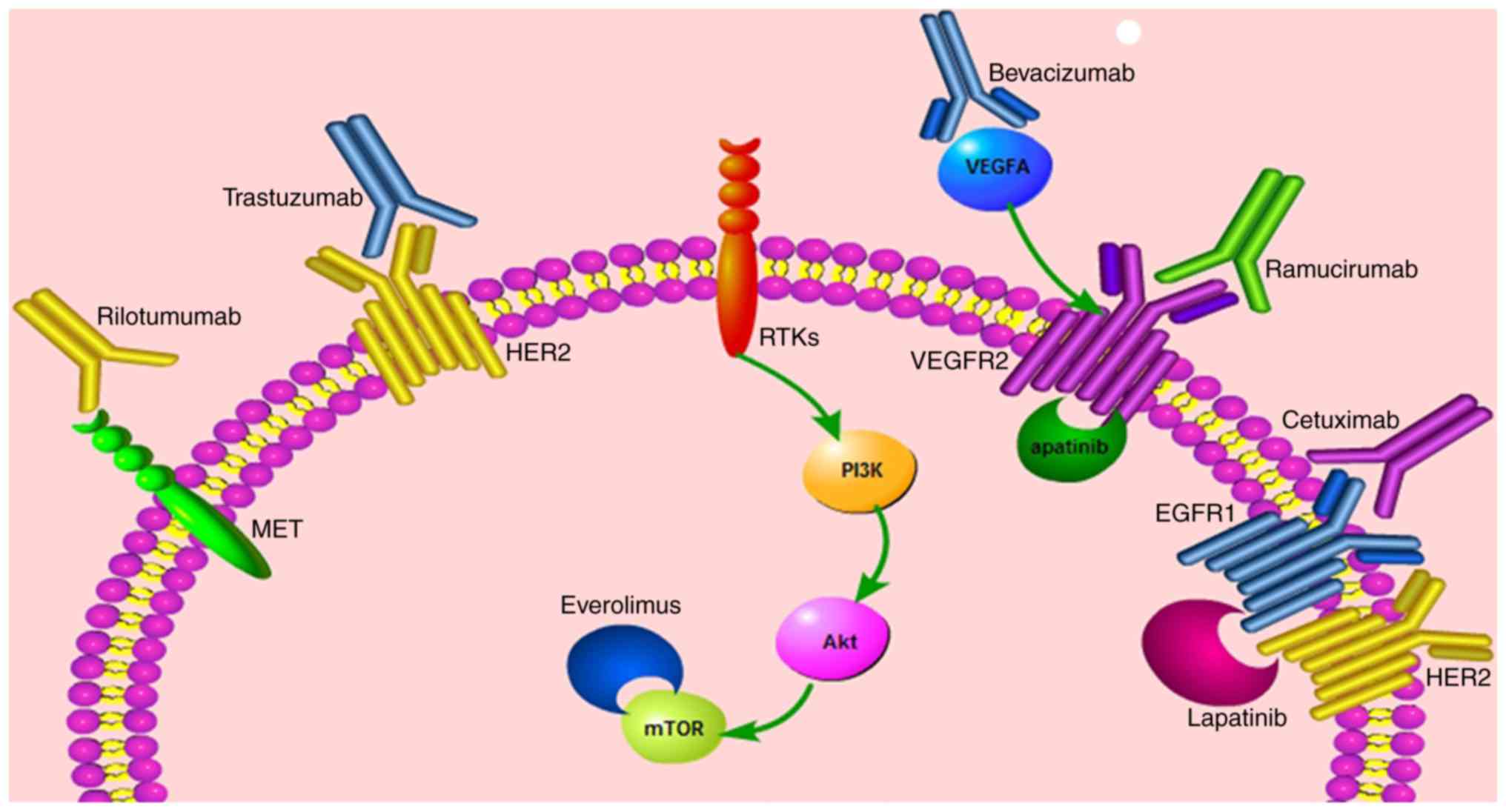
Gastric cancer (GC) is the fourth-most common
malignant neoplasm and the second leading cause of
cancer-associated death worldwide (1). Although surgery is considered the
only curative method for GC, a large number of patients are unable
to undergo surgery because of advanced disease stage at diagnosis
(2–4). Accordingly, chemotherapy is one of
the options for the treatment of advanced gastric cancer (AGC).
However, the response rate of first-line chemotherapy regimens in
GC is ~40% In human epidermal receptor 2 (HER2)-positive patients,
the response rate of first-line chemotherapy regimens using
trastuzumab fails to reach 50% and the improvement of overall
survival is limited (5). In
patients who do not respond to chemotherapy, the benefit of
chemotherapy is clearly limited, whereas the incidence of toxic
reactions associated with the treatment is high (6). Therefore, it is important to identify
patients who are sensitive to certain drugs. In addition, multidrug
resistance (MDR) is an important contributor to drug non-response,
and cellular resistance in AGC may be associated with the function
of the MDR protein (7).
Cetuximab and panitumumab are monoclonal antibodies
against EGFR. Although several phase II clinical trials
demonstrated the benefit of EGFR inhibitors in patients with GC
(8,9), both randomized and open-label phase
III trials of the drugs failed to prove the benefit of EGFR
inhibitors in GC treatment. Cetuximab in Combination with Xeloda
and Cisplatin in Advanced Esophago-gastric Cancer (EXPAND) clinical
trial reported that cetuximab + cisplatin and capecitabine failed
to improve the progression-free survival (PFS) (10). Furthermore, the Trial of Efficacy
of Epirubicin, Oxaliplatin and Capecitabine (EOX) with/without
Panitumumab in Previously Untreated Advanced Oesophagogastric
Cancer (REAL3) showed that panitumumab + EOX did not increase the
OS (11). Therefore, anti-EGFR
treatments offer no survival benefits in non-selected patients with
metastatic GC.
