Introduction
Periodontal disease is a chronic bacterial condition
associated with tooth loss. Periodontitis remains a major cause of
tooth loss in adults worldwide: The World Health Organization
recently reported that severe periodontitis exists in 5–20% of
adult populations (1). In recent
years, the association between periodontal disease and systemic
health has acquired a high level of attention. As medical research
has developed, it has been reported that periodontal disease may
involve the local oral periodontal tissue, and it may also be
associated with cardiovascular disease (2), diabetes (3), rheumatoid arthritis (4), respiratory tract infection (5) and other systemic diseases.
Porphyromonas gingivalis (P.
gingivalis) is the main pathogenic bacteria involved in
periodontal disease (6). Plaque
formation is the initial factor of periodontal disease;
saliva-derived membranes, which may be colonized by bacteria, are
formed at the beginning of production (7). With the progression of periodontal
disease, the pathogenic bacteria in the oral cavity and oropharynx
increases constantly, resulting in various types of pneumonia and
respiratory infections as they arrive in the lower respiratory
tract and lungs (8).
Pneumonia-associated pathogens may be detected in the dental plaque
of patients with pneumonia, and periodontal pathogens may be
cultured from lung lavage fluid (9). Previous clinical studies (1,10)
have indicated that periodontal infection may significantly
increase the incidence of fatal pneumonia in patients on a
ventilator, and there is an increased risk of mortality from
respiratory diseases, such as pneumonia, in elderly patients with
periodontitis. A therapeutic studies has revealed that the
incidence of respiratory infection decreased in elderly patients
with periodontitis who received regular oral treatment for
periodontal disease (11). In
addition, previous investigation has provided information on the
formation and maintenance of good oral health (11). Epidemiological surveys demonstrated
that the risk of respiratory disease is increased due to
mouth-breathing habits and periodontal disease, which may be due to
the airflow via the oral cavity, the lower respiratory tract and
the lungs, thereby introducing periodontal pathogens into the lungs
where an infection may occur (12,13).
In the process of periodontal epithelial tissue and
bone tissue destruction, P. gingivalis serves an important
role; it secretes a large number of toxic factors to stimulate the
host cells into producing the inflammatory cytokines interleukin
(IL)-1, IL-6 and tumor necrosis factor-α (TNF-α), which lead to
physiological and pathological damage (14). Influenza A virus (IAV) H1N1 is a
lethal pathogen that infects humans and animals. It is globally
pervasive and is associated with high rates of morbidity and
mortality (15). Normally, the
number of macrophages in the alveolar cavity is low; a variety of
inflammatory cytokines, including ILs, TNFs, chemokines, cytokines
and nitric oxide (NO), are produced when lung epithelial cells are
severely infected, which is closely associated with lung injury
(16). A study on the modeling of
bacterial or viral infection in mice demonstrated that IAV H1N1 may
increase the risk of mice becoming infected with pneumococcus
(17). Additionally, higher
expression levels of inflammatory cytokines, monocyte chemotactic
protein 1, chemokines and granulocyte colony stimulating factor
were observed.
In the present study, lung epithelial cell
infection, co-infection of P. gingivalis and IAV H1N1, and
the expression levels of inflammatory cytokines and NO were
investigated in order to study the effects of P. gingivalis
on the inflammatory cytokine and NO production within lung
epithelial cells infected with H1N1.
Materials and methods
Cells and viruses
BEAS-2B cells were acquired from the Department of
Immunology and Microbiology of Jinzhou Medical University (Jinzhou,
China), and were grown in RPMI-1640 (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; Zhejiang Tianhang Biotechnology Co., Ltd., Hangzhou, China).
All cells were maintained under the recommended culture conditions
and incubated at 37°C in a humidified environment with 5%
CO2. P. gingivalis was acquired from the
Department of Oral Biology of Jinzhou Medical University and
routinely grown in Brain Heart Infusion (BHI) blood agar medium or
BHI broth (Beijing Aoboxing Biotechnology Co., Ltd., Beijing,
China), supplemented with 0.5% yeast extract, hemin (10 µg/ml) and
vitamin K (1 µg/ml). Following incubation at 37°C for 4 days, the
bacterial number in the culture medium was determined by reading
the optical density values at 600 nm using a spectrophotometer, and
comparing them against a curve derived from a standard plate count.
The influenza PR/8/34 (H1N1) virus employed in the present study
was obtained from the Department of Immunology and Microbiology of
Jinzhou Medical University. The virus was grown in the
chorioallantoic fluid of 10-day-old embryonic hen eggs (Beijing
Merial Vital Laboratory Animal Technology Co., Ltd., Beijing,
China) at 37°C for 2 days. Following harvesting, the allantoic
fluid was filtered with a 0.22-µm cellulose acetate membrane. The
filtered liquid was stored in small aliquots at −70°C until further
use. The BEAS-2B cells were randomly and equally divided into four
groups and treated under the following conditions at 37°C with 5%
CO2: control group (no bacterial and viral infections)
H1N1 virus-treated [multiplicity of infection (MOI)=2:1]; P.
gingivalis-treated and H1N1 virus plus P.
gingivalis-treated (mixed infection) groups. Briefly, cells
were plated in a 6-well plate at a density of 2×106
cells/well and adhered for 24 h at 37°C in a 5% CO2
incubator. Bacteria and viruses were suspended in 2 ml serum and
antibiotic-free RPMI-1640 medium. The P. gingivalis
infection group and mixed infection group cells were infected with
P. gingivalis (MOI=100:1) and were cultured at 37°C with 5%
CO2 for 2 h. Then, bacterial infection liquid was
removed and H1N1 virus (MOI=2:1) was used to infect the virus and
mixed infection group at 37°C with 5% CO2 for 1 h.
Maintenance medium (2 ml; RIPM-1640 with 0.5% FBS) was subsequently
added to each well. Untreated cells were used as a negative control
group. All groups were recorded as 0 h at this time.
