Introduction
Gastric cancer (GC) ranks fifth among all cancer
types and is the third primary cause of cancer-associated mortality
worldwide (1). Despite
improvements in surgical and adjuvant therapies, the 5-year overall
survival rate for patients with GC remains low; a poor prognosis is
closely associated with the high metastasis and recurrence rate of
GC. Furthermore, due to the development of drug resistance, as well
as other factors, the benefits of chemotherapy and radiotherapy are
limited (2,3). Therefore, there is a requirement for
the development of novel treatment methods for GC.
It was previously demonstrated that 25–48% of the
currently available Food and Drug Administration-approved medical
treatments are derived from plants (4–6), and
a number of these plant-derived compounds exhibit anticancer
properties. Rottlerin, also referred to as mallotoxin, is a
polyphenolic compound derived from the kamala tree (Mallotus
philipensis) (7). Initial
studies demonstrated that rottlerin is a protein kinase C δ (PKCδ)
inhibitor (8–10). However, an increasing amount of
evidence indicates that rottlerin may not only act as a specific
PKCδ inhibitor, but that it has multiple molecular targets and
anticancer properties, including the inhibition of cell
proliferation, promotion of apoptosis and inhibition of cell
migration and invasion (11–13).
However, the exact molecular mechanisms underlying the actions and
anticancer effects of rottlerin remain to be elucidated.
In eukaryotic cells, autophagy, which causes
organelle and protein degradation, constitutes an adaptive response
under conditions of starvation or cellular stress (14). It is necessary to recycle the
unnecessary or dysfunctional organelles and the misfolded or
damaged proteins through basal autophagy in order to maintain
genomic integrity and cellular homeostasis (15). The activation of autophagy is
implicated in a variety of stress conditions, whereas its
dysregulation is implicated in numerous pathophysiological
processes, including cardiovascular diseases and cancer (16). In cancer, autophagy may serve two
opposing roles. Specifically, autophagy may act as a prosurvival
mechanism when tumor cells are subjected to injury by chemical or
physical treatment, but it may also promote cancer cell death,
acting as a tumor suppressor (17). A previous study demonstrated that
autophagy is constitutively activated in cancer in response to
treatment and may be involved in therapy-triggered apoptosis.
Therefore, it is necessary to investigate the exact underlying
mechanisms of autophagy in cancer (18).
Moretti et al (19) demonstrated that autophagy improved
the effectiveness of anticancer therapy. Previous studies have
demonstrated that rottlerin may promote apoptosis and autophagy in
several types of cancer (20–22),
but it remains unknown whether it exerts an anticancer effect on
GC. To the best of our knowledge, the present study is the first to
report antitumor effects of rottlerin on human GC cell lines.
Furthermore, the molecular mechanism underlying the antitumor
activity of rottlerin through activation of autophagy in GC cells
was investigated, and the results indicate that rottlerin-induced
autophagy may promote anticancer activity through cancer cell
apoptosis.
Materials and methods
Cell culture and reagents
The SGC-7901 and MGC-803 human GC cell lines were
purchased from the Type Culture Collection of the Chinese Academy
of Sciences, Shanghai, China. The cells were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.)
and 1% streptomycin and penicillin in a humidified incubator at 5%
CO2 and 37°C. Rabbit primary antibodies against S-phase
kinase-associated protein 2 (Skp2; cat. no. ab183039), mechanistic
target of rapamycin kinase (mTOR; cat. no. ab32028),
microtubule-associated protein 1 light chain 3β (LC3)-II (cat. no.
ab51520), caspase-3 (cat. no. ab13847), cleaved-caspase-3 (cat. no.
ab2302), poly(ADP ribose) polymerase (PARP; cat. no. ab32138) and
cleaved-PARP (cat. no. ab32064) were purchased from Abcam
(Cambridge, UK). Rabbit primary antibodies against β-actin (cat.
no. 4970) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Secondary antibody (goat anti-rabbit; cat. no.
sc2004) was obtained from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). Rottlerin and dimethyl sulfoxide (DMSO) were acquired
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Rottlerin was
dissolved in DMSO to generate a 10 mM stock solution. Cells
cultured with only 0.1% DMSO served as the control group.
Cell proliferation assay
Cell proliferation was measured with a Cell Counting
Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). SGC-7901 and MGC-803 cells were seeded in 96-well
plates at a density of 2,000 cells/well and incubated in a humid
environment at 5% CO2 and 37°C for 4 h. Subsequently,
the cells were exposed to 0, 1, 2, 4, 8 and 16 µM rottlerin for 12,
24, 48 and 72 h. CCK-8 reagent was then added and incubated for 30
min at 37°C. Absorbance of the colored formazan product, formed by
mitochondrial dehydrogenases, was measured at a wavelength of 450
nm.