A previous study reported that wild-type KRAS
proto-oncogene, GTPase (KRAS) could be a positive predictor of
cetuximab efficacy in EGFR-positive gastric cancer cell lines
(12) However, a prospective
multi-center phase II trial assessed biomarkers in patients with
gastroesophageal cancer treated with cetuximab + irinotecan,
folinic acid and 5-FU (13). The
study analyzed mutations of the KRAS (exons 12 and 13), B-Raf
proto-oncogene, serine/threonine kinase (V600E), and
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit a
(PIK3CA; exons 9 and 20) oncogenes, concluding that KRAS mutations
were not suitable predictors of cetuximab efficacy (13).
Taken together, phase III clinical trials failed to
confirm the efficacy of EGFR inhibitors in patients with GC and a
phase II translational study did not identify any effective
biomarkers for the prediction of cetuximab efficacy.
Trastuzumab is a monoclonal antibody which binds to
the extracellular domain of HER2 receptor (14). Trastuzumab is also the first
approved targeted agent for the treatment of metastatic GC
(5). HER2 is a promising
predictive biomarker for the efficacy of trastuzumab efficacy
(5).
In the Trastuzumab for GC (ToGA) phase III study,
594 GC/gastroesophageal junction cancer (GEJ), HER2
immunohistochemistry (IHC) 3+, or fluorescent in situ
hybridization (FISH)-positive patients were randomly assigned to
receive either chemotherapy (cisplatin combined with FU or
capecitabine) + trastuzumab or chemotherapy alone (5). The OS and PFS significantly improved
in patients treated with trastuzumab + chemotherapy. A post hoc
analysis of OS by subgroups differentiated by protein expression
level of HER2 showed the increased efficacy of trastuzumab
associated with high expression of the protein (5). ToGA was a landmark study which
utilized a targeted agent based on the measurement of a predictive
biomarker, leading to a consensus about which patients may benefit
from trastuzumab, the criteria for HER2-positive status being IHC3+
or IHC2+ and FISH+.
However, not all HER2-positive patients with GC
benefit from trastuzumab. The efficacy prediction of trastuzumab
has been investigated for years (15–19).
In 2013, Gomez-Martin et al (16) reported that HER2 amplification,
determined by the HER2/ centromeric probe for chromosome 17 (CEP17;
17p11.1-q11.1) ratio and HER2 copy number, demonstrated a
significant positive correlation with sensitivity to HER2-targeted
therapy and, therefore, could be considered a predictive factor of
trastuzumab efficacy. Recently, Ock et al (17) reported a similar result to
Gomez-Martin et al (16)
indicating that HER2 gene amplification, identified using
HER2/CEP17 ratio and HER2 copy number, may be a positive predictive
factor for treatment outcome of trastuzumab-based chemotherapy,
especially in patients with HER2 IHC <2+. However, another
recent study evaluated the genomic alteration of HER2 using three
different methods, including IHC, copy number variation and
Ampliseq sequencing, and reported that the concomitant genomic
alteration does not correlate with the treatment outcomes of
HER2-targeted chemotherapy (18).
In addition, Oyama et al (19) also tested the serum levels of
HER2-extracellular domain (ECD) in patients with GC by
chemiluminescent immunoassay and observed a correlation between
HER2-ECD level and tissue HER2 status. The study also reported that
alterations in the HER2-ECD level during chemotherapy positively
correlated with therapy response in HER2-positive patients treated
with trastuzumab-based chemotherapy. Accordingly, the researchers
concluded that the serum HER2-ECD level could be a potential
biomarker and monitoring marker for response to trastuzumab
(19).
Lapatinib is a dual EGFR and HER2 inhibitor, the
anticancer efficacy of which has been demonstrated in breast cancer
(20), but not in GC. The
Lapatinib Optimization Study in HER2 Positive GC (LOGiC) and
Lapatinib Plus Paclitaxel Versus Paclitaxel Alone in the
Second-Line Treatment of HER2-Amplified Advanced GC in Asian
Populations (TyTAN) phase III trials were designed to evaluate the
efficacy of lapatinib in patients with GC as first- and second-line
treatments, respectively (21,22).
Both trials failed to achieve the primary endpoint, although the
subgroup analysis of the LOGiC cohort showed a significant OS
benefit in Asian patients and patients <60 years of age.
Similarly, the efficacy of treatment with lapatinib + paclitaxel
improved in IHC3+ patients compared with IHC0/1+ and 2+ patients in
the TyTAN trial (21,22). Because of the disappointing results
of the aforementioned lapatinib clinical trials, a number of
studies have investigated the molecular mechanisms (23–26).
HER2-targeted therapy has served an important role
in GC drug treatments for a number of years (27–29),
however, there are challenges associated with both intrinsic and
acquired drug resistance. In 2014, Eto et al (24) analyzed the over-expression and
suppression of the microRNA-21 (miR-21)/phosphatase and tensin
homolog (PTEN) pathway in GC cell lines revealing that
over-expression of miR-21 led to decreased sensitivity of GC cells
to trastuzumab, while the suppression of miR-21 restored the
trastuzumab-resistance in GC cell lines. Recently, Zhang et
al (25) reported that PTEN
deficiency is a predictive factor for the early resistance to
HER2-targeted therapy, including trastuzumab and lapatinib. In
2014, Hong et al (26)
established a lapatinib-resistant OE19 subclone from HER2-positive
lapatinib-sensitive cell lines, in order to analyze the function of
lapatinib resistance. The authors identified a novel acquired
mutation, SrcE527K, that activated both the phosphatidylinositide
3-kinase (PI3K) and mitogen-activated protein kinase (MAPK)
pathways, and which may be a potential mechanism of lapatinib
resistance, however, these findings require validation in primary
tumor cells (26). Study by Hong
et al (26) reported that
the activation of the PI3K/protein kinase B (Akt) and MAPK pathways
may be involved in HER2-targeted drug resistance.