ELISA for TNF-α, IL-1β and IL-6
At 4, 8, 12 and 24 h post-infection, the cell
culture media of all groups were collected and centrifuged at 800 ×
g for 20 min at 4°C to remove debris. The supernatants were
collected (400 ml) to measure the concentrations of TNF-α, IL-1β
and IL-6 with an ELISA-based capture assay, using commercial TNF-α
(cat. no. PT512), IL-1β (cat. no. P1301) and IL-6 (cat. no. P1326)
ELISA kits (Beyotime Institute of Biotechnology, Shanghai, China)
according to the manufacturer's instruction. For all cytokines, the
optical density was determined within 15 min upon the addition of
50 µl Stop Solution (2 N sulfuric acid), using a microplate reader
(ChroMate® 4300, Awareness Technology Inc., Palm City,
Florida, USA) set to 450 nm, with the reference at 630 nm. Standard
curves were constructed using ChroMate® 4300 Manager
software (Awareness Technology Inc.). Each experiment was performed
≥3 times.
Nitrate (NO3−) reductase
assay. In the cell, NO undergoes a series of reactions with
numerous molecules present in biological fluids and is eventually
metabolized to nitrite (NO2−) and
NO3−(18).
At 4, 8, 12 and 24 h post-infection, cells of all groups were
centrifuged at 800 × g for 20 min at 4°C to collect the supernatant
(1 ml) and were stored at −80°C. After 30 min, the supernatant
samples were assayed for NO levels using a Microplate Assay from
Active Motif (Carlsbad, CA, USA) and a NO Quantitation kit (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) according to
the manufacturer's instructions. Each experiment was performed in
triplicate.
Western blot assay
Cells in each treatment group were centrifuged at
300 × g for 5 min at 4°C, followed by incubation with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) on ice. Protein concentration was quantified with a
Bradford assay. Then, 50 mg total protein extracts were separated
by 10% SDS-PAGE and transferred to polyvinylidene difluoride
membranes (GE Healthcare, Chicago, IL, USA), followed by blocking
for 1 h at room temperature in blocking buffer (cat. no. P0023B;
Beyotime Institute of Biotechnology). The membranes were incubated
with the following primary antibodies overnight at 4°C: Rabbit
anti-inducible NO synthase (iNOS; cat. no. ab3523; 1:1,000; Abcam,
Cambridge, MA, USA), rabbit anti-B-cell lymphoma-2 (Bcl-2; cat. no.
ab196495; 1:1,000), rabbit anti-Bcl-2-associated X protein (Bax;
cat. no. ab53154; 1:1,000), rabbit anti-caspase-3 (cat. no. ab4051;
1:1,000) and mouse anti-GAPDH (cat. no. ab8245; 1:1,000) or mouse
anti-β-actin (cat. no. ab6276; 1:1,000; all Abcam). Following
subsequent incubation with goat anti-rabbit (cat. no. ab205718) or
goat anti-mouse (cat. no. ab205719) horseradish
peroxidase-conjugated secondary antibody for 1 h at room
temperature. bands were detected using an Enhanced
Chemiluminescence kit (Beyotime Institute of Biotechnology). Image
J software version 1.48 (National Institutes of Health, Bethesda,
MD, USA) was used to analyze relative protein band density. iNOS
was measured at 4, 8, 12 and 24 h post-infection; Bax, Bcl-2 and
caspase-3 were measured at 24 h post-infection only. Each sample
was analyzed in triplicate.
Apoptosis analysis
Treated cells were collected by low-speed
centrifugation (300 × g, for 5 min at 4°C), and cell pellets were
re-suspended in 1 ml PBS solution. Apoptosis was analyzed using an
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) according to
the manufacturer's instructions. In brief, cells were washed,
centrifuged (300 × g, 4°C, 5 min) and re-suspended in PI staining
buffer containing 50 µl/ml PI. The cell mixture was subsequently
incubated at 4°C for 30 min in a dark environment and stained with
5 µl Annexin V-FITC to detect apoptosis with a flow cytometer
(Beckman Coulter, Inc., Brea, CA, USA). FlowJo software 7.6.5 (Tree
Star, Inc., Ashland, OR, USA) was used to analyze the results of
the flow cytometry. All experiments were performed in
triplicate.
Statistical analysis
All of the results were expressed as the mean ±
standard deviation and all statistical analyses were performed
using the SPSS 18.0 version (SPSS, Inc., Chicago, IL, USA).
Statistical comparisons were conducted using one-way analysis of
variance followed by Dunnett's post-hoc test for multiple
comparisons. Non-linear regression models were employed to
calculate the concentration of each cytokine in the culture
supernatants. P<0.05 was considered to indicate a statistically
significant difference.
Results
P. gingivalis promotes the secretion
of TNF-α, IL-1β and IL-6 from BEAS-2B cells infected with H1N1
To study the effects of P. gingivalis on the
secretion of TNF-α, IL-1β and IL-6 from BEAS-2B cells infected with
H1N1, ELISAs were performed to detect their concentrations at
various time points (4, 8, 12 and 24 h) in all groups. The results
of the present study revealed that following infection of BEAS-2B
cells with P. gingivalis and H1N1, H1N1 only or P.
gingivalis alone, the concentrations of TNF-α, IL-1β and IL-6
in the supernatant were significantly increased at each time point,
when compared with the control group (at 4, 8, 12 and 24 h;
Fig. 1). Following BEAS-2B cell
infection with P. gingivalis and H1N1, the concentrations of
TNF-α, IL-1β and IL-6 in the supernatant were significantly
increased at each time point, compared with the H1N1 and P.
gingivalis alone groups (at 4, 8, 12 and 24 h; Fig. 1). These results demonstrated that
lung epithelial cells infected with H1N1 and P. gingivalis
promoted the production of inflammatory cytokines.
