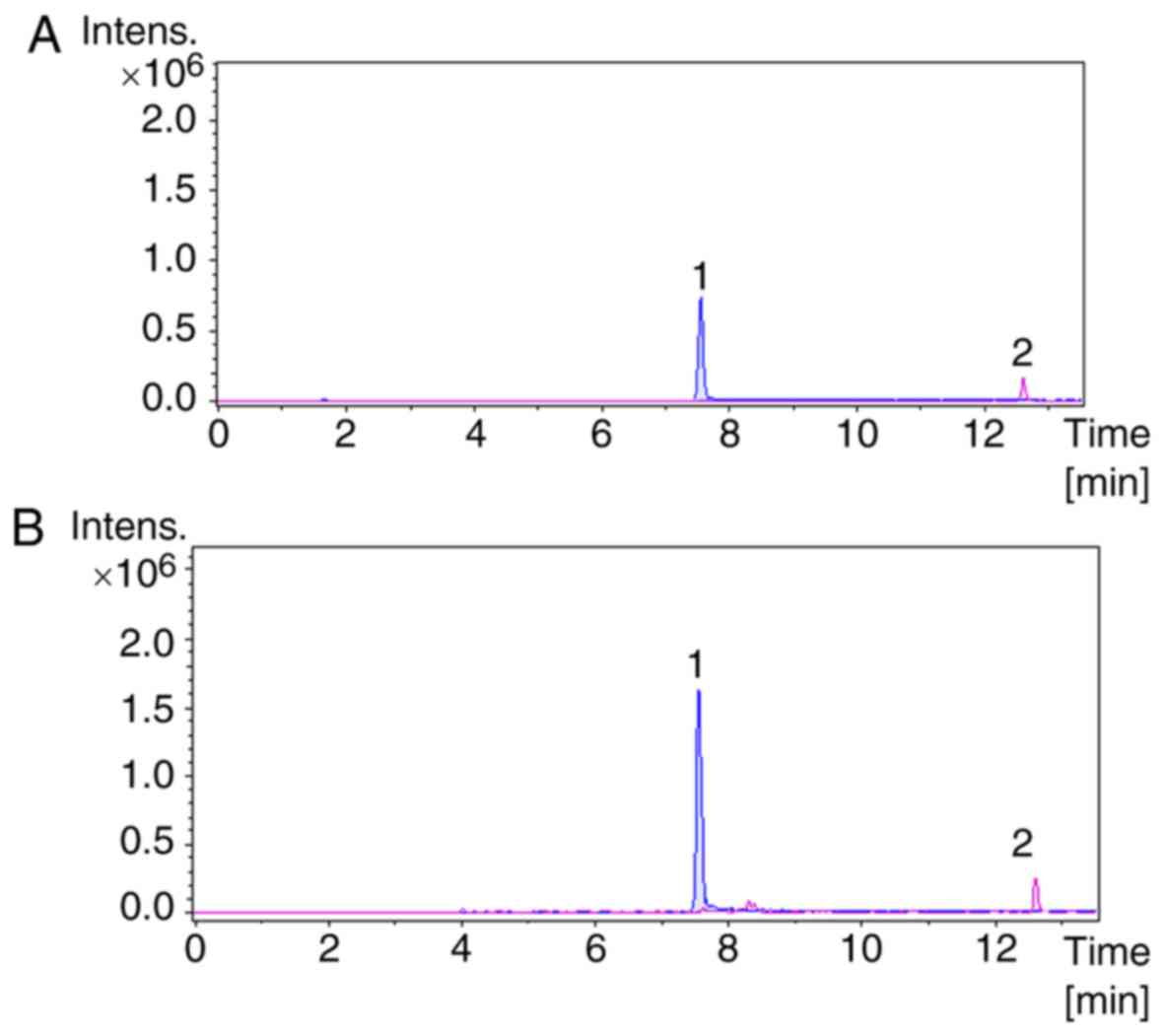
peak chromatograms of sample C8 are presented in Fig. 1. Extraction ion chromatograms of
the two standards and C8 are presented in Fig. 2. The results (Table II) obtained from UHPLC-Q-TOF-MS
analysis determined that the pterodontic acid and pterodondiol
content in C8 was 107.98 and 111.3 mg/g, respectively. These
results provided essential data required for the identification and
quality control of C8 obtained from L. pterodonta.
 | Table II.Content of the two sesquiterpenes in
C8. |
Table II.
Content of the two sesquiterpenes in
C8.
| Sample | Retention time,
min | Content, mg/g | RSD, % |
|---|
| Pterodondiol | 7.58 | 111.3 | 1.3 |
| Pterodontic
acid | 12.59 | 107.98 | 4.7 |
Cytotoxicity and anti-influenza
activity of C8
C8 was examined for its cytotoxic ability in
confluent MDCK cell cultures. No significant cytotoxic effects were
observed at >200 µg/ml. To evaluate the anti-influenza activity
of C8, MDCK cells were infected with influenza virus (100
TCID50) and C8 was added at increasing concentrations.