The mesenchymal-epithelial transition (MET) pathway
also serves a role in HER2-targeted drug resistance (30,31).
Kim et al (30) established
a lapatinib-resistant SNU216 cell line with an
epithelial-mesenchymal transition (EMT) phenotype. The researchers
analyzed EMT-associated extracellular molecules and genes and
identified testican-1, an extracellular molecule, the inhibition of
which decreased testican-1-induced, MET-dependent downstream
signaling and restored sensitivity to lapatinib (30). Therefore, this molecule may
contribute to lapatinib resistance. A recent multicenter clinical
trial conducted by De Silva et al (31) collected biopsy samples from
patients with oesophagogastric adenocarcinoma (OGA) treated with
neoadjuvant chemotherapy + lapatinib. The molecular analysis showed
a significant positive correlation between MET activation and
phosphor(p)-Erk level (P=0.0005) and p-PI3K/total-PI3K ratio
(P=0.0037), which indicated that MET contributed to lapatinib
resistance, whereas neither insulin-like growth factor receptor
(IGFR) nor receptor tyrosine-protein kinase erbB-3 were associated
with lapatinib resistance (31).
Based on the significant improvement in PFS in the
AVAGAST trial, many oncologists believe it is important to identify
patients who are sensitive to bevacizumab (38–41).
In 2012, Yamashita-Kashima et al (38) reported that tumor VEGF levels and
VEGF/basic fibroblast growth factor ratios were associated with
bevacizumab sensitivity in vitro. Subsequently, Van Cutsem
et al (39) hypothesized
that angiogenic markers may be potential predictors for bevacizumab
treatment outcome and conducted a study based on patients with AGC
in the AVAGAST trial. They evaluated several angiogenic markers and
found that plasma VEGF-A and neuropilin-1 were potential biomarkers
for the prediction of treatment outcome in patients with AGC
treated with chemotherapy + bevacizumab (39). In 2014, Han et al (40) analyzed bevacizumab pharmacokinetics
in patients with AGC in the AVAGAST. The median clearance of
bevacizumab was faster in patients with AGC compared with patients
with other cancers (4.5 vs. 3 ml/day/kg), however, the mechanism of
clearance remains unknown. The high clearance may contribute to the
adverse treatment outcomes of bevacizumab (40). Recently, Hacker et al
(41) evaluated angiopoietin-2
(Ang-2) expression in patients enrolled in the AVAGAS trial,
reporting baseline plasma Ang-2 levels to be a prognostic biomarker
for OS that was strongly positively associated with lymph node
metastasis in AGC but not for bevacizumab efficacy. Therefore,
although plasma VEGF-A and neuropilin-1 are potential biomarkers
for bevacizumab treatment outcome, they remain to be validated
(41).
Ramucirumab, a fully human monoclonal antibody
against VEGFR-2, is the first drug approved by US Food and Drug
Administration (FDA) for second-line treatment of AGC (42). A phase III clinical trial RAINBOW
showed improved OS in the ramucirumab + paclitaxel arm compared
with paclitaxel used as a single agent (43). Another phase III trial, REGARD,
reported that ramucirumab as a single drug improved OS compared
with that for the best supportive care [median OS: 5.2 months vs.
3.8 months, hazard ratio (HR) 0.776, 95% CI 0.603–0.998; P=0.047]
(44). However, the predictive
factors of treatment efficacy are lacking for ramucirumab (45).
Apatinib, a VEGFR-2 tyrosine kinase inhibitor, was
approved by Chinese Food and Drug Administration (CFDA) for
patients with AGC refractory to >2 lines of prior chemotherapy.
The phase III trial showed that OS and PFS time improved in the
apatinib group compared with the placebo group (46).
MET receptor, a tyrosine kinase receptor activated
by hepatocyte growth factor (HGF) or scatter factor (SF), results
in the activation of downstream pathways, including the PI3K/Akt
and RAS-MAPK pathways (47).
Rilotumumab, an investigational monoclonal antibody,
inhibits the MET signaling pathway (48). Although a phase II trial reported
that rilotumumab could improve PFS and OS in MET-positive GC/GEJ
patients (49), a phase III study,
RELOMET1, failed to confirm the OS or PFS benefit from rilotumumab
(50). Another phase III study
also failed to prove the OS benefit of MET inhibitor onartuzumab
administered in combination with mFolFox6 (fluorouracil,
oxaliplatin and leucovorin) in MET-positive patients with GC
(51). Foretinib, an inhibitor
targeting both receptor tyrosine kinase (RTK) encoded by MET
proto-oncogene (cMET) and VEGFR2, was effective in MET-positive
patients with AGC in a 2013 phase II study (52).
The frequency of MET inhibitor responsiveness is
low, the molecular mechanism of which has been analyzed. A 2015
study reported that short-form recepteur d'origine nantais (RON)
pathways conferred intrinsic cMET-targeted therapy resistance in
vivo and in vitro (53). Another recent study demonstrated
that co-amplification of driver oncogenes, including HER2 and MET,
contributed to the acquired resistance to MET inhibitors (54). Ji et al (55) reported that high PI3K p110 α
expression contributed to tyrosine kinase inhibitor resistance in
GC xenografts. Musiani et al (56) reported that the induction of
heat-shock protein 27 may limit the MET inhibitor efficacy in
MET-positive patients with GC.