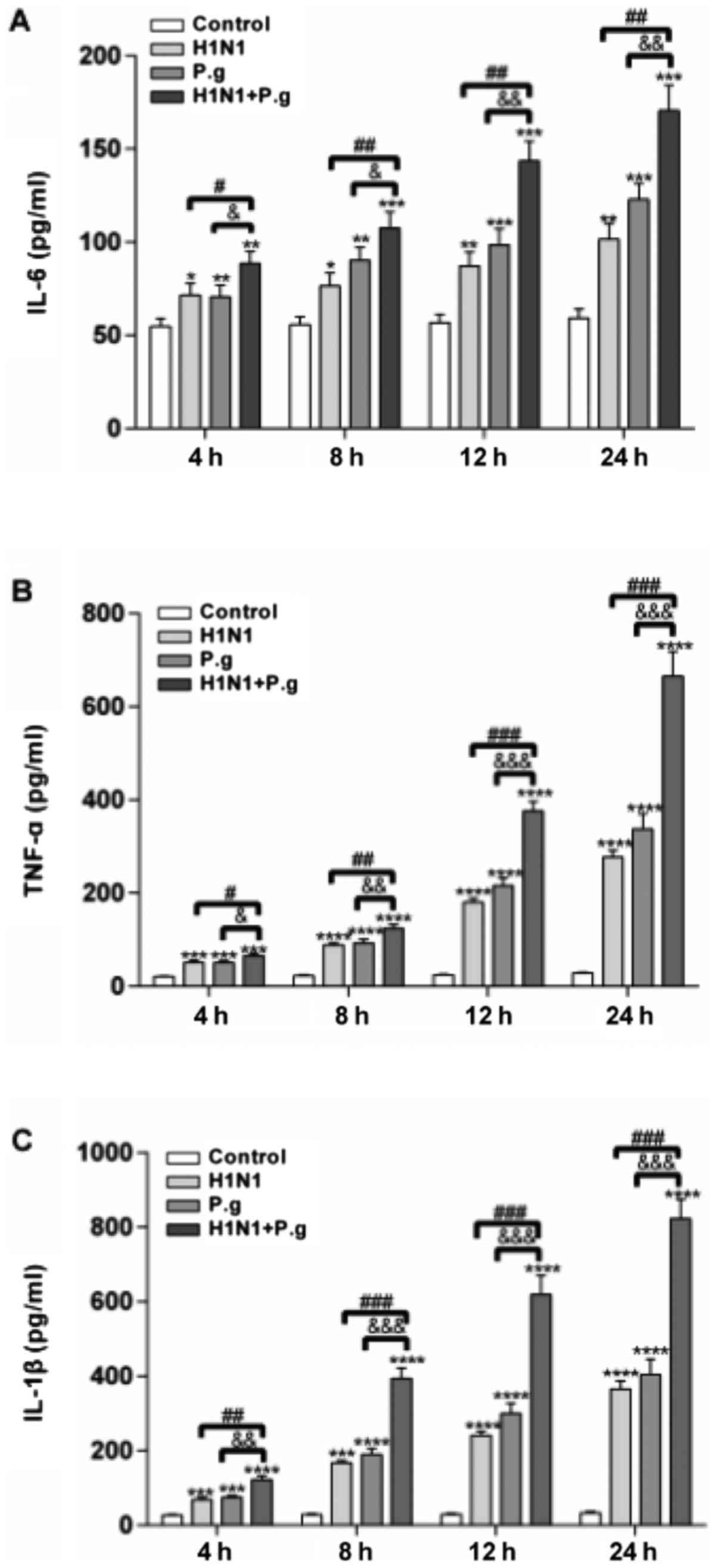 | Figure 1.Effects of P.g. on the expression
levels of TNF-α, IL-1β and IL-6 in BEAS-2B cells infected with
influenza virus. The concentrations of (A) IL-6, (B) TNF-α and (C)
IL-1β in the control, H1N1, P.g. and H1N1+P.g. groups following
infection of BEAS-2B for 4, 8, 12 and 24 h, were detected by ELISA.
The values are presented as the mean ± standard deviation.
*P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001 vs.
control group at the same time point; #P<0.05,
##P<0.01 and ###P<0.001 vs. H1N1 group
at the same time point; &P<0.05,
&&P<0.01 and
&&&P<0.001 vs. P.g. group at the same
time point. IL, interleukin; H1N1, influenza 1 virus H1N1; P.g.,
Porphyromonas gingivalis; TNF-α, tumor necrosis
factor-α. |
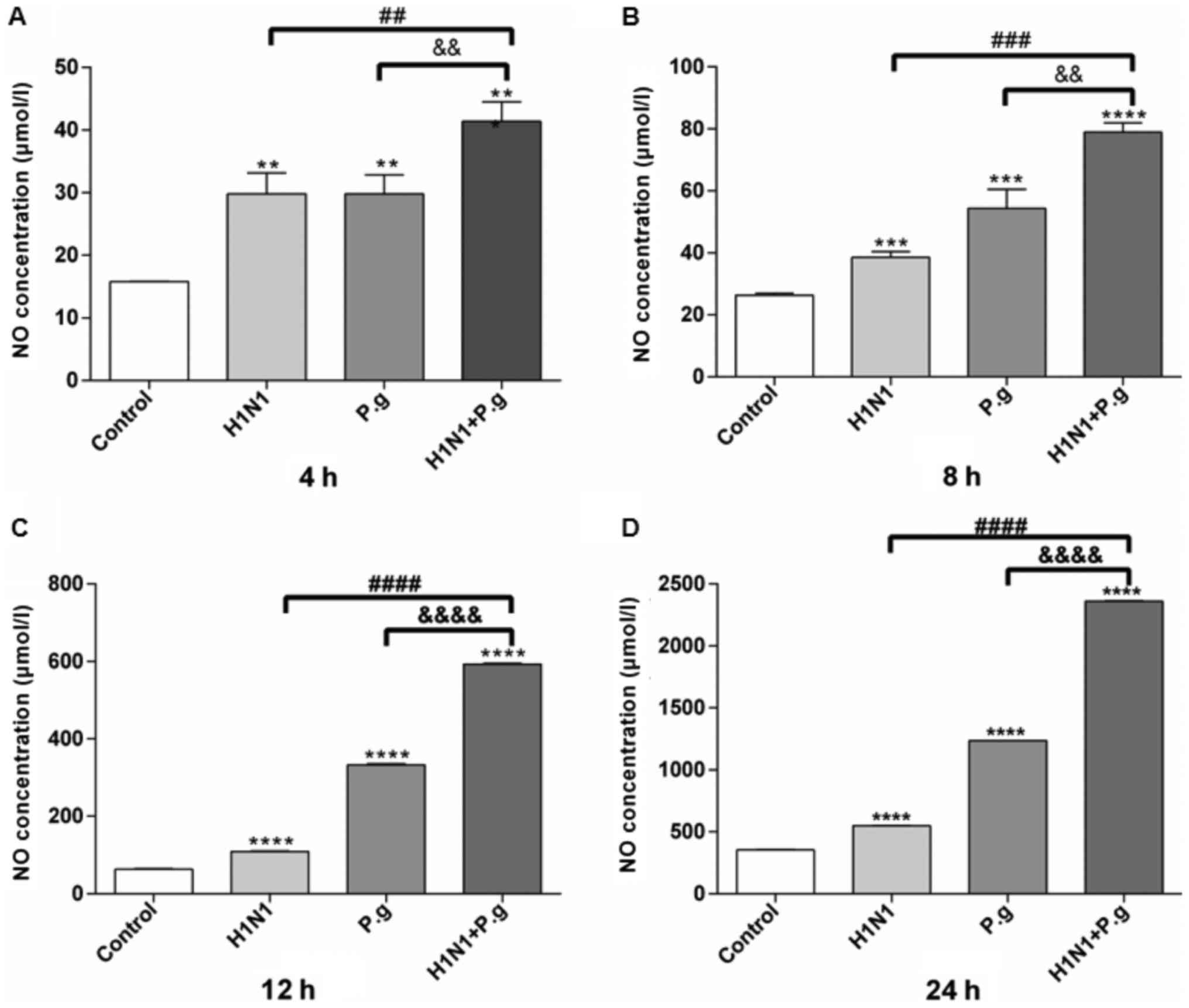
P. gingivalis increases the NO
expression levels in BEAS-2B cells infected with H1N1
In order to observe the effects of P.