Following treatment for 48 h, the antiviral effect of C8 was
evaluated. C8 exhibited an antiviral effect on a number of
influenza virus strains, with IC50 values of 19.9–91.4
µg/ml (Table III).
 | Table III.Anti-influenza spectrum of C8. |
Table III.
Anti-influenza spectrum of C8.
| Strain | TC50,
µg/ml | IC50,
µg/ml | SI |
|---|
| A/PR/8/34,
H1N1 | >200 | 25 | >8 |
|
A/Guangzhou/GIRD/07/09, H1N1 | >200 | 50 | >4 |
|
A/Guangzhou/GIRD/02/09, H1N1 | >200 |
19.9 | >10.1 |
| Influenza B | >200 | 50 | >4 |
|
A/Duck/Guangdong/2009, H6N2 | >200 |
84.8 | >2.36 |
|
A/Duck/Guangdong/1994, H7N3 | >200 |
80.2 | >2.49 |
|
A/Chicken/Guangdong/1996, H9N2 | >200 |
91.4 | >2.19 |
Inhibition of the
TLR7/MyD88/TRAF6/NF-κB signaling pathway
TLR7 recognizes influenza single-stranded RNA and
subsequently combines with the adapter protein MyD88, which induces
the phosphorylation of IL-1 receptor-associated kinase 1 (IRAK)
through IRAK4. Following this, IRAK1 interacts with TRAF6, which is
able to activate the NF-κB signaling pathway (18,19).
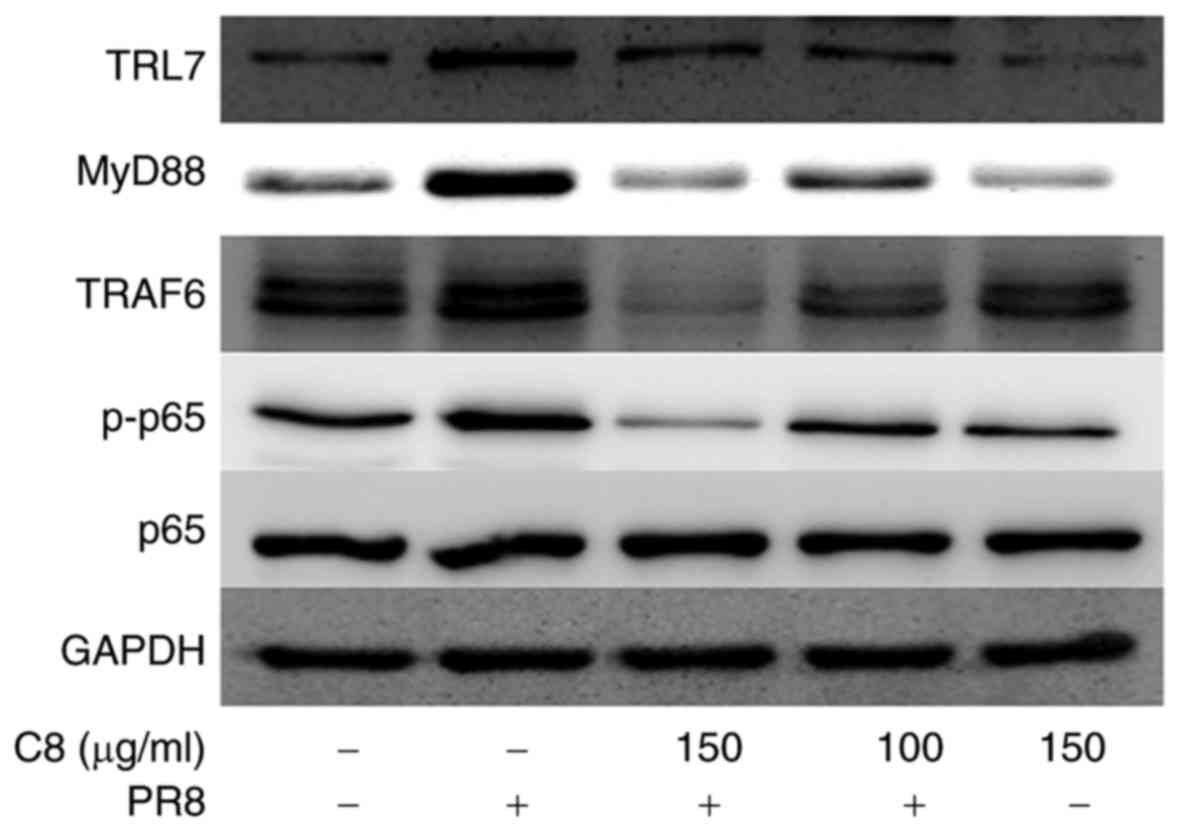
Western blot analysis indicated that C8 inhibited TLR7, MyD88,
TRAF6 and p-p65 phosphorylation levels at 100 and 150 µg/ml
(Fig. 3).