Furthermore, MET has cross-interactions with HER2 to
modulate trastuzumab resistance (24), lapatinib resistance (57,58)
and HER2 expression (59). Several
in vivo and in vitro experiments (24–26)
have shown that drug resistance in HER2-targeted treatments may be
associated with the MET pathway, which has been explained in detail
in the anti-HER2 agents section of the present article. In 2014, a
review by Sukawa et al (58) reported that HER2 over-expression
was significantly positively correlated with p-Akt expression in GC
tissues. Furthermore, p-Akt expression correlated with poor
prognosis. These results suggest that the PI3K/Akt pathway serves a
role in HER2-positive GC. Furthermore, PIK3CA mutations and/or PTEN
inactivation may affect the effectiveness of HER2-targeted therapy
(58).
The above studies indicated that efficacy of MET
inhibitors has not been confirmed in phase III clinical trials and
conclusive evidence of predictive factors remains lacking. However,
the MET pathway could partially explain the resistance to HER2
inhibitors.
mTOR, involved in the PI3K/Akt pathway, is a
serine/threonine kinase that increases the production of proteins
that stimulate cell growth, proliferation, angiogenesis and cell
metabolism (60).
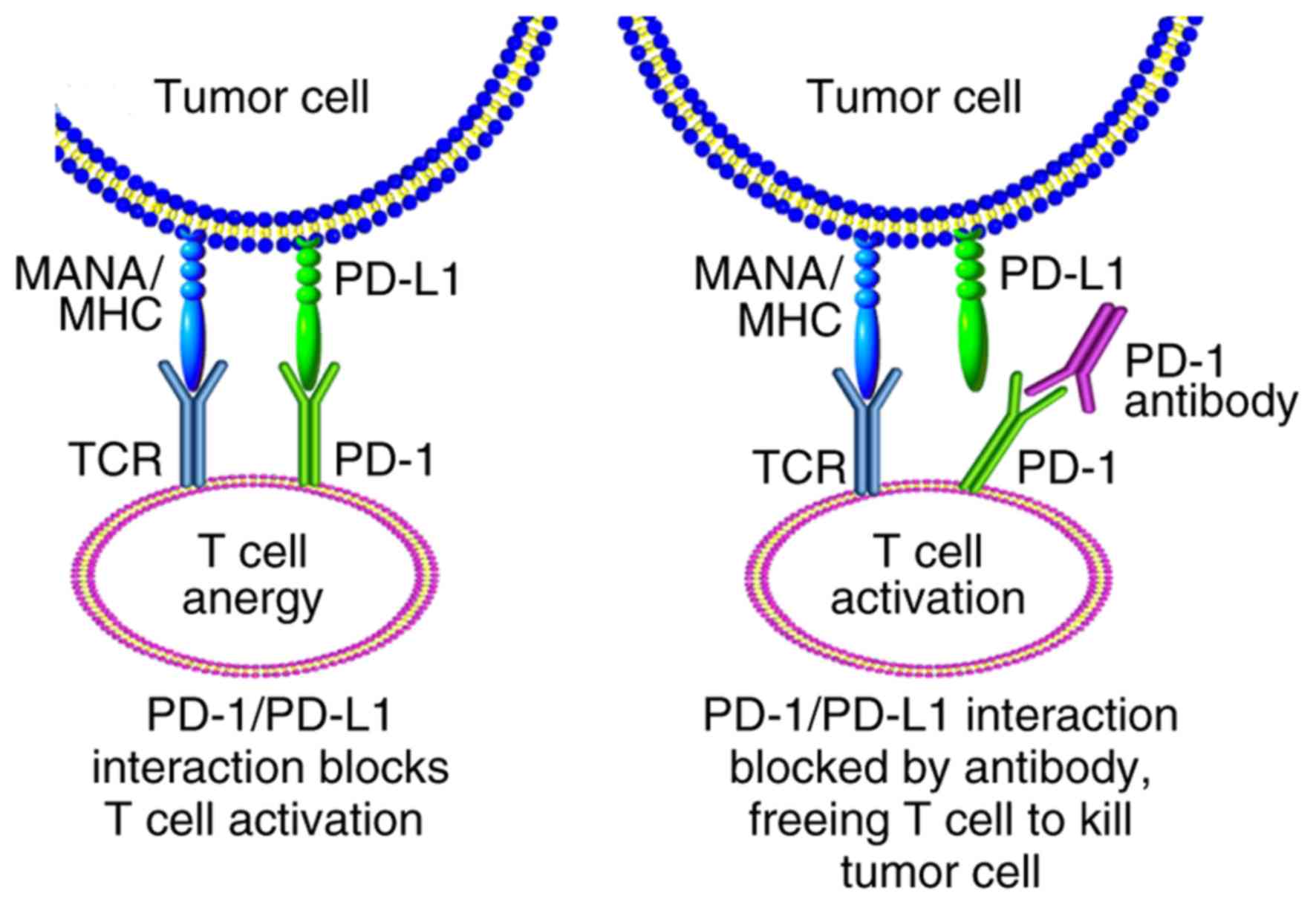
PD-1 is a negative costimulatory receptor expressed
primarily on the surface of activated T cells (67). The binding of PD-1 to one of its
ligands, PD-L1 or PD-L2, inhibits cytotoxic T-cell response
(68). Phase Ib trial Keynote-012
has reported manageable toxicity and promising antitumor activity
of pembrolizumab (pembro) in patients with AGC (69). Phase II Keynote-059 reported that
pembro exhibited encouraging efficacy and manageable safety in
patients with gastric/gastroesophageal junction cancer (G/GEJ) who
had received >2 prior lines of therapy, although the survival
and bio-marker data have yet to be published (70). According to Keynote-059, pembro has
been approved by FDA for the treatment of patients with G/GEJ who
had received >2 prior lines of therapy. Further phase III trials
(Keynote-061 and 062) (71,72)
have yet to be published. On December 14th, Merck & Co., Inc.
