Introduction
Tea is one of the most widely consumed beverages
worldwide, the health benefits of which have been recorded against
numerous diseases in ancient China (1). In terms of worldwide distribution,
black tea is mainly consumed in Western countries, whereas green
tea is more common in Asian countries. It has been reported that
tea polyphenols can inhibit osteoclast formation and
differentiation in rats (2);
however, the mechanism underlying the protective effects of tea
polyphenols on cartilage cells have yet to be elucidated.
Theaflavins (TFs) are the primary active polyphenols in black tea,
which include theaflavin-3-gallate, theaflavin-3′-gallate and
theaflavin-3-3′-digallate (3). TFs
have been reported to possess numerous properties including
antioxidant, antiviral and anticancer activities, in various
biological processes (4,5). Cartilage degeneration is associated
with the progression of osteoarthritis, and is mainly induced by
oxidative stress (6). The present
study aimed to explore the potential effect of TFs, in particular
theaflavin-3-3′-digallate, on an in vitro model of cartilage
degeneration and the related mechanisms.
Progressive cartilage destruction can be attributed
to several factors (7). Among
these factors, reactive oxygen species (ROS) are responsible for
the maintenance of cartilage homeostasis; ROS act as the critical
signaling intermediate of intracellular signaling pathways,
including the phosphoinositide 3-kinase/protein kinase B and c-Jun
N-terminal kinase pathways (8,9).
Over-accumulation of ROS may lead to the disruption of cartilage
homeostasis (10,11). In addition, apoptosis is considered
to be associated with cartilage degeneration (12,13).
It has been reported that overproduction of ROS can trigger
intracellular DNA damage, which serves as a cellular stress factor
(8). Furthermore, it has been
demonstrated that Forkhead box (FOX) transcription factors are
implicated in cell cycle progression, immune regulation, tumor
growth and the aging process (14). FOXO proteins are able to regulate
oxidative stress resistance through controlling downstream
antioxidant targets, including glutathione peroxidase 1 (Gpx1) and
catalase (CAT) (15,16). In addition, AKT serine/threonine
kinase (AKT) serves important roles in cell survival and its
activation can phosphorylate several downstream proteins, including
FOXO3a. The transcriptional inactivation of FOXO3a can be induced
by phosphorylation via AKT (17).
Based on these findings, it is likely that AKT/FOXO signaling may
be associated with the protective effect of TFs in cartilage
cells.
The present study aimed to investigate the
protective effect of TFs on cartilage cells and attempted to
explore the underlying mechanisms.
Materials and methods
Cell culture and grouping
Human chondrocytes (cat. no. 4650; ScienCell
Research Laboratories, Inc., San Diego, CA, USA) were cultured in a
6-well plate (1×105/well) at 37°C in a 5% CO2
incubator with Dulbecco's modified Eagle medium containing 10%
fetal bovine serum (both Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and 1% penicillin and streptomycin (Sangon
Biotech Co., Ltd., Shanghai, China). Cells were fixed with 95%
ethanol for 15 sec at room temperature and then stained with 1%
toluidine blue (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China) for 5 min at room temperature and observed
under a light microscope (magnification, ×100).
Theaflavin-3-3′-digallate (purity >90%) was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). At ~80% confluence,
the cells were serum-starved overnight and were then divided into
three treatment groups for comparison: i) Control group; ii) model
group, in which cells were treated with 0.3 mM
H2O2 for 6 h; and iii) TF pretreatment
groups, in which cells were pretreated with various doses of TFs
(10 and 20 µg/ml) for 12 h, followed by 6 h
H2O2 incubation. The TF concentrations
employed were based on previous literature (18,19).
For the activation of AKT, cells were pretreated with 50 ng/ml
insulin-like growth factor I (IGF-I; R&D Systems, Inc.,
Minneapolis, MN, USA) for 10 min prior to
H2O2 or TF treatment, according to previous
studies (20,21).
Cell Counting kit (CCK)-8 assay
The cells were serum starved overnight in a 96-well
plate (1×105 cells/well), and were then treated with
H2O2 (0.1–0.5 µM) in serum-free medium for
various durations (6, 12, 24 and 48 h). A CKK-8 kit was used to
detect cell viability, according to the manufacturer's protocol
(Beijing Kangwei Century Biotechnology Co., Ltd., Beijing, China).