Inhibition of p65/NF-κB nuclear
translocation
p65-p50 constitutes the typical NF-κB inhibitor
(IκB) proteins, the p50 and p65 complex translocates to the nucleus
to promote the inflammatory response (20,21).
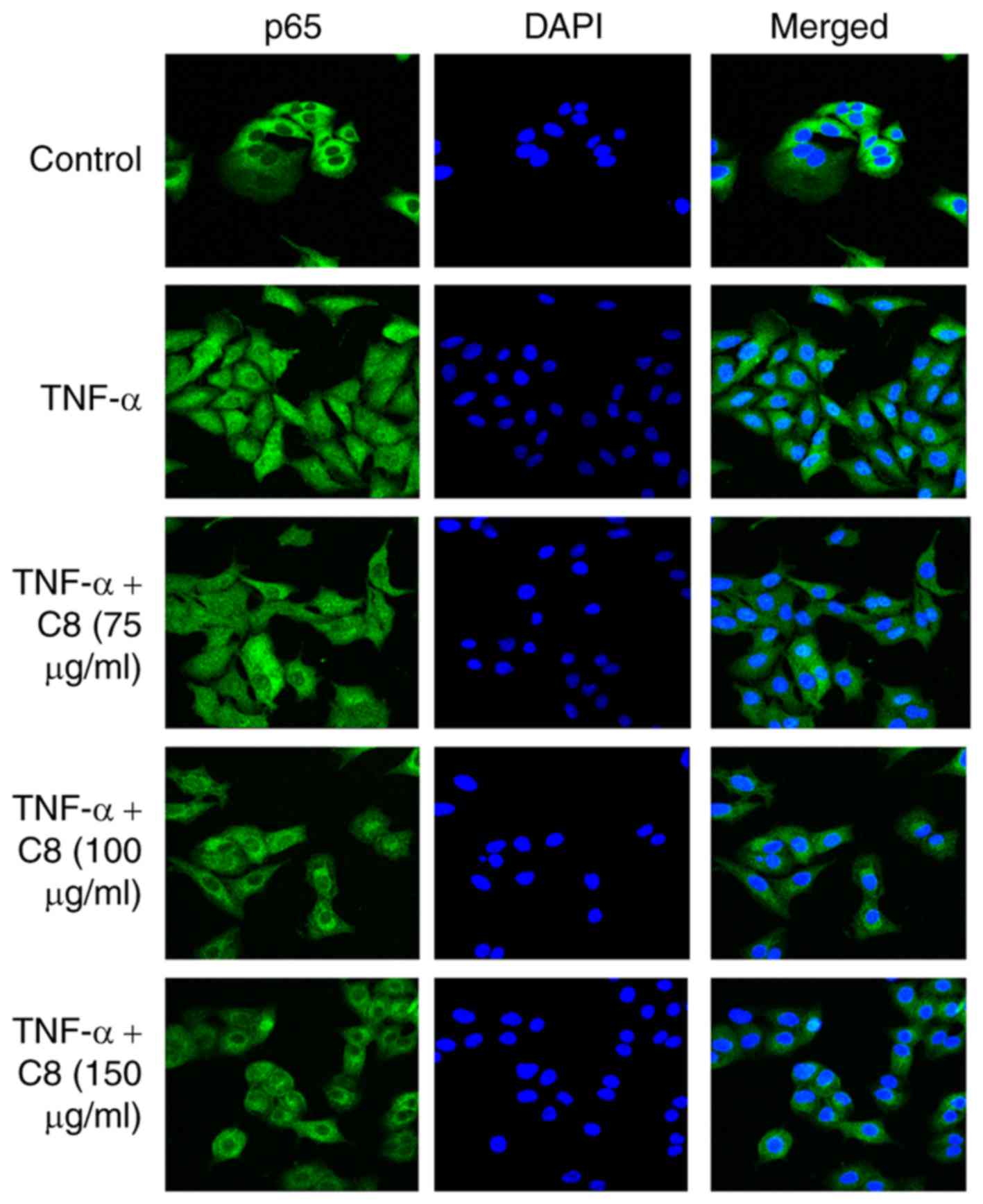
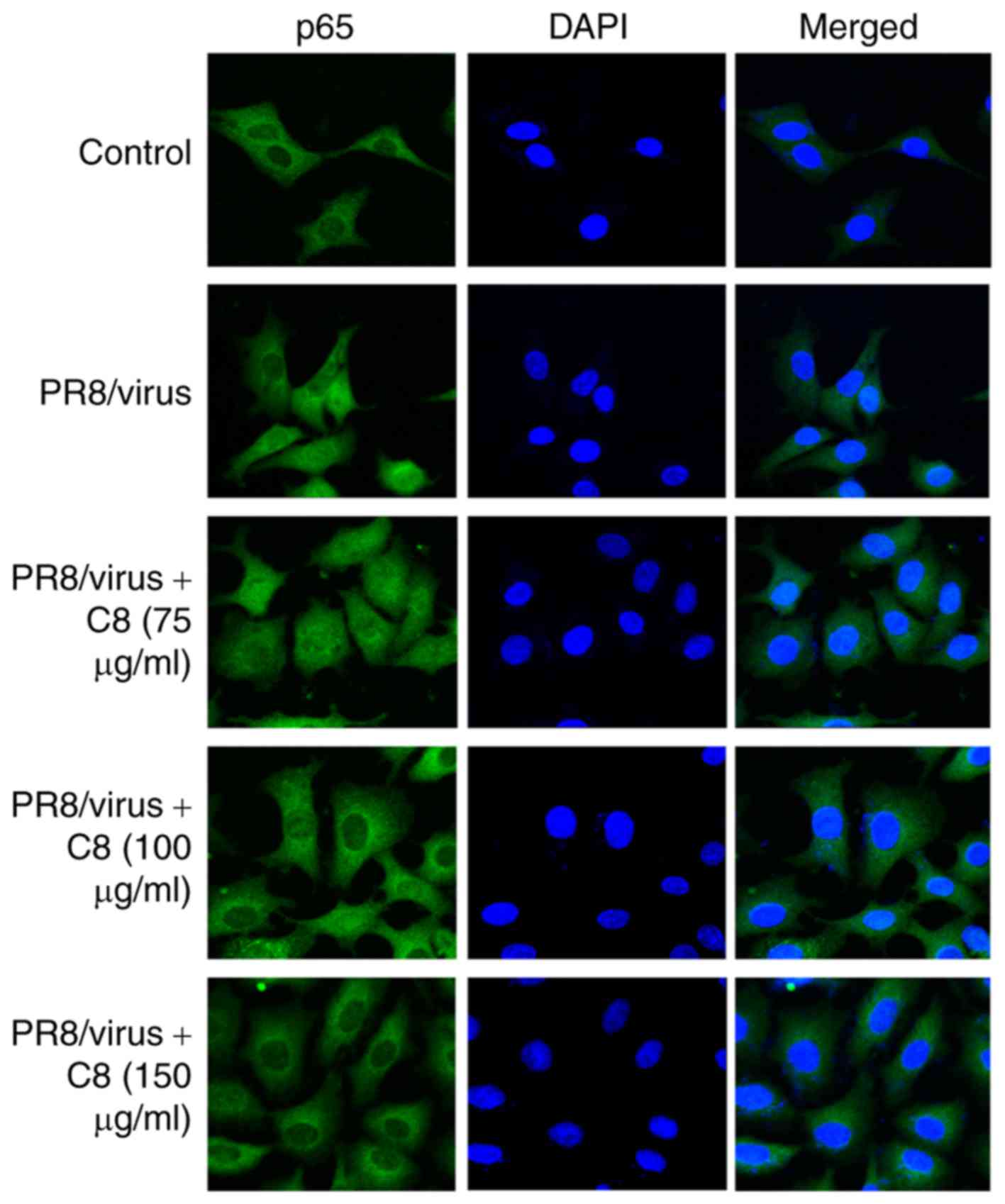
The results revealed that C8 inhibited p65 nuclear translocation
induced by TNF-α (Fig. 4) and
influenza virus (Fig. 5).