(Whitehouse Station, NJ, USA) officially announced the failure of
Keynote-061. The company announced that the clinical trial did not
achieve the primary endpoint of OS and the difference between PFS
was not statistically significant. The results of Keynote-061 and
062 have yet to be published. In addition to pembro, a phase III
trial for another PD1-targeted drug, nivolumab, is also in progress
and the result are not available.
Ipilimumab is an anti-CTLA-4 antibody, the
anti-tumor mechanism of which is based on upregulation of antitumor
immunity (73). A phase III
clinical trial CheckMate649 aimed to evaluate ipilimumab +
nivolumab (nivo) or nivo + chemotherapy vs. chemotherapy alone as
first-line treatment for advanced G/GEJ, of which the results have
not been reported (74).
The present article reviewed the efficacy predictors
of targeted agents in GC. A total of four drugs have been approved
by FDA and CFDA. Firstly, HER2 is a bio-marker for trastuzumab in
patients with GC and, therefore, HER2 analysis is recommended in
patients with GC using standard test methods (HER2-positive status
being IHC3+ or IHC2+ and FISH+) (5). HER2 amplification determined using
HER2/CEP17 (17p11.1-q11.1) ratio and HER2 copy number may be a
predictor in HER2-targeted chemotherapy, which requires
verification in further prospective studies (16,17).
In addition, the MET/PIK3CA/Akt pathway may be partially associated
with HER2 resistance (24–26,30,31).
Secondly, administration of ramucirumab, a VEGFR-2 antibody, is a
standard therapy for second-line treatment of AGC (43,44).
However, the predictive factors of treatment efficacy are lacking
for ramucirumab. Thirdly, apatinib, a VEGFR-2 tyrosine kinase
inhibitor, was approved by CFDA for the treatment of patients with
AGC refractory to ≥2 lines of prior chemotherapy (46). Fourthly, pembro, a PD-1 antibody,
has been approved by FDA in patients with G/GEJ who had received
>2 prior lines of therapy (70). Lastly, the results of phase III
studies suggest a modest and limited response to both cetuximab and
bevacizumab in patients with GC (10,36,37),
however, efficacy predictions are currently lacking.
Not applicable.
This study was funded by The National Key Research
and Development Program of China (grant no. 2017YFC1308900).
All data generated or analyzed during this study are
included in this published article.
FW performed the literature search and wrote the
article. ZX designed the review and drafted and revised the
article.
No applicable.
No applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ajani JA, Mayer RJ, Ota DM, Steele GD,
Evans D, Roh M, Sugarbaker DJ, Dumas P, Gray C, Vena DA, et al:
Preoperative and postoperative combination chemotherapy for
potentially resectable gastric carcinoma. J Natl Cancer Inst.
85:1839–1844. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leichman L, Silberman H, Leichman CG,
Spears CP, Ray M, Muggia FM, Kiyabu M, Radin R, Laine L, Stain S,
et al: Preoperative systemic chemotherapy followed by adjuvant
postoperative intraperitoneal therapy for gastric cancer: A
University of Southern California pilot program. J Clin Oncol.
10:1933–1942. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chau I, Norman AR, Cunningham D, Waters
JS, Oates J and Ross PJ: Multivariate prognostic factor analysis in
locally advanced and metastatic esophago-gastric cancer-pooled
analysis from three multicenter, randomized, controlled trials
using individual patient data. J Clin Oncol. 22:2395–2403. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Livshits Z, Rao RB and Smith SW: An
approach to chemotherapy-associated toxicity. Emerg Med Clin North
Am. 32:167–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mangia A, Caldarola L, Dell'Endice S,
Scarpi E, Saragoni L, Monti M, Santini D, Brunetti O, Simone G and
Silvestris N: The potential predictive role of nuclear NHERF1
expression in advanced gastric cancer patients treated with
epirubicin/oxaliplatin/capecitabine first line chemotherapy. Cancer
Biol Ther. 16:1140–1147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tebbutt NC, Parry MM, Zannino D,
Strickland AH, Van Hazel GA, Pavlakis N, Ganju V, Mellor D,
Dobrovic A and Gebski VJ: Australasian Gastro-Intestinal Trials
Group (AGITG): Docetaxel plus cetuximab as second-line treatment
for docetaxel-refractory oesophagogastric cancer: The AGITG ATTAX2
trial. Br J Cancer. 108:771–774. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang ZD, Kong Y, Yang W, Zhang B, Zhang
YL, Ma EM, Liu HX, Chen XB and Hua YW: Clinical evaluation of
cetuximab combined with an S-1 and oxaliplatin regimen for Chinese
patients with advanced gastric cancer. World J Surg Oncol.
12:1152014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lordick F, Kang YK, Chung HC, Salman P, Oh
SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, et
al: Capecitabine and cisplatin with or without cetuximab for
patients with previously untreated advanced gastric cancer
(EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol.
14:490–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Waddell T, Chau I, Cunningham D, Gonzalez
D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G,
Wadsley J, et al: Epirubicin, oxaliplatin, and capecitabine with or
without panitumumab for patients with previously untreated advanced
oesophagogastric cancer (REAL3): A randomised, open-label phase 3
trial. Lancet Oncol. 14:481–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hotz B, Keilholz U, Fusi A, Buhr HJ and
Hotz HG: In vitro and in vivo antitumor activity of cetuximab in
human gastric cancer cell lines in relation to epidermal growth
factor receptor (EGFR) expression and mutational phenotype. Gastric
Cancer. 15:252–264. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moehler M, Mueller A, Trarbach T, Lordick
F, Seufferlein T, Kubicka S, Geissler M, Schwarz S, Galle PR and
Kanzler S: German Arbeitsgemeinschaft Internistische Onkologie:
Cetuximab with irinotecan, folinic acid and 5-fluorouracil as
first-line treatment in advanced gastroesophageal cancer: A
prospective multi-center biomarker-oriented phase II study. Ann
Oncol. 22:1358–1366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi AH, Kim J and Chao J: Perioperative
chemotherapy for resectable gastric cancer: MAGIC and beyond. World
J Gastroenterol. 21:7343–7348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pietrantonio F, De Braud F, Da Prat V,
Perrone F, Pierotti MA, Gariboldi M, Fanetti G, Biondani P,
Pellegrinelli A, Bossi I and Di Bartolomeo M: A review on
biomarkers for prediction of treatment outcome in gastric cancer.