gingivalis on the NO expression levels in BEAS-2B cells
infected with H1N1, a NO3− reductase assay
was performed to determine the NO expression levels at various time
points in all groups. The results of the present study demonstrated
that following the infection of BEAS-2B cells with P.
gingivalis and H1N1, H1N1 only or P. gingivalis alone,
the expression levels of NO in the supernatant significantly
increased at each time point in all groups, when compared with the
control group (Fig. 2). In
addition, BEAS-2B cells infected with P. gingivalis and H1N1
exhibited significantly increased expression levels of NO in the
supernatant at each time point when compared with the H1N1 only and
P. gingivalis alone groups (at 4, 8, 12 and 24 h; Fig. 2A-D). These results indicated that
P. gingivalis may have promoted the production of NO in lung
epithelial cells infected with influenza virus H1N1.
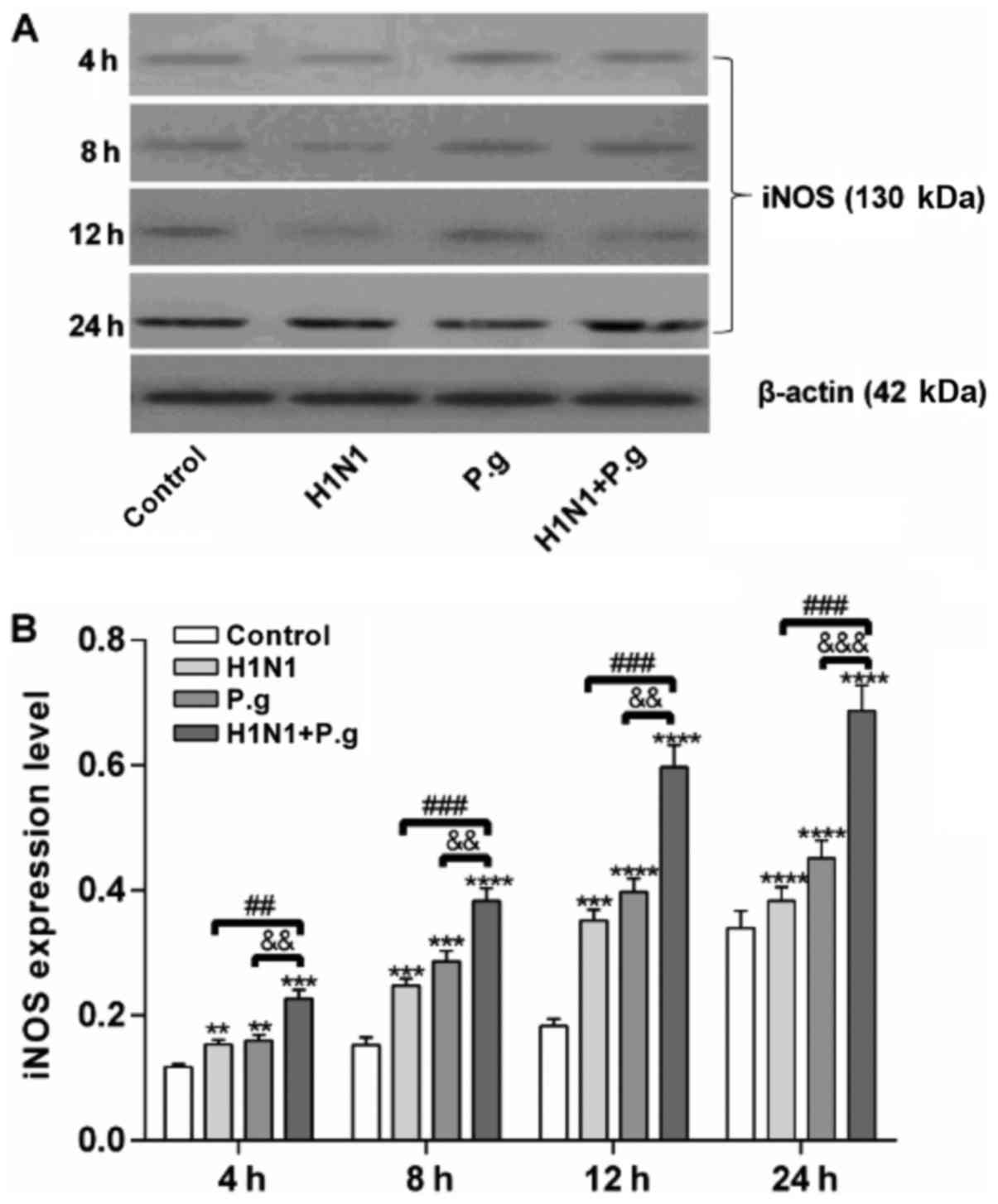
P. gingivalis increases the protein
expression levels of iNOS in BEAS-2B cells infected with H1N1
To further confirm the effects of P.
gingivalis on the protein expression of iNOS in BEAS-2B cells
infected with H1N1, western blot analysis was conducted. The
present study reported that the BEAS-2B cells infected with P.
gingivalis and H1N1, H1N1 only or P. gingivalis alone
exhibited significantly increased protein expression levels of iNOS
at each time point when compared with the control group (at 4, 8,
12 and 24 h; Fig. 3A and B). In
addition, BEAS-2B cells infected with P. gingivalis and H1N1
exhibited significantly increased protein expression levels of iNOS
at each time point when compared with the H1N1 and P.
gingivalis only groups (at 4, 8, 12 and 24 h; Fig. 3A and B), indicating that lung
epithelial cells co-infected with H1N1 and P. gingivalis may
have further induced the expression of iNOS.