Colony formation assay
SGC-7901 and MGC-803 cells were cultured in a 6-well
plate at a density of 500 cells/well with 0, 2, 4 and 8 µM
rottlerin at 37°C for 2 weeks. Cells treated with rottlerin-free
medium served as the control group. After 2 weeks, the cells were
fixed in 4% methanol for 15 min at room temperature. Cells were
then stained with 0.1% crystal violet for 5 min at room temperature
and imaged using a light microscope (Olympus Corporation, Tokyo,
Japan) at ×40 magnification.
Cell cycle assay
SGC-7901 and MGC-803 cells were seeded at a density
of 1×106/ml, and then harvested following treatment with
0, 2 4 and 8 µM rottlerin at 37°C for 24 h. The cells were fixed in
70% ethanol at 4°C overnight. The fixed cells were centrifuged at
1,000 × g for 15 min at room temperature and washed with cold PBS
three times. The cells were incubated with 50 µg/ml RNase A at 37°C
for 30 min. Then cells were incubated with 100 µg/ml propidium
iodide (PI) in the dark at 4°C for 30 min. The DNA content was
quantified by FCM (BD CellQuest Pro; BD Biosciences, Franklin
Lakes, NJ, USA). The percentages of cells in the
G0-G1, S and G2-M phases were
compared with the control group.
Apoptosis assay
SGC-7901 and MGC-803 cells were cultured at
1×106/ml in 6-well plates following treatment with 0, 2
4 and 8 µM rottlerin at 37°C for 24 h. Cells were collected, washed
twice with cold PBS and resuspended in 100 µl binding buffer
containing 5 µl fluorescein isothiocyanate-conjugated anti-Annexin
V antibody and 5 µl PI using a FITC-Annexin V Apoptosis Detection
kit (BD Biosciences). Apoptosis was assessed using a FACS Calibur
flow cytometer (BD CellQuest Pro; BD Biosciences). The percentages
of apoptotic cells were compared with the control group.
Cell migration and invasion
assays
SGC-7901 and MGC-803 cells were cultured at
1×106/ml in 6-well plates. Migration was assessed using
a wound healing assay that was performed following treatment with
0, 2 4 and 8 µM rottlerin at 37°C for 0 and 24 h. A scratch was
created in a culture plate using the tip of a pipette (Thermo
Fisher Scientific, Inc.). Cells were incubated at 37°C and images
were captured after 0 and 24 h. For the cell invasion assay, GC
cells were incubated with 0, 2, 4 and 8 µM rottlerin at 37°C for 24
h and then harvested. 5×104 cells were added to the
upper chambers of a Transwell assay plate, which were coated with
Matrigel, containing 200 µl serum-free medium. The lower chambers
contained complete medium with 0, 2, 4 and 8 µM rottlerin. After 48
h, the cells in the lower chamber were fixed in cold methanol,
stained with 0.1% crystal violet and images were captured.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
SGC-7901 and MGC-803 cells were incubated with 0, 2,
4 and 8 µM rottlerin at 37°C for 24 h. Total RNA was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. cDNA was synthesized
using a PrimeScript™ RT Reagent kit (Takara Biotechnology Co.,
Ltd., Dalian, China) at 37°C for 15 min, followed by 85°C for 5
sec, determined at 4°C and qPCR was conducted using a
SYBR® Premix Ex Taq™ kit (Takara Biotechnology Co.,
Ltd.) on a Bio-Rad iQ5 Real-Time PCR system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The following thermocycling conditions
were used for the PCR: Initial denaturation at 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The
primer sequences for amplification were as follows: mTOR,
5′-AGGCCGCATTGTCTCTATCAA-3′ (forward) and
5′-GCAGTAAATGCAGGTAGTCATCCA-3′ (reverse); Skp2,
5′-GCTGCTAAAGGTCTCTGGGT-3′ (forward) and 5′-AGGCTTAGATTCTGCACTTG-3′
(reverse); and GAPDH, 5′-ACCCAGAAGACTGTGGATGG-3′ (forward) and
5′-CAGTGAGCTTCCCGTTCAG-3′ (reverse). The relative expression was
calculated using the 2−ΔΔCq method, with GAPDH used as
the internal control (23).
Autophagy assay
SGC-7901 and MGC-803 cells were seeded in 96-well
plates at a density of 2,000 cells/well in rottlerin-containing
medium (0, 2, 4 and 8 µM rottlerin) at 37°C for 24 h, followed by
incubation with the Autophagosome Detection kit (Sigma-Aldrich;
Merck KGaA). Medium was removed from the cells and 100 µl of the
autophagosome detection reagent working solution was added to each
well and cells were incubated at 37°C with 5% CO2 for 30
min. Subsequently cells were washed with Wash Buffer 3 times by
gently adding 100 µl of Wash Buffer to each well and cells were
immediately imaged using a fluorescence microscope (Olympus
Corporation, Tokyo, Japan). Autophagy was visualized as the bright
blue staining of autophagic vacuoles.