Inhibition of the mRNA and protein
expression of inflammatory cytokines
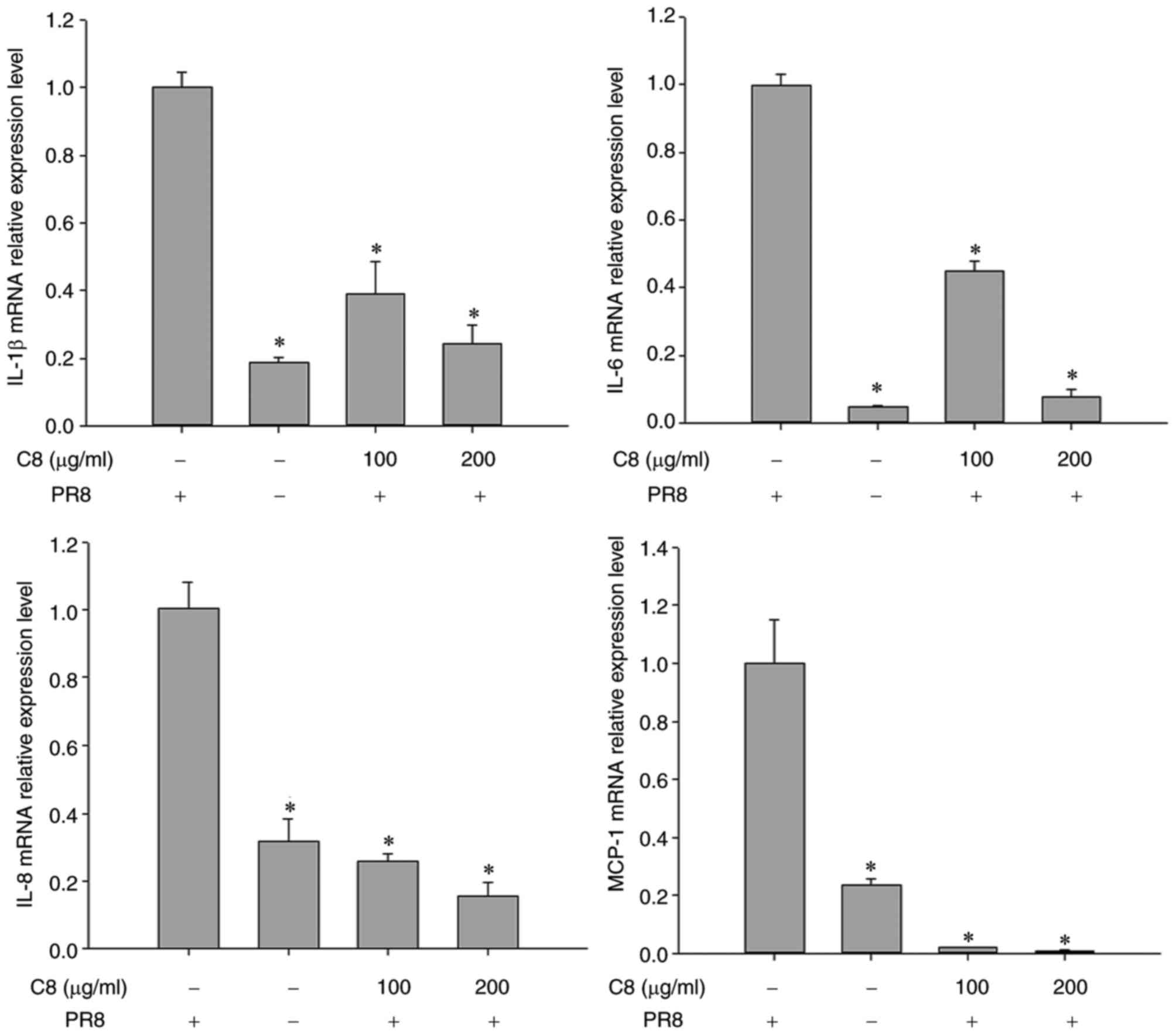
The effects of C8 on inducing cytokine production
were determined. The results revealed that the IL-1β, IL-6, IL-8
and MCP-1 mRNA expression was significantly reduced in C8-treated
cells after 24 h (P<0.05; Fig
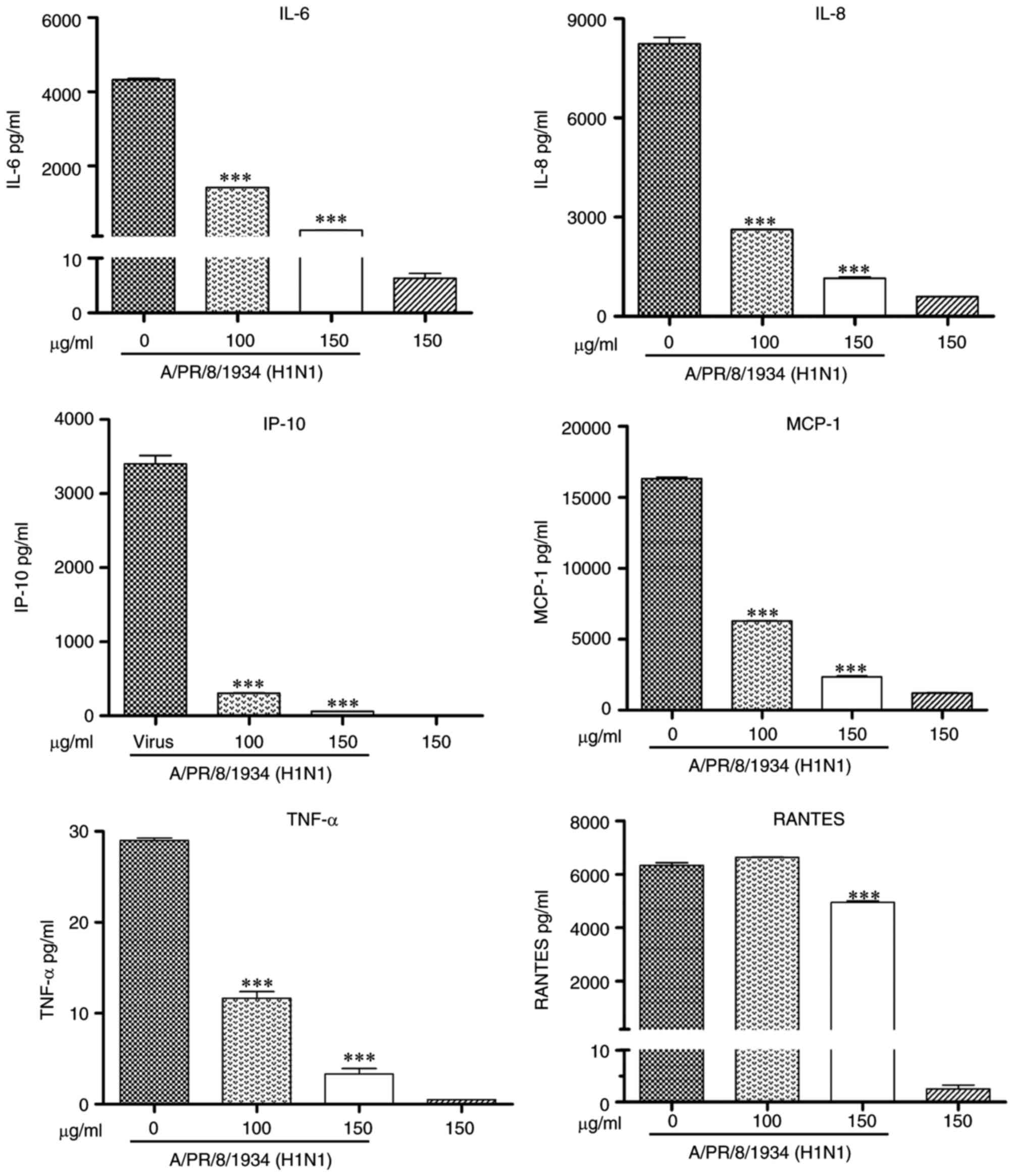
6). The results of the Bio-Plex assay demonstrated that the
protein expression of IL-6, IL-8, TNF-α, IP-10, MCP-1 and RANTES
was inhibited (P<0.001; Fig.
7).
Discussion
Influenza viruses have the ability to cause severe
pandemic or epidemic outbreaks, particularly through the
transmission of avian influenza viruses to humans, which may lead
to the development of novel virus strains capable of causing a
pandemic outbreak. The majority of currently available antiviral
drugs target the viral proteins of influenza. However, the clinical
use of these drugs is limited due to increasing drug resistance,
and alternative antiviral targets are required. Influenza viruses
take advantage of host cellular functions to support efficient
viral replication. Numerous studies have reported that cell
signaling pathways are involved in the influenza virus life cycle,
such as NF-κB (21),
Raf/mitogen-activated protein kinase/extracellular signal-regulated
kinase (22), phosphatidylinositol
3-kinase/protein kinase B (23)
and p38/MAPK (24) pathways. The
results of a genome-wide RNA interference screen in mammalian cells
revealed that 219 of the 295 factors were identified to be required
for efficient wild-type influenza virus replication. Therefore, the
development of drugs that are able inhibit host factors essential