P. gingivalis increases the BEAS-2B
cellular apoptosis rate following infection with H1N1
To further study the effects of P. gingivalis
on the apoptosis rate of BEAS-2B cells infected with H1N1, flow
cytometric analysis was conducted. The results of the present study
demonstrated that BEAS-2B cells infected with P. gingivalis
and H1N1, H1N1 only or P. gingivalis alone, exhibited
significantly increased rates of apoptosis at each time point
compared with the Control group (at 4, 8, 12 and 24 h; Fig. 4A-E). Additionally, BEAS-2B cells
infected with P. gingivalis and H1N1 exhibited a
significantly increased apoptotic rate at each time point when
compared with the H1N1 and P. gingivalis only groups (at 4,
8, 12 and 24 h; Fig. 4A-E). These
results revealed that P. gingivalis may have induced an
inflammatory response and subsequently promoted the induction of
apoptosis.
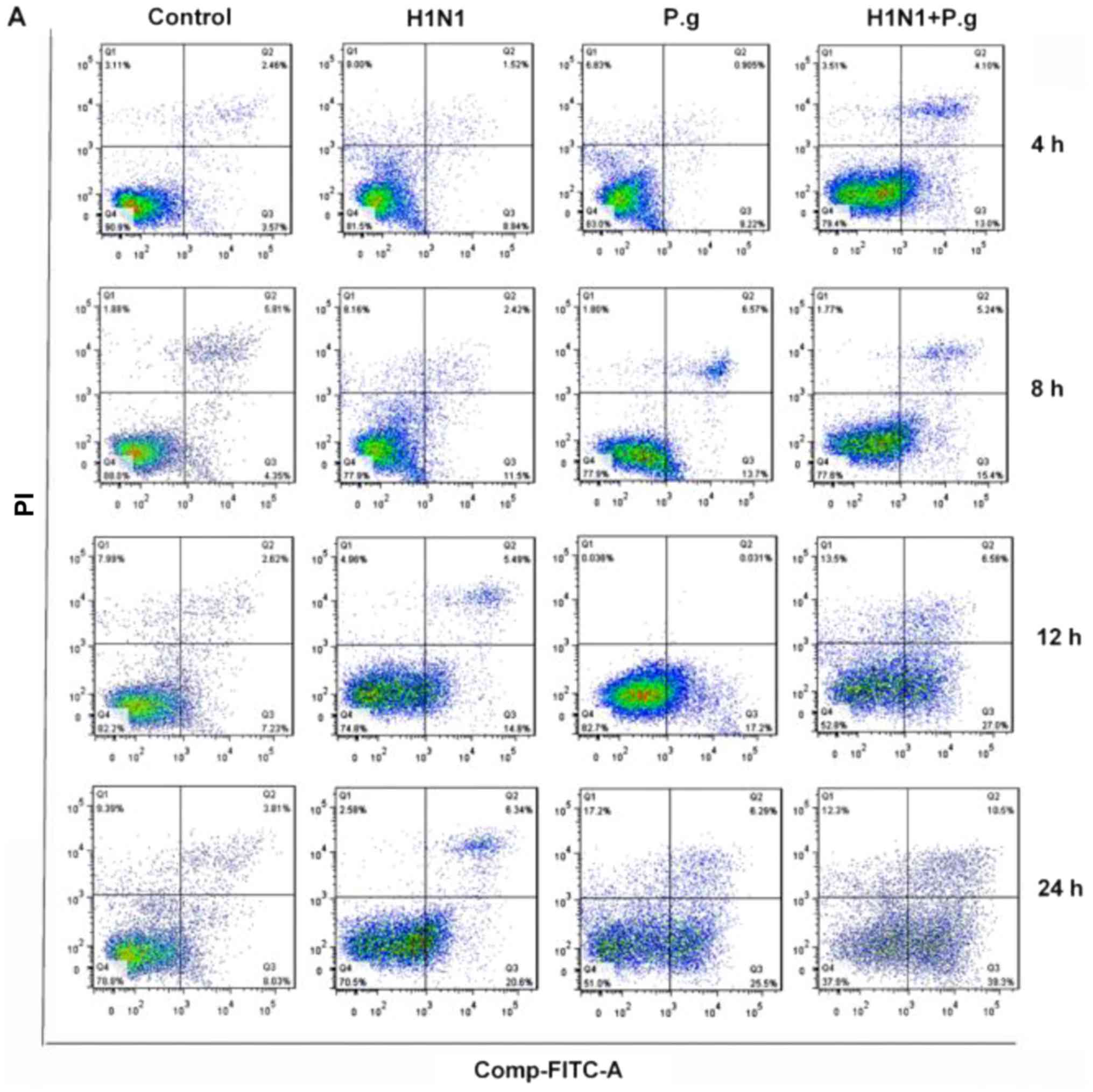 | Figure 4.Effects of P.g. on the cell apoptosis
rate in BEAS-2B cells infected with influenza virus. (A) BEAS-2B
cell apoptosis in the control, H1N1, P.g. and H1N1+ P.g. groups
following infection of BEAS-2B cells for 4, 8, 12 and 24 h,
detected by flow cytometry analysis. The apoptotic rates of BEAS-2B
cells in the control, H1N1, P.g. and H1N1+ P.g. groups following
infection of BEAS-2B for (B) 4, (C) 8, (D) 12 and (E) 24 h, as
detected by flow cytometry analysis. The values are presented as
the mean ± standard deviation. ***P<0.001 and ****P<0.0001
vs. control group; ##P<0.01, and
####P<0.0001 vs. H1N1 group;
&&P<0.01 and
&&&&P<0.0001 vs. P.g. group. H1N1,
influenza 1 virus H1N1; FITC, fluorescein isothiocyanate; PI,
propidium iodide; P.g., Porphyromonas gingivalis. |
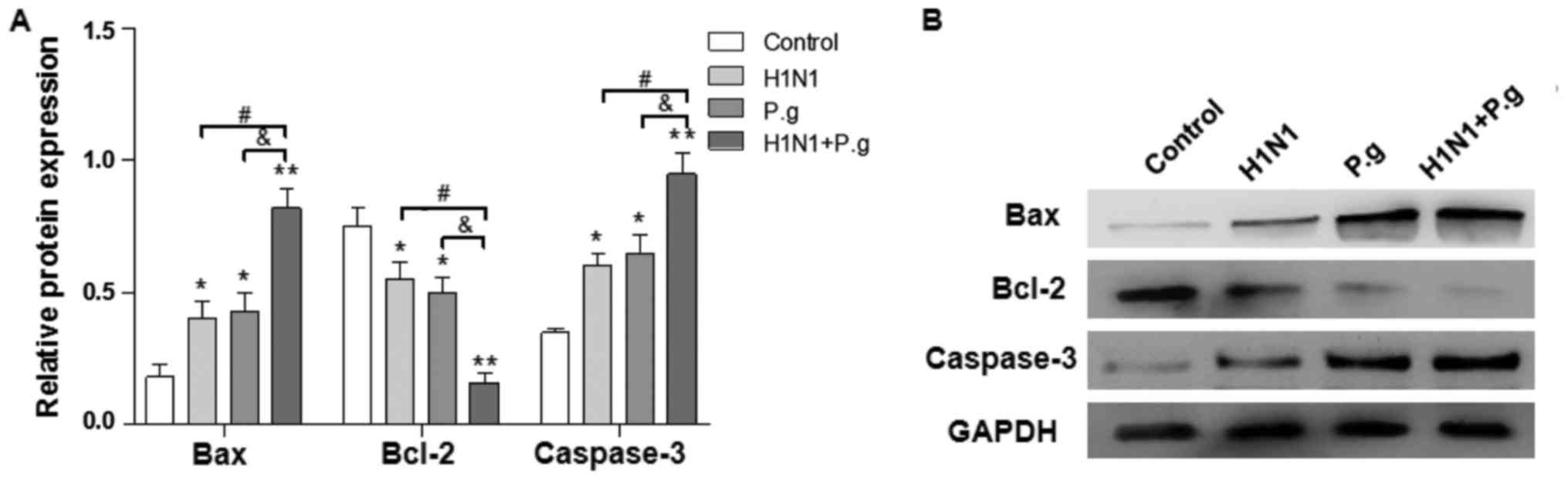
Effects of P. gingivalis on the
Bcl-2/Bax/caspase-3 signaling pathway in BEAS-2B cells infected
with influenza virus
The Bcl-2 and caspase families serve an important
role in the regulation of cellular apoptosis (19). In order to characterize the
mechanism by which the co-infection with P. gingivalis and
H1N1 induces BEAS-2B cellular apoptosis, the protein expression
levels of Bcl-2, Bax and caspase-3 were detected by western
blotting. Compared with the control, the relative protein
expression levels of caspase-3 and Bax were significantly
increased, and the relative expression of Bcl-2 protein decreased
significantly in lung epithelial cells following co-infection with
P. gingivalis and H1N1 for 24 h (Fig. 5A and B). These results revealed
that co-infection with P. gingivalis and H1N1 may have