Western blot analysis
Following treatment with 0, 2, 4 and 8 µM rottlerin
at 37°C for 24 h, radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China) was added for
the extraction of proteins from GC cells for 40 min on ice. The
supernatants incubated in 4°C for 30 min and centrifuged at 13,000
× g at 4°C for 15 min, the protein concentrations were measured by
Enhanced BCA Protein Assay kit (Beyotime Institute of
Biotechnology, Shanghai, China). Protein (20 µg) was loaded and
separated via 12.5% SDS-PAGE, with a volume of 20 µl per well. The
proteins were transferred onto 0.22-µm polyvinylidene fluoride
membranes. The membranes were subsequently incubated with primary
antibodies at a dilution of 1:1,000 at 4°C overnight, followed by
incubation with secondary antibodies diluted in TBS-Tween-20 (0.1%
Tween-20) at room temperature for 1.5 h. Enhanced chemiluminescence
reagent (Pierce; Thermo Fisher Scientific, Inc.) was used to
visualize the bound antibodies using the ChemiDoc imaging system
(Abcam). Protein band intensities were semi-quantitated with ImageJ
software (version 1.46r; National Institutes of Health, Bethesda,
MD, USA) and normalized to β-actin.
Statistical analysis
Data are presented as the mean ± standard error.
Each experiment was performed three times independently. Data
analysis was performed by ANOVA using GraphPad Prism 5 software
(GraphPad Software, Inc., La Jolla, CA, USA). Bonferroni's test was
used as a post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Rottlerin suppresses the proliferation
of GC cells in a time- and dose-dependent manner
To investigate whether rottlerin suppressed the
proliferation of GC cells, a CCK-8 assay was performed using
SGC-7901 and MGC-803 cells following rottlerin treatment. The
results demonstrated that rottlerin inhibited GC cell proliferation
in a dose-dependent manner (Fig. 1A
and B). Relative cell viability is presented in Fig. 1C. Rottlerin at 1 µM exerted no
obvious suppressive effect on GC cells, 16 µM rottlerin caused cell
death, and 2, 4 and 8 µM rottlerin was used as the experimental
group.
Rottlerin on clonogenic capacity in GC
cells
The colony formation assay revealed that rottlerin
suppressed the clonogenic capacity of GC cells compared with the
control group (Fig. 1D).
Rottlerin promotes apoptosis and cell
cycle arrest in GC cells
It was previously reported that rottlerin promoted
cell cycle arrest and apoptosis in breast cancer cells (11). To further elucidate the effects of
rottlerin on cell cycle distribution and apoptosis in GC cells, the
percentage of GC cells in each cell cycle phase following rottlerin
treatment was determined through PI staining and flow cytometry. As
presented in Fig. 2A and B,
rottlerin led to evident G0/G1 phase arrest
in GC cells. Rottlerin at 2, 4 and 8 µM led to a G1 cell
population increase from 45.13±2.21% to 53.43±1.81, 60.45±3.07 and
66.79±1.94%, respectively, in SGC-7901 cells. Similarly, 2, 4 and 8
µM rottlerin caused a G1 cell population increase from
42.21±1.86% to 50.41±1.87, 56.42±2.38 and 65.82±2.22%,
respectively, in MGC-803 cells. Furthermore, flow cytometry
following annexin V-FITC and PI staining revealed that, following
treatment with rottlerin at 2, 4 and 8 µM, the apoptosis rate of
MGC-803 cells were increased from 2.68±1.00% to 8.57±0.80,
12.27±1.06 and 15.19±0.92%, the SGC-7901 cell apoptosis rate from
3.20±1.60% to 16.01±1.15, 23.83±1.67 and 29.99±1.64%, respectively
(Fig. 2C and D). Taken together,
these results indicate that rottlerin promoted cell cycle arrest
and apoptosis in GC cells.
Rottlerin inhibits the migration and
invasion of GC cells
To determine the effects of rottlerin on the
migratory activity of GC cells, a wound-healing assay was
conducted. The results demonstrated that rottlerin inhibited
migration in both types of GC cells in a dose-dependent manner
(Fig. 3A and B). To confirm the
effect of rottlerin on the invasion ability of GC cells, a
Transwell assay was conducted using Matrigel (Fig. 3C and D). Rottlerin reduced the
number of GC cells invading through the Matrigel, compared with the
control group (Fig. 3C and D).
These results indicate that rottlerin inhibited the migration and
invasion ability of GC cells.
Effect of rottlerin on cell
morphology
GC cells treated with 2, 4 and 8 µM rottlerin were
examined under a light microscope (Fig. 4A). Cells treated with rottlerin
were fewer compared with the control group, as well as smaller and
more spindle-shaped, with less cytoplasm. Membrane ruffling was
reduced, with a contractive cell appearance. Therefore, the
morphology of GC cells treated with rottlerin was clearly affected.
The observations were consistent with those in rat C6 glioblastoma
cells, as reported by Parmer et al (24).