Briefly, the CCK-8 solution was added to each well and the cells
were incubated for 4 h at 37°C, after which, absorbance was
measured at 450 nm using a microplate reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
ELISA assay
The cells were seeded at a density of
1×104 cells/well in a 96-well plate and were treated as
aforementioned. Matrix metalloproteinase (MMP)-13 (cat. no. DY511)
and interleukin (IL)-1β (cat. no. DLB50) activities were detected
using ELISA kits (R&D Systems, Inc.), according to the
manufacturer's protocols. The ELISA kit used to measure the
expression of cartilage glycoprotein 39 (Cgp-39; cat. no. HC021)
was purchased from Shanghai GeFan Biotechnology, Co., Ltd.
(Shanghai, China).
Flow cytometric analysis of ROS
levels
The cells (1×104 cells/well) were treated
as aforementioned. Subsequently, the cells were stained with
H2DCHF-DA (Invitrogen; Thermo Fisher Scientific, Inc.) for ROS
measurement, as previously described (22). BD FACSCanto II (BD Biosciences,
Franklin Lakes, NJ, USA) running BD CellQuest™ software
version 3.3 (BD Biosciences) was used to perform flow cytometric
analysis. All data are representatives of at least three
independent experiments.
Flow cytometric analysis of
apoptosis
The cells (1×105/well) were cultured in
6-well plates. An Annexin V/propidium iodide (PI) apoptosis kit
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to detect
apoptosis. According to the manufacturer's protocol, Annexin-V and
PI staining was analyzed using analyzed using a flow cytometer (BD
FACSCanto II) with FACSDiva software version 6.1 (both BD
Biosciences).
Flow cytometric analysis of DNA
damage
According to a previous study (23), DNA damage was estimated using flow
cytometry-based detection of γ-H2A histone family, member X
(γ-H2AX). Briefly, the collected cells were suspended in BD
Cytofix/Cytoperm fixation and permeabilization solution (BD
Biosciences). After a 15-min incubation at 37°C, the cells were
washed using PBS and blocked with 10% normal goat serum (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C for 1 h. Cells were
incubated with anti-γ-H2AX (pS139) antibody (cat. no. ab26350;
1:100; Abcam, Cambridge, UK) at 4°C overnight and then incubated
with fluorescein isothiocyanate-conjugated secondary antibodies
(cat. no. ab7064; 1:1,000; Abcam) for 45 min at 37°C. Subsequently,
the fluorescent signals were measured using a BD flow cytometer (BD
Biosciences).
Total RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using RNAiso reagent (Takara
Bio, Inc., Otsu, Japan). Subsequently, cDNA was reverse transcribed
from total RNA using ReverTra Ace (Toyobo Life Science, Osaka,
Japan) and oligo-dT (Takara Bio, Inc.), according to manufacturer's
protocol. The mRNA expression levels were quantified using an ABI
7500 Real-time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using AceQ qPCR SYBR Green Master Mix (Vazyme,
Piscataway, NJ, USA). The thermocycling conditions were as follows:
95°C for 5 min, followed by 30 cycles of 94°C for 15 sec and 60°C
for 30 sec, and final extension at 72°C for 5 min. Relative
expression levels were calculated using the 2−ΔΔCq
method (24). Primer sequences for
RT-qPCR were as follows: ATR serine/threonine kinase (ATR) forward,
5′-GGAATCACGACTCGCTGA; AC-3′ reverse, 5′-AAATCGGCCCACTAGTAGCA-3′;
ATM serine/threonine kinase (ATM) forward,
5′-CGAGGCGTACAATGGTGAAG-3′ and reverse, 5′-CCTCCGGCTAAGCGAAATTC-3′;
B-cell lymphoma 2-associated X protein (Bax) forward,
5′-GAGCGGCGGTGATGGA-3′ and reverse, 5′-TGGATGAAACCCTGAAGCAAA-3′;
and β-actin forward, 5′-CTCTTCCAGCCTTCCTTCC-3′; and reverse,
5′-AGCACTGTGTTGGCGTACAG-3′.