promoted cellular apoptosis in a manner associated with the
regulation of the Bcl-2/Bax/caspase-3 signaling pathway.
Discussion
Periodontal disease is a chronic inflammatory
process associated with infection with P. gingivalis,
causing a local and systemic inflammatory response (20). Numerous studies have reported that
periodontal disease may be mediated by P. gingivalis and
systemic diseases, including cardiovascular and cerebrovascular
diseases, diabetes and respiratory infections (21–23).
Oropharyngeal secretions enter the human respiratory system via
breathing and swallowing mechanisms, and may therefore lead to
pulmonary disease associated with periodontal pathogens such as
P. gingivalis (24). The
influenza virus, such as H1N1, is a common pathogen causing lung
infection in humans (25).
Clinical research suggests that infection of lung epithelial cells
by P. gingivalis or influenza viruses does not induce
apoptosis and necrosis of respiratory epithelial cells in a short
period of time (26,27). However, upon co-infection with
P. gingivalis and influenza viruses, a large proportion of
apoptotic and necrotic respiratory epithelial cells were observed
within a short period (28). In
the present study, using lung epithelial cells co-infected with
P. gingivalis and the influenza virus H1N1, the expression
levels of inflammatory cytokines and NO were detected to
investigate the mechanism underlying the association between
periodontal disease and respiratory infection.
NOS catalyzes the synthesis of NO in vivo;
under normal physiological states, NO synthesis mediated by
endothelial NOS serves a major role in the regulation of vascular
endothelial cells (29). Low or
absent expression of iNOS in endothelial cells leads to the
secretion of large amounts of NO, and the expression of iNOS
increases correspondingly following severe infection (30,31).
Cytotoxic products, such as lipopolysaccharide, of P.
gingivalis induce host cells to release inflammatory cytokines,
of which TNF-α, IL-1β and IL-6 are of the most importance (32,33).
P. gingivalis-infected cells expressed TNF-α, IL-1β and
IL-6, along with other inflammatory cytokines; therefore, the
synthesis of NOS is induced to increase the production of NO, which
leads to endothelial cell dysfunction (34). Research suggests that excess NO
production may be closely associated with the invasion of vascular
endothelial cells by P. gingivalis (18). NO production may cause cytotoxicity
and mediate pulmonary epithelial cell damage (19,35,36).