Rottlerin induces autophagy in GC
cells
GC cells treated with 2, 4 and 8 µM rottlerin were
used to investigate the association between rottlerin and
autophagy. LC3-II expression levels were detected by western
blotting and fluorescence microscopy was used to evaluate
autophagosome formation. As demonstrated by examination with a
fluorescence microscope, the number of autophagosomes was increased
in the rottlerin-treated GC cells compared with the control group,
as demonstrated by increased light blue fluorescence, whereas the
light green cells were those without autophagosomes. (Fig. 4B). During autophagosome formation,
the microtubule-associated LC3-I is converted to the membrane-bound
form LC3-II (25). The LC3-II
expression level was increased in GC cells following treatment with
rottlerin and the quantitative results revealed significant
differences compared with the control cells (Fig. 4C and D). Therefore, the results
indicated that rottlerin may promote autophagy in GC cells.
Rottlerin causes apoptotic cell death
independently of caspase
Rottlerin-induced apoptosis has been reported in
several cancer cell lines (11,26).
The caspase cascade functions in apoptosis induction and completion
(27,28). Western blotting was conducted to
evaluate caspase-3 and PARP expression, which exhibited no obvious
increase in GC cells following treatment with rottlerin (Fig. 5). Cleaved-caspase-3 and PARP were
also evaluated, and no cleaved-caspase-3 expression was detected in
GC cells treated with rottlerin (Fig.
5). Cleaved-PARP was detected in GC cells, but the difference
between rottlerin-treated GC cells and control cells was not
statistically significant (Fig.
5). Similar observations were reported by Torricelli et
al (29). Therefore, rottlerin
may promote apoptosis independently of caspase in GC cells.
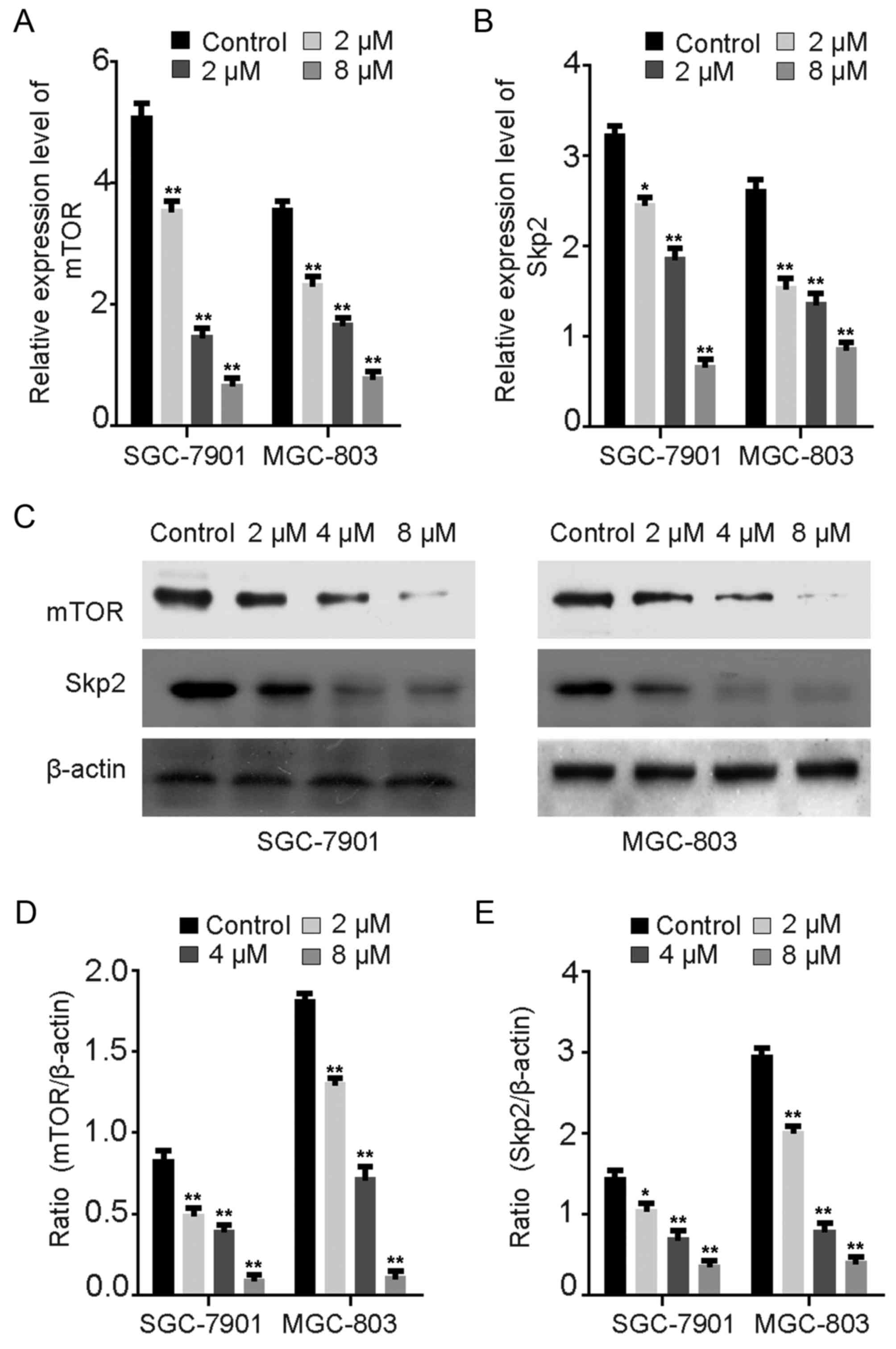
Rottlerin downregulates Skp2 and mTOR
protein expression
Several studies have reported that rottlerin
inhibits Skp2 protein expression in pancreatic and breast cancer
(11,13). Wu et al (30) observed that Skp2 promoted autophagy
through inhibition of mTOR complex 1 (mTORC1). The expression
levels of mTOR and Skp2 were evaluated via RT-qPCR (Fig. 6A and B) and western blot assays
(Fig. 6C-E) in GC cells treated
with rottlerin. The mRNA levels of mTOR and Skp2 were downregulated
following treatment with rottlerin for 24 h (Fig. 6A and B). The protein levels of mTOR
and Skp2 following rottlerin treatment were consistent with the
results for mRNA expression levels in GC cells (Fig. 6C-E). Consistent with previous
studies, these results demonstrate that rottlerin may reduce the
expression of Skp2 via the inhibition of mTOR expression in order
to promote autophagy in GC cells (11,13).