Western blot analysis
A Total Extraction Sample kit (Sigma-Aldrich; Merck
KGaA) was used to extract total proteins. Protein concentration was
determined using the Pierce™ BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.). The proteins (20 µg/lane) were
separated by 10% SDS-PAGE and were then transferred onto a
polyvinylidene fluoride membrane. To block non-specific proteins,
non-fat milk (3%) was used to incubate the membrane for 2 h at room
temperature. Following incubation with primary antibodies overnight
at 4°C, the membrane was incubated with secondary antibodies for 2
h at room temperature, and the bands were developed using an
enhanced chemiluminescence reagent (GE Healthcare Life Sciences,
Little Chalfont, UK). Blot density was determined using Quantity
One software version 4.6.9 (Bio-Rad Laboratories, Inc.). The
primary antibodies used were as follows: Anti-ATR (cat. no. ab2905;
1:1,000), anti-Bax (cat. no. ab53154; 1:1,000; both Abcam,
Cambridge, UK), anti-cleaved caspase-3 (cat. no. 9664; 1:1,000;
Cell Signaling Technology, Inc., Danvers, MA, USA), anti-ATM (cat.
no. ab78; 1:2,000), anti-γH2AX (cat. no. ab11175; 1:8,000; both
Abcam), anti-phosphorylated (p)-AKT (cat. no. 13038; 1:1,000; Cell
Signaling Technology, Inc.), anti-AKT1/2 (cat. no. ab182729;
1:5,000), anti-p-FOXO3a (cat. no. ab53287; 1:1,000; both Abcam),
anti-FOXO3a (cat. no. 2497; 1:1,000; CST), anti-Gpx1 (cat. no.
ab22604; 1:1,000), anti-CAT (cat. no. ab16731; 1:2,000; both Abcam)
and anti-β-actin (cat. no. 4970; 1:1,000; Cell Signaling
Technology, Inc.). Horseradish peroxidase-conjugated secondary
antibodies (goat anti-rabbit; cat. no. ab205718; 1:2,000 and goat
anti-mouse; cat. no. ab205719; 1:5,000) were obtained from
Abcam.
Statistical analysis
All experiments were independently performed ≥3
times. Data are presented as the means ± standard deviation.
GraphPad software version 6.0 (GraphPad Software, Inc., La Jolla,
CA, USA) was used to compare differences between groups by one-way