The results of the present study revealed that P. gingivalis
may induce the production of a large number of inflammatory
cytokines in lung epithelial cells. It was hypothesized that in the
present study, P. gingivalis may have induced an
inflammatory reaction in lung epithelial cells, promoting the
accumulation of NO, which may have resulted in damage to lung
epithelial cells and the induction of apoptosis. When compared with
the control group, and the viral and bacterial infection groups,
apoptotic cell numbers increased by the 4 h time point, and the
highest levels were observed at 24 h in the group co-infected with
P. gingivalis and H1N1; the percentage of apoptotic lung
epithelial cells corresponded to the increase in NO production. In
the present study, P. gingivalis may have promoted the
production of NO in lung epithelial cells infected with influenza
virus H1N1, which may have led to an increase in the inflammatory
reaction and lung epithelial cell injury.
Regarding the characterization of the mechanism by
which the co-infection of P. gingivalis and H1N1 induces
BEAS-2B cellular apoptosis, the present study reported that
compared with the control, the relative protein expression levels
of caspase-3 and Bax increased significantly, and that the relative
expression levels of Bcl-2 protein decreased significantly, within
24 h in BEAS-2B cells following co-infection with P.
gingivalis and H1N1. The results of the present study revealed
that co-infection with P. gingivalis and H1N1 may have
promoted cellular apoptosis by modulating the Bcl-2/Bax/caspase-3
signaling pathway.
In conclusion, the present study demonstrated that
lung epithelial cells infected with H1N1 and P. gingivalis
led to the promoted production of inflammatory cytokines and the
expression of iNOS, which may have also increased the accumulation
of NO, resulting in an increased proportion of lung epithelial
cells undergoing apoptosis via the Bcl-2/Bax/caspase-3 signaling
pathway. The present study provided the experimental foundation for
research into periodontal pathogens in the injury and apoptosis of
lung epithelial cells, in order to confirm whether there is an
association between periodontal disease and respiratory
infection.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Liaoning
Province Key Laboratory of Oral Diseases Fund Program (grant no.
LPKLOD-15-03), Liaoning Science & Technology Fund Program
(grant no. 2016010294) and Jinzhou Medical University's 2017
Undergraduate Innovation and Entrepreneurship Training Program
(grant no. 201705).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YP and XL designed the study. YC, RZ, ZY, YL, YF, YZ
and PL performed the experiments. YC analyzed the data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jin LJ, Armitage GC, Klinge B, Lang NP,
Tonetti M and Williams RC: Global oral health inequalities: Task
group-periodontal disease. Adv Dent Res. 23:221–226. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Noguchi S, Toyokawa S, Miyoshi Y, Suyama
Y, Inoue K and Kobayashi Y: Five-year follow-up study of the
association between periodontal disease and myocardial infarction
among Japanese male workers: MY Health Up study. J Public Health
(Oxf). 37:605–611. 2015.PubMed/NCBI
|
|
3
|
Chapple IL and Genco R: Working group 2 of
the joint EFP/AAP workshop: Diabetes and periodontal diseases:
Consensus report of the Joint EFP/AAP workshop on periodontitis and
systemic diseases. J Periodontol. 84 4 Suppl:S106–S112. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mikuls TR, Payne JB, Yu F, Thiele GM,
Reynolds RJ, Cannon GW, Markt J, McGowan D, Kerr GS, Redman RS, et
al: Periodontitis and porphyromonas gingivalis in patients
with rheumatoid arthritis. Arthritis Rheumatol. 66:1090–1100. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fernández-Plata R, Olmedo-Torres D,
Martínez-Briseño D, García-Sancho C, Franco-Marina F and
González-Cruz H: Prevalence of severe periodontal disease and its
association with respiratory disease in hospitalized adult patients
in a tertiary care center. Gac Med Mex. 151:608–613. 2015.(In
Spanish). PubMed/NCBI
|
|
6
|
Cho BH, Jung YH, Kim DJ, Woo BH, Jung JE,
Lee JH, Choi YW and Park HR: Acetylshikonin suppresses invasion of
Porphyromonas gingivalis-infected YD10B oral cancer cells by
modulating the interleukin-8/matrix metalloproteinase axis. Mol Med
Rep. 17:2327–2334. 2018.PubMed/NCBI
|
|
7
|
Anand PS, Kamath KP and Anil S: Role of
dental plaque, saliva and periodontal disease in Helicobacter
pylori infection. World J Gastroentero. 20:5639–5653. 2014.
View Article : Google Scholar
|
|
8
|
Sharma N and Shamsuddin H: Association
between respiratory disease in hospitalized patients and
periodontal disease: A cross-sectional study. J Periodontol.
82:1155–1160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bousbia S, Papazian L, La Scola B and
Raoult D: Detection of plant DNA in the bronchoalveolar lavage of
patients with ventilator-associated pneumonia. PLoS One.
5:e112982010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Rabbany M, Zaghlol N, Bhandari M and
Azarpazhooh A: Prophylactic oral health procedures to prevent
hospital-acquired and ventilator-associated pneumonia: A systematic
review. Int J Nurs Stud. 52:452–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bansal M, Khatri M and Taneja V: Potential
role of periodontal infection in respiratory diseases-a review. J
Med Life. 6:244–248. 2013.PubMed/NCBI
|
|
12
|
Lee SY, Bae CS, Seo JH, Cho SS, Bae MS, Oh
DS and Park DH: Mycoleptodonoides aitchisonii suppresses asthma via
Th2 and Th1 cell regulation in an ovalbumin-induced asthma mouse
model. Mol Med Rep. 17:11–20. 2018.PubMed/NCBI
|
|
13
|
Liu Y, Xie L, Yang M, Tan X, Zeng Y, Zheng
G, Chen Y and Chen P: PPAR-α improves the recovery of lung function
following acute respiratory distress syndrome by suppressing the
level of TGF-β1. Mol Med Rep. 16:49–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu B, Wang J, Cheng L and Liang J: Role
of JNK and NF-κB pathways in Porphyromonas gingivalis
LPS-induced vascular cell adhesion molecule-1 expression in human
aortic endothelial cells. Mol Med Rep. 8:1594–1600. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chambers C, Skowronski DM, Sabaiduc S,
Winter AL, Dickinson JA, De Serres G, Gubbay JB, Drews SJ,
Martineau C, Eshaghi A, et al: Interim estimates of 2015/16 vaccine
effectiveness against influenza A(H1N1)pdm09, Canada, February
2016. Euro Surveill. 21:301682016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J and Kubes P: A reservoir of mature
cavity macrophages that can rapidly invade visceral organs to
affect tissue repair. Cell. 165:668–678. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shah NS, Greenberg JA, McNulty MC, Gregg
KS, Riddell J IV, Mangino JE, Weber DM, Hebert CL, Marzec NS,
Barron MA, et al: Bacterial and viral co-infections complicating
severe influenza: Incidence and impact among 507 U.S. patients,
2013–14. J Clin Virol. 80:12–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alayan J, Ivanovski S, Gemmell E, Ford P,
Hamlet S and Farah CS: Deficiency of iNOS contributes to
Porphyromonas gingivalis-induced tissue damage. Oral
Microbiol Immunol. 21:360–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahamed M, Akhtar MJ, Khan MAM, Alhadlaq HA
and Aldalbahi A: Nanocubes of indium oxide induce cytotoxicity and
apoptosis through oxidative stress in human lung epithelial cells.