Discussion
Due to the development of chemotherapy resistance,
numerous phytochemical compounds derived from edible plants and
their synthetic derivatives have attracted attention due to their
unique anticancer properties and have been recommended for cancer
therapy (31–33). However, information focusing on the
anticancer properties of phytochemical compounds is limited;
therefore, it is necessary to elucidate the mechanisms underlying
their antitumor effects (34). The
anticancer effects of phytochemical compounds are widely
recognized, and phytochemicals may enhance the effectiveness of
chemotherapy and decrease the toxic side effects (35,36).
Rottlerin is a traditional Indian medicinal plant that was first
identified as a PKCδ-selective inhibitor by Gschwendt et al
(7) in 1994 and has been largely
overlooked, despite its potential involvement in multiple signaling
pathways. Further research demonstrated that rottlerin may inhibit
several pathways in a PKCδ-independent manner. Basu et al
(37) demonstrated that rottlerin
was able to induce the apoptosis of HeLa cells through
downregulation of caspase-2 via a PKCδ-independent pathway. Lin
et al (12) reported that
rottlerin inhibited follicular thyroid carcinoma cell migration
independent of PKCδ. Yin et al (11) reported that rottlerin exerted
anticancer effects partially through Skp2 inactivation in breast
cancer cells, while Su et al (13) demonstrated that rottlerin inhibited
the proliferation and invasion of pancreatic cancer cells via the
inactivation of Skp2. In addition, Zhao et al (38) demonstrated that rottlerin
suppressed non-small-cell lung cancer through the downregulation of
transcriptional co-activator with PDZ-binding motif. Similarly, the
results of the current study revealed that rottlerin suppressed
cancer cell proliferation, promoted apoptosis, and inhibited
migration and invasion.
Apoptosis, a process that is crucial for the
maintenance of normal tissue homeostasis, usually occurs as a
consequence of either extracellular stimuli (extrinsic pathway) or
intracellular stimuli (intrinsic or mitochondrial pathway)
(39,40) and is closely associated with the
design of chemotherapeutics. Shukla et al (41) observed that apigenin promoted the
interaction between Ku70 and Bcl-2-associated X, and induced
prostate cancer cell apoptosis via apoptosis protein suppression.
Caspases are involved in cell proliferation, migration,
cytoskeletal organization and immunological effects (42,43).
Disorders of the activity of caspases have been associated with
multiple pathological processes, including cancer (44). Rottlerin may induce apoptosis
through suppression of phosphoinositide 3-kinase (PI3K)/Akt/mTOR
signaling or caspase cascade activation (45). The present study revealed no
significant alterations in caspase-3, PARP, cleaved-caspase-3 and
cleaved-PARP protein expression, indicating that rottlerin may
promote apoptosis in GC cells through a caspase-independent
pathway. Furthermore, the present results demonstrated that the
percentage of SGC-7901 and MGC-803 cells in the G1 phase
increased as the concentration of rottlerin increased, but there
was no significant difference between the control and any rottlerin
groups for S and G2 phases. It suggested that rottlerin
may have caused cell cycle arrest in the G1 phase. And
rottlerin may have enhanced the G1/S checkpoint
activities and weakened the G2/M checkpoint activities,
causing more SGC-7901 and MGC-803 cells to remain in G1
phase, and thus performing a function of preventing cell
proliferation. But this needs to be verified with further
experiments in future studies.
Autophagy, which involves the degradation of
superfluous or damaged organelles and proteins, is an
evolutionarily conserved lysosomal degradation process (46,47).