Anticancer Res. 33:1257–1266. 2013.PubMed/NCBI
|
|
16
|
Gomez-Martin C, Plaza JC, Pazo-Cid R,
Salud A, Pons F, Fonseca P, Leon A, Alsina M, Visa L, Rivera F, et
al: Level of HER2 gene amplification predicts response and overall
survival in HER2-positive advanced gastric cancer treated with
trastuzumab. J Clin Oncol. 31:4445–4452. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ock CY, Lee KW, Kim JW, Kim JS, Kim TY,
Lee KH, Han SW, Im SA, Kim TY, Kim WH, et al: Optimal patient
selection for trastuzumab treatment in HER2-positive advanced
gastric cancer. Clin Cancer Res. 21:2520–2529. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JY, Hong M, Kim ST, Park SH, Kang WK,
Kim KM and Lee J: The impact of concomitant genomic alterations on
treatment outcome for trastuzumab therapy in HER2-positive gastric
cancer. Sci Rep. 5:92892015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oyama K, Fushida S, Tsukada T, Kinoshita
J, Watanabe T, Shoji M, Nakanuma S, Okamoto K, Sakai S, Makino I,
et al: Evaluation of serum HER2-ECD levels in patients with gastric
cancer. J Gastroenterol. 50:41–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Azambuja E, Holmes AP, Piccart-Gebhart
M, Holmes E, Di Cosimo S, Swaby RF, Untch M, Jackisch C, Lang I,
Smith I, et al: Lapatinib with trastuzumab for HER2-positive early
breast cancer (NeoALTTO): Survival outcomes of a randomised,
open-label, multicentre, phase 3 trial and their association with
pathological complete response. Lancet Oncol. 15:1137–1146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hecht JR, Bang YJ, Qin SK, Chung HC, Xu
JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero A, et al:
Lapatinib in combination with capecitabine plus oxaliplatin in
human epidermal growth factor receptor 2-positive advanced or
metastatic gastric, esophageal, or gastroesophageal adenocarcinoma:
TRIO-013/LOGiC-A randomized phase III trial. J Clin Oncol.
34:443–451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Satoh T, Xu RH, Chung HC, Sun GP, Doi T,
Xu JM, Tsuji A, Omuro Y, Li J, Wang JW, et al: Lapatinib plus
paclitaxel versus paclitaxel alone in the second-line treatment of
HER2-amplified advanced gastric cancer in Asian populations:
TyTAN-a randomized, phase III study. J Clin Oncol. 32:2039–2049.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oshima Y, Tanaka H, Murakami H, Ito Y,
Furuya T, Kondo E, Kodera Y and Nakanishi H: Lapatinib
sensitivities of two novel trastuzumab-resistant HER2
gene-amplified gastric cancer cell lines. Gastric Cancer.
17:450–462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eto K, Iwatsuki M, Watanabe M, Ida S,
Ishimoto T, Iwagami S, Baba Y, Sakamoto Y, Miyamoto Y, Yoshida N
and Baba H: The microRNA-21/PTEN pathway regulates the sensitivity
of HER2-positive gastric cancer cells to trastuzumab. Ann Surg
Oncol. 21:343–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang X, Park JS, Park KH, Kim KH, Jung M,
Chung HC, Rha SY and Kim HS: PTEN deficiency as a predictive
biomarker of resistance to HER2-targeted therapy in advanced
gastric cancer. Oncology. 88:76–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hong YS, Kim J, Pectasides E, Fox C, Hong
SW, Ma Q, Wong GS, Peng S, Stachler MD, Thorner AR, et al: Src
mutation induces acquired lapatinib resistance in ERBB2-amplified
human gastroesophageal adenocarcinoma models. PLoS One.
9:e1094402014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sanford M: Trastuzumab: A review of its
use in HER2-positive advanced gastric cancer. Drugs. 73:1605–1615.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gomez-Martin C, Lopez-Rios F, Aparicio J,
Barriuso J, García-Carbonero R, Pazo R, Rivera F, Salgado M, Salud
A, Vázquez-Sequeiros E and Lordick F: A critical review of
HER2-positive gastric cancer evaluation and treatment: from
trastuzumab, and beyond. Cancer Lett. 351:30–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sanford M: Subcutaneous trastuzumab: A
review of its use in HER2-positive breast cancer. Target Oncol.
9:85–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim HP, Han SW, Song SH, Jeong EG, Lee MY,
Hwang D, Im SA, Bang YJ and Kim TY: Testican-1-mediated
epithelial-mesenchymal transition signaling confers acquired
resistance to lapatinib in HER2-positive gastric cancer. Oncogene.
33:3334–3341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
De Silva N, Schulz L, Paterson A, Qain W,
Secrier M, Godfrey E, Cheow H, O'Donovan M, Lao-Sirieix P,
Jobanputra M, et al: Molecular effects of Lapatinib in the
treatment of HER2 overexpressing oesophago-gastric adenocarcinoma.