for influenza virus replication may be an alternative and more
effective therapeutic strategy (25,26).
In the present study, the antiviral component C8 was
isolated from the traditional Chinese medicine L. pterodonta
and its activity against influenza virus was evaluated. The results
revealed that C8 was effective against different influenza virus
stains, including human and avian, with IC50 values of
19.9–91.4 µg/ml. The anti-inflammatory activity of C8 was
subsequently examined.
The host innate inflammatory response is mediated by
pattern recognition receptors (PRRs). The TLR family is an
important class of PRRs that recognizes pathogen-associated
molecular patterns. There are ten TLRs in humans: TLR2, TLR4, TLR5,
TLR6 and TLR11 are located at the cell surface; TLR3, TLR7, TLR9
and TLR13 are intracellular receptors. Additionally, TLR7 is able
to recognize single-stranded RNA (18,19).
The influenza virus infects the host cell and
releases its RNA, which is recognized by TLR7. MyD88 is a TLR7
adapter protein, which induces the phosphorylation of IRAK1 via
IRAK4. IRAK1 subsequently interacts with TRAF6, which is able to
activate NF-κB (19).
Activation of the NF-κB signaling pathway is
required for efficient influenza virus replication. In mammals, it
contains five known members, including p50 (NF-κB1), p52 (NF-κB2),
p65 (RelA or NF-κB3), RelB, and c-Rel. The NF-κB signaling pathway
has a central role in host immune response regulation, cell
adhesion, differentiation and apoptosis (20). A previous study reported that
active NF-κB signaling is a prerequisite for influenza virus
infection (21).