Macroautophagy, chaperone-mediated autophagy and microautophagy,
the major identified types of autophagy, vary in terms of delivery
method of the cargo to the lysosome and physiological function
(48). Autophagy is closely
associated with cell survival, maintenance and various pathological
processes, among which cancer has received the most attention
(49–52). However, the role of autophagy in
tumors remains controversial (53). Song et al (21) demonstrated that rottlerin induced
autophagy and apoptotic cell death in human fibrosarcoma cells in a
PKCδ-independent manner. In the present study, western blotting and
autophagosome staining revealed that rottlerin promoted autophagy
in a dose-dependent manner, indicating the potential anticancer
function of rottlerin in GC.
The connection between apoptosis and autophagy has
not been fully elucidated, however, a growing body of evidence
indicates the presence of a molecular crosstalk between these two
pathways (54,55). Our results demonstrated that
rottlerin increased cell apoptosis and promoted autophagy, but
future investigations should focus on the interplay of apoptosis
and autophagy caused by rottlerin in GC. Several studies have
demonstrated that rottlerin may inhibit cancer cell proliferation
and progression via downregulation of the Skp2 protein (11,13).
Wu et al (30) observed
that Skp2 promoted autophagy through inhibition of mTORC1.
Similarly, the present study identified Skp2 downregulation in GC
cells following treatment with rottlerin. Kumar et al
(20) demonstrated that rottlerin
promoted apoptosis and autophagy in prostate cancer via
PI3K/Akt/mTOR signaling. A similar result was reported by Singh
et al (45) in pancreatic
cancer. The present study demonstrated that the mRNA and protein
levels of mTOR and Skp2 were both decreased following rottlerin
treatment. It is not clear whether there is a connection between
them, and this should be further investigated in future research.
Future investigations should focus on upstream and downstream
effectors of PI3K/Akt/mTOR signaling and the Skp2 protein, in order
to further elucidate the specific mechanism of action underlying
the role of rottlerin in GC, alone or in combination with
chemotherapeutic drugs, and to investigate the crosstalk between
autophagy and apoptosis signaling pathways, as well as apoptosis
pathways other than the caspase cascade. Furthermore, in
vivo experiments are required to verify these results of
rottlerin treatment observed in vitro.
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate that rottlerin induces
apoptosis and autophagy in GC and, therefore, that treatment with
rottlerin may be an effective approach to GC treatment.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Changzhou Health
and Family Planning Commission Project (grant nos. ZD201606 and
QN201711) and the Nanjing Medical University School Fund (grant
nos. 2016NJMUZD081 and 2017NJMU043).
Availability of data and materials
All data generated and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JS and LT designed the study and wrote the
manuscript. JS, YZ, YG and HL carried out the experiments and
performed the statistical analysis. JS and YZ made contributions in
modification the manuscript. All the authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GC
|
gastric cancer
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
mTOR
|
mechanistic target of rapamycin
kinase
|
|
Skp2
|
S-phase kinase-associated protein
2
|
|
PKCδ
|
protein kinase C δ
|
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeng YJ, Zhang CD and Dai DQ: Impact of
lymph node micrometastasis on gastric carcinoma prognosis: A
meta-analysis. World J Gastroenterol. 21:1628–1635. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deng JY and Liang H: Clinical significance
of lymph node metastasis in gastric cancer. World J Gastroenterol.
20:3967–3975. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Russo GL: Ins and outs of dietary
phytochemicals in cancer chemoprevention. Biochem Pharmacol.
74:533–544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Orlikova B and Diederich M: Power from the
garden: Plant compounds as inhibitors of the hallmarks of cancer.
Curr Med Chem. 19:2061–2087. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji S, Orlikova B and Diederich M:
Non-edible plants as an attractive source of compounds with
chemopreventive potential. J Cancer Prev. 19:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gschwendt M, Müller HJ, Kielbassa K, Zang
R, Kittstein W, Rincke G and Marks F: Rottlerin, a novel protein
kinase inhibitor. Biochem Biophys Res Commun. 199:93–98. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maioli E, Torricelli C and Valacchi G:
Rottlerin and cancer: Novel evidence and mechanisms.
ScientificWorldJournal. 2012:3508262012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nguyen BT, Park M, Pyun JC, Yoo YS and
Kang MJ: Efficient PKC inhibitor screening achieved using a
quantitative CE-LIF assay. Electrophoresis. 37:3146–3153. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Tan C, Wang G, Cai JJ, Wang LP,
Imperato-McGinley J and Zhu YS: Dual action of NSC606985 on cell
growth and apoptosis mediated through PKCδ in prostatic cancer
cells. Int J Oncol. 51:1601–1610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin X, Zhang Y, Su J, Hou Y, Wang L, Ye X,
Zhao Z, Zhou X, Li Y and Wang Z: Rottlerin exerts its anti-tumor
activity through inhibition of Skp2 in breast cancer cells.