Br J Cancer. 113:1305–1312. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abdel-Rahman O: Targeting vascular
endothelial growth factor (VEGF) pathway in gastric cancer:
Preclinical and clinical aspects. Crit Rev Oncol Hematol. 93:18–27.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Escudier B, Pluzanska A, Koralewski P,
Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar
B, Bajetta E, et al: Bevacizumab plus interferon alfa-2a for
treatment of metastatic renal cell carcinoma: A randomised,
double-blind phase III trial. Lancet. 370:2103–2111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qu CY, Zheng Y, Zhou M, Zhang Y, Shen F,
Cao J and Xu LM: Value of bevacizumab in treatment of colorectal
cancer: A meta-analysis. World J Gastroenterol. 21:5072–5080. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lauro S, Onesti CE, Righini R and
Marchetti P: The use of bevacizumab in non-small cell lung cancer:
An update. Anticancer Res. 34:1537–1545. 2014.PubMed/NCBI
|
|
36
|
Ohtsu A, Shah MA, Van Cutsem E, Rha SY,
Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, et al:
Bevacizumab in combination with chemotherapy as first-line therapy
in advanced gastric cancer: A randomized, double-blind,
placebo-controlled phase III study. J Clin Oncol. 29:3968–3976.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shen L, Li J, Xu J, Pan H, Dai G, Qin S,
Wang L, Wang J, Yang Z, Shu Y, et al: Bevacizumab plus capecitabine
and cisplatin in Chinese patients with inoperable locally advanced
or metastatic gastric or gastroesophageal junction cancer:
Randomized, double-blind, phase III study (AVATAR study). Gastric
Cancer. 18:168–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamashita-Kashima Y, Fujimoto-Ouchi K,
Yorozu K, Kurasawa M, Yanagisawa M, Yasuno H and Mori K: Biomarkers
for antitumor activity of bevacizumab in gastric cancer models. BMC
Cancer. 12:372012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Van Cutsem E, de Haas S, Kang YK, Ohtsu A,
Tebbutt NC, Xu Ming J, Yong Peng W, Langer B, Delmar P, Scherer SJ
and Shah MA: Bevacizumab in combination with chemotherapy as
first-line therapy in advanced gastric cancer: A biomarker
evaluation from the AVAGAST randomized phase III trial. J Clin
Oncol. 30:2119–2127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han K, Jin J, Maia M, Lowe J, Sersch MA
and Allison DE: Lower exposure and faster clearance of bevacizumab
in gastric cancer and the impact of patient variables: Analysis of
individual data from AVAGAST phase III trial. AAPS J. 16:1056–1063.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hacker UT, Escalona-Espinosa L, Consalvo
N, Goede V, Schiffmann L, Scherer SJ, Hedge P, Van Cutsem E,
Coutelle O and Büning H: Evaluation of Angiopoietin-2 as a
biomarker in gastric cancer: results from the randomised phase III
AVAGAST trial. Br J Cancer. 114:855–862. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Poole RM and Vaidya A: Ramucirumab: First
global approval. Drugs. 74:1047–1058. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fuchs CS, Tomasek J, Yong CJ, Dumitru F,
Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry
DR, et al: Ramucirumab monotherapy for previously treated advanced
gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An
international, randomised, multicentre, placebo-controlled, phase 3
trial. Lancet. 383:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Iacovelli R, Pietrantonio F, Farcomeni A,
Maggi C, Palazzo A, Ricchini F, de Braud F and Di Bartolomeo M:
Chemotherapy or targeted therapy as second-line treatment of
advanced gastric cancer. A systematic review and meta-analysis of
published studies. PLoS One. 9:e1089402014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y,
Liu W, Tong J, Liu Y, Xu R, et al: Randomized, double-blind,
placebo-controlled phase III trial of apatinib in patients with
chemotherapy-refractory advanced or metastatic adenocarcinoma of
the stomach or gastroesophageal junction. J Clin Oncol.
34:1448–1454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fioroni I, Dell'Aquila E, Pantano F,
Intagliata S, Caricato M, Vincenzi B, Coppola R, Santini D and
Tonini G: Role of c-mesenchymal-epithelial transition pathway in
gastric cancer. Expert Opin Pharmacother. 16:1195–1207. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang Y, Doshi S and Zhu M:
Pharmacokinetics and pharmacodynamics of rilotumumab: a decade of
experience in preclinical and clinical cancer research. Br J Clin
Pharmacol. 80:957–964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Iveson T, Donehower RC, Davidenko I,
Tjulandin S, Deptala A, Harrison M, Nirni S, Lakshmaiah K, Thomas
A, Jiang Y, et al: Rilotumumab in combination with epirubicin,
cisplatin, and capecitabine as first-line treatment for gastric or
oesophagogastric junction adenocarcinoma: An open-label, dose
de-escalation phase 1b study and a double-blind, randomised phase 2
study. Lancet Oncol. 15:1007–1018. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Catenacci DVT, Tebbutt NC, Davidenko I,
Murad AM, Al-Batran SE, Ilson DH, Tjulandin S, Gotovkin E,
Karaszewska B, Bondarenko I, et al: Rilotumumab plus epirubicin,
cisplatin, and capecitabine as first-line therapy in advanced
MET-positive gastric or gastro-oesophageal junction cancer
(RILOMET-1): A randomised, double-blind, placebo-controlled, phase
3 trial. Lancet Oncol. 18:1467–1482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shah MA, Bang YJ, Lordick F, Alsina M,
Chen M, Hack SP, Bruey JM, Smith D, McCaffery I, Shames DS, et al:
Effect of fluorouracil, leucovorin, and oxaliplatin with or without
onartuzumab in HER2-negative, MET-positive gastroesophageal
adenocarcinoma: The METGastric randomized clinical trial. JAMA
Oncol. 3:620–627. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shah MA, Wainberg ZA, Catenacci DV,
Hochster HS, Ford J, Kunz P, Lee FC, Kallender H, Cecchi F, Rabe
DC, et al: Phase II study evaluating 2 dosing schedules of oral
foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with
metastatic gastric cancer. PLoS One. 8:e540142013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu Z, Zhang Z, Ge X, Lin Y, Dai C, Chang
J, Liu X, Geng R, Wang C, Chen H, et al: Identification of
short-form RON as a novel intrinsic resistance mechanism for
anti-MET therapy in MET-positive gastric cancer. Oncotarget.