The results of the western blot analysis in the
present study demonstrated that C8 inhibited TLR7/MyD88/TRAF6
expression and p65 phosphorylation. Additionally, the western blot
analysis results demonstrated that C8 may inhibit the nuclear
translocation of p65 at different concentrations (100 and 150
µg/ml). p65 is an important member of the NF-κB pathway; following
proteasomal degradation of cytosolic IκB proteins, the p50 and p65
complex translocates into the nucleus to promote the inflammatory
response. These results suggested that C8 may inhibit the NF-κB
signaling pathway through two different mechanisms.
Following viral infection, cell death may activate
the immune response, and the production of cytokines and
chemokines, including TNF-α, MCP-1, RANTES, IP-10 and IL-8
(27). IL-6 is a pro-inflammatory
cytokine that is able to activate T cells. Previous studies have
demonstrated that IL-6 may be a potential disease severity
biomarker for severe pandemic H1N1 influenza A infection (27,28).
Additionally, NF-κB is involved in producing an effective immune
and inflammatory response against viral infections and induces the
transcription of pro-inflammatory cytokines, including TNF-α, IL-6
and IL-8 (21). The results of the
present study demonstrated that the expression of IL-1β, IL-6, IL-8
and MCP-1 mRNA was decreased following treatment with C8.
Furthermore, the Bio-Plex results revealed that protein levels of
IL-6, IL-8, TNF-α, IP-10, MCP-1 and RANTES had decreased.
NF-κB inhibition may result in substantial clinical
benefit and it is a potential target for the development of novel
anti-influenza virus therapies. However, inhibition may
additionally lead to detrimental effects on health (29) and its detailed mechanism requires
investigation in future research.
Although the present study demonstrated the
potential immunoregulatory mechanisms of C8, C8 is a component
containing a number of monomer compounds. Thus, further study is
required to verify the exact targets and mechanisms of each monomer
compound isolated from C8.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. U1502226 and
81460593), the Engineering Technology Research Center of Guangdong
General Universities (grant no. GCZX-A1408), Key Projects of
Applied Basic Research in Yunnan Province (grant no. 2014FA031),
Guangzhou Municipal Science and Technology Program-Technology
Benefiting Special (grant no. 2014Y2-00031), Collaborative
Innovation Major Projects of Guangzhou Health Care (grant no.
201400000002), and the Science Research Project of the Guangdong
Province (grant no. 2016A050503047).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW performed data collection and analysis, and was
involved in the drafting of the manuscript. JL, XP and RZ
participated in fraction isolation and characterization analysis.
YW, WY and QC participated in the virological experimentation. ZJ
and XW designed the study and were involved in the revising of the
manuscript. All of the authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publications
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marois I, Cloutier A, Meunier I, Weingartl
HM, Cantin AM and Richter MV: Inhibition of influenza virus
replication by targeting broad host cell pathways. PLoS One.
9:e1106312014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saladino R, Barontini M, Crucianelli M,
Nencioni L, Sgarbanti R and Palamara AT: Current advances in
anti-influenza therapy. Curr Med Chem. 17:2101–2140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hussain M, Galvin HD, Haw TY, Nutsford AN
and Husain M: Drug resistance in influenza a virus: The
epidemiology and management. Infect Drug Resist. 10:121–134. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Clercq E: Antiviral agents active
against influenza A viruses. Nat Rev Drug Discov. 5:1015–1025.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Jia W, Zhao A and Wang X:
Anti-influenza agents from plants and traditional Chinese medicine.
Phytother Res. 20:335–341. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu Y, Zhou C, Li X, Song L, Wu X, Lin W,
Chen H, Bai H, Zhao J, Zhang R, et al: Evaluation of
antiinflammatory activity of the total flavonoids of Laggera
pterodonta on acute and chronic inflammation models. Phytother
Res. 20:585–590. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao C, Liu B, Zeng C, Lu Y, Chen S, Yang
L, Li B and Li Y and Li Y: A polymethoxyflavone from Laggera
pterodonta induces apoptosis in imatinib-resistant K562R cells
via activation of the intrinsic apoptosis pathway. Cancer Cell Int.
14:1372014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi S, Huang K, Zhang Y, Zhao Y and Du Q:
Purification and identification of antiviral components from
Laggera pterodonta by high-speed counter-current
chromatography. J Chromatogr B Analyt Technol Biomed Life Sci.