Oncotarget. 7:66512–66524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin CJ, Lin CY, Chen Y, Huang SH and Wang
SM: Rottlerin inhibits migration of follicular thyroid carcinoma
cells by PKCdelta-independent destabilization of the focal adhesion
complex. J Cell Biochem. 110:428–437. 2010.PubMed/NCBI
|
|
13
|
Su J, Wang L, Yin X, Zhao Z, Hou Y, Ye X,
Zhou X and Wang Z: Rottlerin exhibits anti-cancer effect through
inactivation of S phase kinase-associated protein 2 in pancreatic
cancer cells. Am J Cancer Res. 6:2178–2191. 2016.PubMed/NCBI
|
|
14
|
Mizushima N and Klionsky DJ: Protein
turnover via autophagy: Implications for metabolism. Annu Rev Nutr.
27:19–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi KS: Autophagy and cancer. Exp Mol
Med. 44:109–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi AM, Ryter SW and Levine B: Autophagy
in human health and disease. N Engl J Med. 368:651–662. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morselli E, Galluzzi L, Kepp O, Vicencio
JM, Criollo A, Maiuri MC and Kroemer G: Anti- and pro-tumor
functions of autophagy. Biochim Biophys Acta. 1793:1524–1532. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brech A, Ahlquist T, Lothe RA and Stenmark
H: Autophagy in tumour suppression and promotion. Mol Oncol.
3:366–375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moretti L, Yang ES, Kim KW and Lu B:
Autophagy signaling in cancer and its potential as novel target to
improve anticancer therapy. Drug Resist Updat. 10:135–143. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar D, Shankar S and Srivastava RK:
Rottlerin induces autophagy and apoptosis in prostate cancer stem
cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett.
343:179–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song KS, Kim JS, Yun EJ, Kim YR, Seo KS,
Park JH, Jung YJ, Park JI, Kweon GR, Yoon WH, et al: Rottlerin
induces autophagy and apoptotic cell death through a
PKC-delta-independent pathway in HT1080 human fibrosarcoma cells:
The protective role of autophagy in apoptosis. Autophagy.
4:650–658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Torricelli C, Daveri E, Salvadori S,
Valacchi G, Ietta F, Muscettola M, Carlucci F and Maioli E:
Phosphorylation-independent mTORC1 inhibition by the autophagy
inducer Rottlerin. Cancer Lett. 360:17–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Parmer TG, Ward MD and Hait WN: Effects of
rottlerin, an inhibitor of calmodulin-dependent protein kinase III,
on cellular proliferation, viability, and cell cycle distribution
in malignant glioma cells. Cell Growth Differ. 8:327–334.
1997.PubMed/NCBI
|
|
25
|
Deretic V, Delgado M, Vergne I, Master S,
De Haro S, Ponpuak M and Singh S: Autophagy in immunity against
mycobacterium tuberculosis: A model system to dissect immunological
roles of autophagy. Curr Top Microbiol Immunol. 335:169–188.
2009.PubMed/NCBI
|
|
26
|
Ohno I, Eibl G, Odinokova I, Edderkaoui M,
Damoiseaux RD, Yazbec M, Abrol R, Goddard WA III, Yokosuka O,
Pandol SJ and Gukovskaya AS: Rottlerin stimulates apoptosis in
pancreatic cancer cells through interactions with proteins of the
Bcl-2 family. Am J Physiol Gastrointest Liver Physiol. 298:G63–G73.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsui N, Yoshioka R, Nozawa A, Kobayashi
N, Shichijo Y, Yoshikawa T and Akagi M: Caspase-independent
apoptosis induced by reperfusion following ischemia without bile
duct occlusion in rat liver. Biol Pharm Bull. 40:104–107. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang M, Harashima N, Moritani T, Huang W
and Harada M: The roles of ROS and caspases in TRAIL-induced
apoptosis and necroptosis in human pancreatic cancer cells. PLoS
One. 10:e01273862015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Torricelli C, Salvadori S, Valacchi G,
Souček K, Slabáková E, Muscettola M, Volpi N and Maioli E:
Alternative pathways of cancer cell death by rottlerin: Apoptosis
versus autophagy. Evid Based Complement Alternat Med.
2012:9806582012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu H, Wang Y, Wang X, Li R and Yin D:
MicroRNA-365 accelerates cardiac hypertrophy by inhibiting
autophagy via the modulation of Skp2 expression. Biochem Biophys
Res Commun. 484:304–310. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang HM, Okwuosa TM, Scarabelli T,
Moudgil R and Yeh ETH: Cardiovascular complications of cancer
therapy: Best practices in diagnosis, prevention, and management:
Part 2. J Am Coll Cardiol. 70:2552–2565. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kidane B, Sulman J, Xu W, Kong QQ, Wong R,
Knox JJ and Darling GE: Baseline measure of health-related quality
of life (Functional Assessment of Cancer Therapy-Esophagus) is
associated with overall survival in patients with esophageal
cancer. J Thorac Cardiovasc Surg. 151:1571–1580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ngo D, Jia JB, Green CS, Gulati AT and
Lall C: Cancer therapy related complications in the liver,
pancreas, and biliary system: An imaging perspective. Insights
Imaging. 6:665–677. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tuorkey MJ: Cancer therapy with
phytochemicals: Present and future perspectives. Biomed Environ
Sci. 28:808–819. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rao CV, Wang CX, Simi B, Lubet R, Kelloff
G, Steele V and Reddy BS: Enhancement of experimental colon cancer
by genistein. Cancer Res. 57:3717–3722. 1997.PubMed/NCBI
|
|
36
|
Siddique YH, Ara G, Beg T, Gupta J and
Afzal M: Assessment of cell viability, lipid peroxidation and
quantification of DNA fragmentation after the treatment of
anticancerous drug mitomycin C and curcumin in cultured human blood
lymphocytes. Exp Toxicol Pathol. 62:503–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Basu A, Adkins B and Basu C:
Down-regulation of caspase-2 by rottlerin via protein kinase
C-delta-independent pathway. Cancer Res. 68:2795–2802. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao Z, Zheng N, Wang L, Hou Y, Zhou X and
Wang Z: Rottlerin exhibits antitumor activity via down-regulation
of TAZ in non-small cell lung cancer. Oncotarget. 8:7827–7838.