6:40519–40534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kwak EL, Ahronian LG, Siravegna G,
Mussolin B, Godfrey JT, Clark JW, Blaszkowsky LS, Ryan DP, Lennerz
JK, Iafrate AJ, et al: Molecular heterogeneity and receptor
coamplification drive resistance to targeted therapy in
MET-amplified esophagogastric cancer. Cancer Discov. 5:1271–1281.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ji F, Liu X, Wu Y, Fang X and Huang G:
Overexpression of PI3K p110α contributes to acquired resistance to
MET inhibitor, in MET-amplified SNU-5 gastric xenografts. Drug Des
Devel Ther. 9:5697–5704. 2015.PubMed/NCBI
|
|
56
|
Musiani D, Konda JD, Pavan S, Torchiaro E,
Sassi F, Noghero A, Erriquez J, Perera T, Olivero M and Di Renzo
MF: Heat-shock protein 27 (HSP27, HSPB1) is up-regulated by MET
kinase inhibitors and confers resistance to MET-targeted therapy.
FASEB J. 28:4055–4067. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen CT, Kim H, Liska D, Gao S,
Christensen JG and Weiser MR: MET activation mediates resistance to
lapatinib inhibition of HER2-amplified gastric cancer cells. Mol
Cancer Ther. 11:660–669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sukawa Y, Yamamoto H, Nosho K, Ito M,
Igarashi H, Naito T, Mitsuhashi K, Matsunaga Y, Takahashi T, Mikami
M, et al: HER2 expression and PI3K-Akt pathway alterations in
gastric cancer. Digestion. 89:12–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
An X, Wang F, Shao Q, Wang FH, Wang ZQ,
Wang ZQ, Chen C, Li C, Luo HY, Zhang DS, et al: MET amplification
is not rare and predicts unfavorable clinical outcomes in patients
with recurrent/metastatic gastric cancer after chemotherapy.
Cancer. 120:675–682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xu K, Liu P and Wei W: mTOR signaling in
tumorigenesis. Biochim Biophys Acta. 1846:638–654. 2014.PubMed/NCBI
|
|
61
|
Hasskarl J: Everolimus. Recent Results
Cancer Res. 201:373–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung
HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, et al: Everolimus
for previously treated advanced gastric cancer: results of the
randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol.
31:3935–3943. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wainberg ZA, Soares HP, Patel R, DiCarlo
B, Park DJ, Liem A, Wang HJ, Yonemoto L, Martinez D, Laux I, et al:
Phase II trial of everolimus in patients with refractory metastatic
adenocarcinoma of the esophagus, gastroesophageal junction and
stomach: Possible role for predictive biomarkers. Cancer Chemother
Pharmacol. 76:61–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhu Y, Tian T, Zou J, Wang Q, Li Z, Li Y,
Liu X, Dong B, Li N, Gao J and Shen L: Dual PI3K/mTOR inhibitor
BEZ235 exerts extensive antitumor activity in HER2-positive gastric
cancer. BMC Cancer. 15:8942015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fuereder T, Jaeger-Lansky A, Hoeflmayer D,
Preusser M, Strommer S, Cejka D, Koehrer S, Crevenna R and Wacheck
V: mTOR inhibition by everolimus counteracts VEGF induction by
sunitinib and improves anti-tumor activity against gastric cancer
in vivo. Cancer Lett. 296:249–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jaeger-Lansky A, Cejka D, Ying L, Preusser
M, Hoeflmayer D, Fuereder T, Koehrer S and Wacheck V: Effects of
vatalanib on tumor growth can be potentiated by mTOR blockade in
vivo. Cancer Biol Ther. 9:919–927. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Muro K, Chung HC, Shankaran V, Geva R,
Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al:
Pembrolizumab for patients with PD-L1-positive advanced gastric
cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial.
Lancet Oncol. 17:717–726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T,
Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al: Safety and
efficacy of pembrolizumab monotherapy in patients with previously
treated advanced gastric and gastroesophageal junction cancer:
Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. Mar 15–2018.(Epub
ahead of print). View Article : Google Scholar :
|
|
71
|
Ohtsu A, Tabernero J, Bang YJ, Fuchs CS,
Sun L, Wang Z, Csiki I, Koshiji and Cutsem EV: Pembrolizumab versus
paclitaxel as second-line therapy for advanced gastric or
gastroesophageal junction (GEJ) adenocarcinoma: Phase 3 KEYNOTE-061
study. J Clin Oncol. 34 15 Suppl:TPS41372016. View Article : Google Scholar
|
|
72
|
Tabernero J, Bang YJ, Fuchs CS, Ohtsu A,
Kher U, Lam B, Koshiji M and Cutsem V: KEYNOTE-062: Phase III study
of pembrolizumab alone or in combination with chemotherapy versus
chemotherapy alone as first-line therapy for advanced gastric or
gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol. 34 4
Suppl:TPS1852016. View Article : Google Scholar
|
|
73
|
Camacho LH: CTLA-4 blockade with
ipilimumab: Biology, safety, efficacy, and future considerations.
Cancer Med. 4:661–672. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Moehler MH, Janjigian YY, Adenis A, Aucoin
JS, Boku N, Chau I, Cleary JM, Feeney KT, Franke FA, Mendez GA, et
al: CheckMate 649: A randomized, multicenter, open-label, phase 3
study of nivolumab (nivo) + ipilimumab (ipi) or nivo + chemotherapy
(CTX) vs CTX alone in pts with previously untreated advanced (adv)
gastric (G) or gastroesophageal junction (GEJ) cancer. J Clin
Oncol. 35 15 Suppl:TPS41322017.
|