Colloids Surf B Biointerfaces. 156:157–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gawron K, Montgomery A, Łazarz-Bartyzel K,
Bereta G, Chomyszyn-Gajewska M, Venables P and Potempa J:
Porphyromonas gingivalis peptidyl arginine deiminase: A
Unique bacterial PAD with implications for periodontal disease and
rheumatoid Arthritis. Protein Deimination Human Health Dis. 99–135.
2017. View Article : Google Scholar
|
|
21
|
Olsen I and Yilmaz Ö: Modulation of
inflammasome activity by Porphyromonas gingivalis in
periodontitis and associated systemic diseases. J Oral Microbiol.
8:303852016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Igari K, Inoue Y and Iwai T: An
experimental model of peripheral vascular disease involving the
intravenous injection of oral bacteria. Ann Vasc Dis. 9:267–271.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Card JW, Carey MA, Voltz JW, Bradbury JA,
Ferguson CD, Cohen EA, Schwartz S, Flake GP, Morgan DL, Arbes SJ
Jr, et al: Modulation of allergic airway inflammation by the oral
pathogen Porphyromonas gingivalis. Infect Immun.
78:2488–2496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okuda K, Kimizuka R, Abe S, Kato T and
Ishihara K: Involvement of periodontopathic anaerobes in aspiration
pneumonia. J Periodontol. 76 11 Suppl:S2154–S2160. 2005. View Article : Google Scholar
|
|
25
|
Guo L, Feng K, Wang YC, Mei JJ, Ning RT,
Zheng HW, Wang JJ, Worthen GS, Wang X, Song J, et al: Critical role
of CXCL4 in the lung pathogenesis of influenza (H1N1) respiratory
infection. Mucosal Immunol. 10:1529–1541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Porto AN, Cortelli SC, Borges AH, Matos
FZ, Aquino DR, Miranda TB, Costa Oliveira F, Aranha AF and Cortelli
JR: Oral and endotracheal tubes colonization by periodontal
bacteria: A case-control ICU study. Eur J Clin Microbiol Infect
Dis. 35:343–351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Q, Pan C, Teng D, Lin L, Kou Y, Haase
EM, Scannapieco FA and Pan Y: Porphyromonas gingivalis
modulates Pseudomonas aeruginosa-induced apoptosis of
respiratory epithelial cells through the STAT3 signaling pathway.
Microbes Infect. 16:17–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Connolly E, Millhouse E, Doyle R, Culshaw
S, Ramage G and Moran GP: The Porphyromonas gingivalis
haemagglutinins HagB and HagC are major mediators of adhesion and
biofilm formation. Mol Oral Microbiol. 32:35–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chuaiphichai S, Crabtree MJ, Mcneill E,
Hale AB, Trelfa L, Channon KM and Douglas G: A key role for
tetrahydrobiopterin-dependent endothelial NOS regulation in
resistance arteries: Studies in endothelial cell
tetrahydrobiopterin-deficient mice. Brit J Pharmacol. 174:657–671.
2017. View Article : Google Scholar
|
|
30
|
Assis MC, Freitas C, Saliba AM, D'A
Carvalho AP, Simao TA, Albano RM and Plotkowski MC: Up-regulation
of Fas expression by Pseudomonas aeruginosa-infected
endothelial cells depends on modulation of iNOS and enhanced
production of NO induced by bacterial type III secreted proteins.
Int J Mol Med. 18:355–363. 2006.PubMed/NCBI
|
|
31
|
Yan M, Hou M, Liu J, Zhang S, Liu B, Wu X
and Liu G: Regulation of iNOS-derived ROS generation by HSP90 and
Cav-1 in porcine reproductive and respiratory syndrome
virus-infected swine lung injury. Inflammation. 40:1236–1244. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ke X, Lei L, Li H, Li H and Yan F:
Manipulation of necroptosis by Porphyromonas gingivalis in
periodontitis development. Mol Immunol. 77:8–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yiemwattana I and Kaomongkolgit R:
Alpha-mangostin suppresses IL-6 and IL-8 expression in P.
gingivalis LPS-stimulated human gingival fibroblasts.
Odontology. 103:348–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu C, Guo S, Niu Y, Yang L, Liu B, Jiang
N, Su M and Wang L: Heat-shock protein 60 of Porphyromonas
gingivalis may induce dysfunction of human umbilical
endothelial cells via regulation of endothelial-nitric oxide
synthase and vascular endothelial-cadherin. Biomed Rep. 5:243–247.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Issaad N, Aitlounis A and Larabadjebari F:
Cytotoxicity and actin cytoskeleton damage induced in human
alveolar epithelial cells by Androctonus australis hector venom.
Toxin Rev. 37:67–74. 2017. View Article : Google Scholar
|
|
36
|
Kaviyarasu K, Geetha N, Kanimozhi K,
Magdalane Maria C, Sivaranjani S, Ayeshamariam A, Kennedy J and
Maaza M: In vitro cytotoxicity effect and antibacterial performance
of human lung epithelial cells A549 activity of Zinc oxide doped
TiO2 nanocrystals: Investigation of bio-medical application by
chemical method. Mater Sci Eng C Mater Biol Appl. 74:325–333. 2017.
View Article : Google Scholar : PubMed/NCBI
|