2017.PubMed/NCBI
|
|
39
|
Ashkenazi A and Dixit VM: Death receptors:
Signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Parrish AB, Freel CD and Kornbluth S:
Cellular mechanisms controlling caspase activation and function.
Cold Spring Harb Perspect Biol. 5:a0086722013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shukla S, Fu P and Gupta S: Apigenin
induces apoptosis by targeting inhibitor of apoptosis proteins and
Ku70-Bax interaction in prostate cancer. Apoptosis. 19:883–894.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Podmirseg SR, Jakel H, Ranches GD,
Kullmann MK, Sohm B, Villunger A, Lindner H and Hengst L: Caspases
uncouple p27(Kip1) from cell cycle regulated degradation and
abolish its ability to stimulate cell migration and invasion.
Oncogene. 35:4580–4590. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoneyama M, Shiba T, Yamaguchi T and Ogita
K: Possible involvement of caspases in proliferation of neocortical
neural stem/progenitor cells in the developing mouse brain. Biol
Pharm Bull. 37:1699–1703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Frejlich E, Rudno-Rudzińska J, Janiszewski
K, Salomon L, Kotulski K, Pelzer O, Grzebieniak Z, Tarnawa R and
Kielan W: Caspases and their role in gastric cancer. Adv Clin Exp
Med. 22:593–602. 2013.PubMed/NCBI
|
|
45
|
Singh BN, Kumar D, Shankar S and
Srivastava RK: Rottlerin induces autophagy which leads to apoptotic
cell death through inhibition of PI3K/Akt/mTOR pathway in human
pancreatic cancer stem cells. Biochem Pharmacol. 84:1154–1163.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ouyang L, Zhang L, Fu L and Liu B: A
small-molecule activator induces ULK1-modulating
autophagy-associated cell death in triple negative breast cancer.
Autophagy. 13:777–778. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun L, Hu L, Cogdell D, Lu L, Gao C, Tian
W, Zhang Z, Kang Y, Fleming JB and Zhang W: MIR506 induces
autophagy-related cell death in pancreatic cancer cells by
targeting the STAT3 pathway. Autophagy. 13:703–714. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Parzych KR and Klionsky DJ: An overview of
autophagy: Morphology, mechanism, and regulation. Antioxid Redox
Signal. 20:460–473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yu T, Guo F, Yu Y, Sun T, Ma D, Han J,
Qian Y, Kryczek I, Sun D, Nagarsheth N, et al: Fusobacterium
nucleatum promotes chemoresistance to colorectal cancer by
modulating autophagy. Cell. 170(548–563): e5162017.
|
|
50
|
Panneerdoss S, Viswanadhapalli S,
Abdelfattah N, Onyeagucha BC, Timilsina S, Mohammad TA, Chen Y,
Drake M, Vuori K, Kumar TR and Rao MK: Cross-talk between
miR-471-5p and autophagy component proteins regulates
LC3-associated phagocytosis (LAP) of apoptotic germ cells. Nat
Commun. 8:5982017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Vargas Rivera T, Cai Z, Shen Y, Dosset M,
Benoit-Lizon I, Martin T, Roussey A, Flavell RA, Ghiringhelli F and
Apetoh L: Selective degradation of PU.1 during autophagy represses
the differentiation and antitumour activity of TH9
cells. Nat Commun. 8:5592017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Levy JMM, Towers CG and Thorburn A:
Targeting autophagy in cancer. Nat Rev Cancer. 17:528–542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Goldsmith J, Levine B and Debnath J:
Autophagy and cancer metabolism. Methods Enzymol. 542:25–57. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Edinger AL and Thompson CB: Death by
design: Apoptosis, necrosis and autophagy. Curr Opin Cell Biol.
16:663–669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mills KR, Reginato M, Debnath J, Queenan B
and Brugge JS: Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) is required for induction of autophagy during lumen
formation in vitro. Proc Natl Acad Sci USA. 101:3438–3443. 2004.
View Article : Google Scholar : PubMed/NCBI
|