859:119–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Zhou B, Lu J, Chen Q, Ti H, Huang
W, Li J, Yang Z, Jiang Z and Wang X: Inhibition of influenza virus
via a sesquiterpene fraction isolated from Laggera
pterodonta by targeting the NF-κB and p38 pathways. BMC
Complement Altern Med. 17:252017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Zhou B, Li C, Chen Q, Wang Y, Li Z,
Chen T, Yang C, Jiang Z, Zhong N, et al:
Lariciresinol-4-O-β-D-glucopyranoside from the root of Isatis
indigotica inhibitsinfluenza A virus-induced pro-inflammatory
response. J Ethnopharmacol. 174:379–386. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reed LJ and Muench H: A simple method of
estimating fifty percent endpoints. Am J Hyg. 27:493–497. 1938.
|
|
12
|
Zu M, Yang F, Zhou W, Liu A, Du G and
Zheng L: In vitro anti-influenza virus and anti-inflammatory
activities of theaflavin derivatives. Antiviral Res. 94:217–224.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu W, Li R, Li X, He J, Jiang S, Liu S and
Yang J: Quercetin as an antiviral agent inhibits influenza a virus
(IAV) entry. Viruses. 8:E62016. View
Article : Google Scholar
|
|
14
|
Uetani K, Hiroi M, Meguro T, Ogawa H,
Kamisako T, Ohmori Y and Erzurum SC: Influenza A virus abrogates
IFN-gamma response in respiratory epithelial cells bydisruption of
the Jak/Stat pathway. Eur J Immunol. 38:1559–1573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ding Y, Zeng L, Li R, Chen Q, Zhou B, Chen
Q, Cheng PL, Yutao W, Zheng J, Yang Z and Zhang F: The Chinese
prescription lianhuaqingwen capsule exerts anti-influenza
activitythrough the inhibition of viral propagation and impacts
immune function. BMC Complement Altern Med. 17:1302017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mok CK, Kang SS, Chan RW, Yue PY, Mak NK,
Poon LL, Wong RN, Peiris JS and Chan MC: Anti-inflammatory and
antiviral effects of indirubin derivatives ininfluenza A (H5N1)
virus infected primary human peripheral blood-derived macrophages
and alveolar epithelial cells. Antiviral Res. 106:95–104. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ramirez-Ortiz ZG, Prasad A, Griffith JW,
Pendergraft WF III, Cowley GS, Root DE, Tai M, Luster AD, El Khoury
J, Hacohen N and Means TK: The receptor TREML4 amplifies
TLR7-mediated signaling during antiviral responses and
autoimmunity. Nat Immunol. 16:495–504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Neill LA, Golenbock D and Bowie AG: The
history of Toll-like receptors-redefining innate immunity. Nat Rev
Immunol. 13:453–460. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vitiello M and Galdiero M, Finamore E,
Galdiero S and Galdiero M: NF-κB as a potential therapeutic target
in microbial diseases. Mol Biosyst. 8:1108–1120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nimmerjahn F, Dudziak D, Dirmeier U, Hobom
G, Riedel A, Schlee M, Staudt LM, Rosenwald A, Behrends U, Bornkamm
GW and Mautner J: Active NF-kappaB signalling is a prerequisite for
influenza virus infection. J Gen Virol. 85:2347–2356. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pleschka S, Wolff T, Ehrhardt C, Hobom G,
Planz O, Rapp UR and Ludwig S: Influenza virus propagation is
impaired by inhibition of the Raf/MEK/ERK signaling cascade. Nat
Cell Biol. 3:301–305. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu MS, Yen HR, Chang CW, Peng TY, Hsieh
CF, Chen CJ, Lin TY and Horng JT: Mechanism of action of the
suppression of influenza virus replication by Ko-Ken Tang through
inhibition of the phosphatidylinositol 3-kinase/Akt signaling
pathway and viral RNP nuclear export. J Ethnopharmacol.
134:614–623. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Holzberg M, Boergeling Y, Schräder T,
Ludwig S and Ehrhardt C: Vemurafenib limits influenza a virus
propagation by targeting multiple signaling pathways. Front
Microbiol. 8:24262017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
König R, Stertz S, Zhou Y, Inoue A,
Hoffmann HH, Bhattacharyya S, Alamares JG, Tscherne DM, Ortigoza
MB, Liang Y, et al: Human host factors required for influenza virus
replication. Nature. 463:813–817. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Watanabe T, Watanabe S and Kawaoka Y:
Cellular networks involved in the influenza virus life cycle. Cell
Host Microbe. 7:427–439. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
La Gruta NL, Kedzierska K, Stambas J and
Doherty PC: A question of self-preservation: Immunopathology in
influenza virus infection. Immunol Cell Biol. 85:85–92. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Paquette SG, Banner D, Zhao Z, Fang Y,
Huang SS, Leόn AJ, Ng DC, Almansa R, Martin-Loeches I, Ramirez P,
et al: Interleukin-6 is a potential piomarker for severe pandemic
H1N1 Influenza A infection. PLoS One. 7:e382142012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Planz O: Development of cellular signaling
pathway inhibitors as new antiviral against influenza. Antiviral
Res. 98:457–468. 2013. View Article : Google Scholar : PubMed/NCBI
|