analysis of variance followed by Tukey's multiple comparisons test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
TFs inhibit ROS generation in
cartilage degeneration
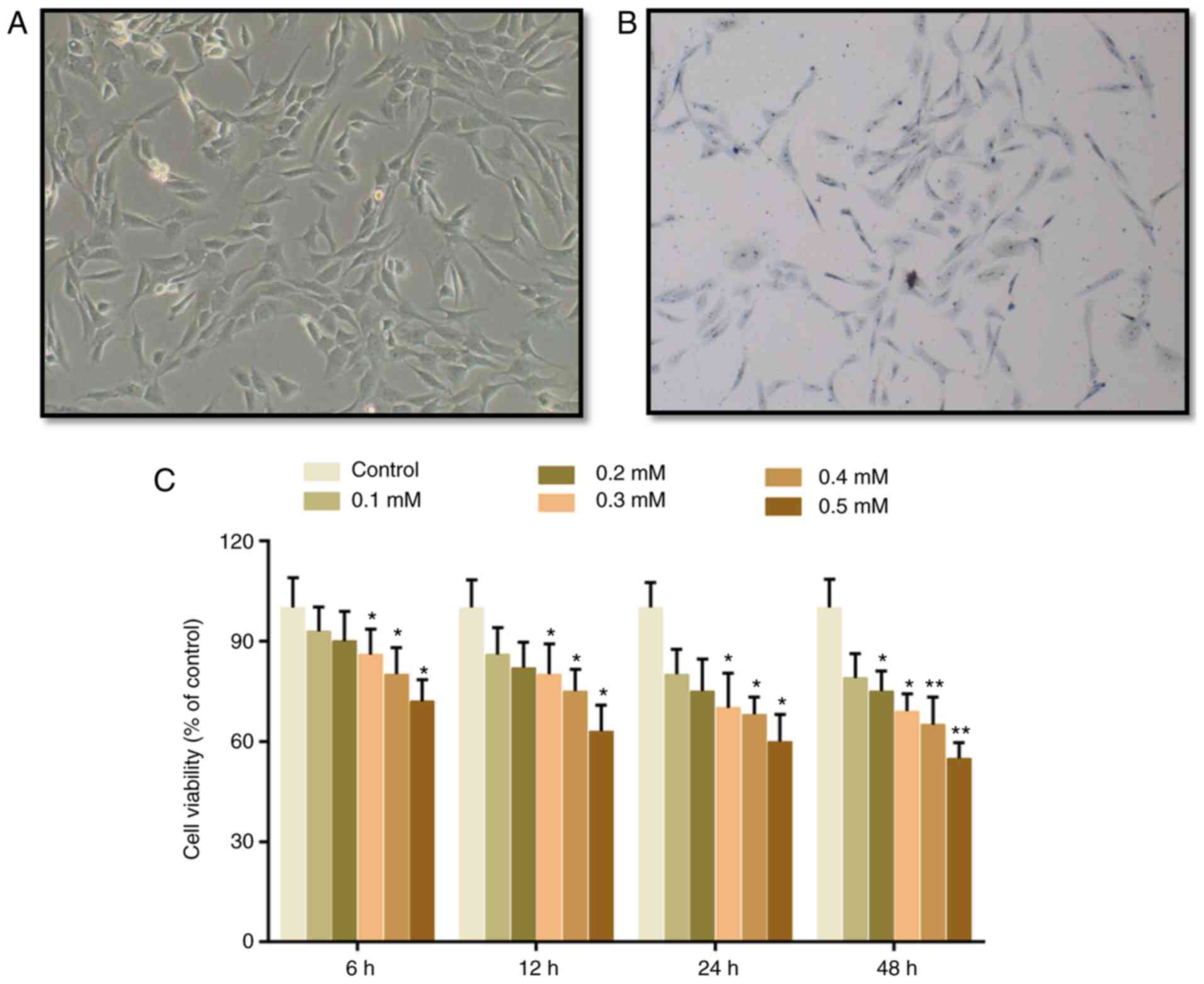
The cartilage cells presented with spindle
morphology, and toluidine blue staining was conducted to identify
the cells. It was identified that the cytoplasm was stained light
blue and the nucleus was stained dark blue, indicating that these
cells were chondrocytes (Fig. 1A and
B). Subsequently, CCK-8 assay was conducted to evaluate the
effects of H2O2 on the viability of cartilage
cells. It was demonstrated that cell viability was suppressed by
H2O2 in a dose-dependent manner. A
significant difference emerged in the group that was treated with
0.3 mM H2O2 for 6 h, in which cell viability
was deceased by 14% (Fig. 1B).
Therefore, 0.3 mM H2O2 was subsequently used
to treat cartilage cells for 6 h, in order to mimic the progression
of cartilage degeneration. To measure cartilage degeneration
following H2O2 treatment, the expression
levels of catabolic factors, MMP-13, IL-1β and Cgp-39, were
detected. It was demonstrated that the expression levels of MMP-13,
IL-1β and Cgp-39 were increased by H2O2, but
were decreased by TF pretreatment (Fig. 2A). These findings suggested that
TFs may inhibit cartilage degeneration. Furthermore, according to
flow cytometric analysis, it was demonstrated that ROS levels were
markedly decreased in the TF pretreatment groups (Fig. 2B and C).
 | Figure 2.(A) Detection of catabolic factors,
MMP-13, IL-1β and Cgp-39. (B) ROS production rate was determined
following treatment with TFs and H2O2. (C)
ROS levels were measured by flow cytometry. **P<0.01 vs.
control; #P<0.05 and ##P<0.01 vs. MG;
^P<0.05, ^^P<0.01 vs. T1 +
H2O2. Cgp, cartilage glycoprotein; IL,
interleukin; MG, model group; MMP, matrix metalloproteinase; ROS,
reactive oxygen species; T1, pretreatment with 10 µg/ml TFs; T2,
pretreatment with 20 µg/ml TFs; TFs, theaflavins. |
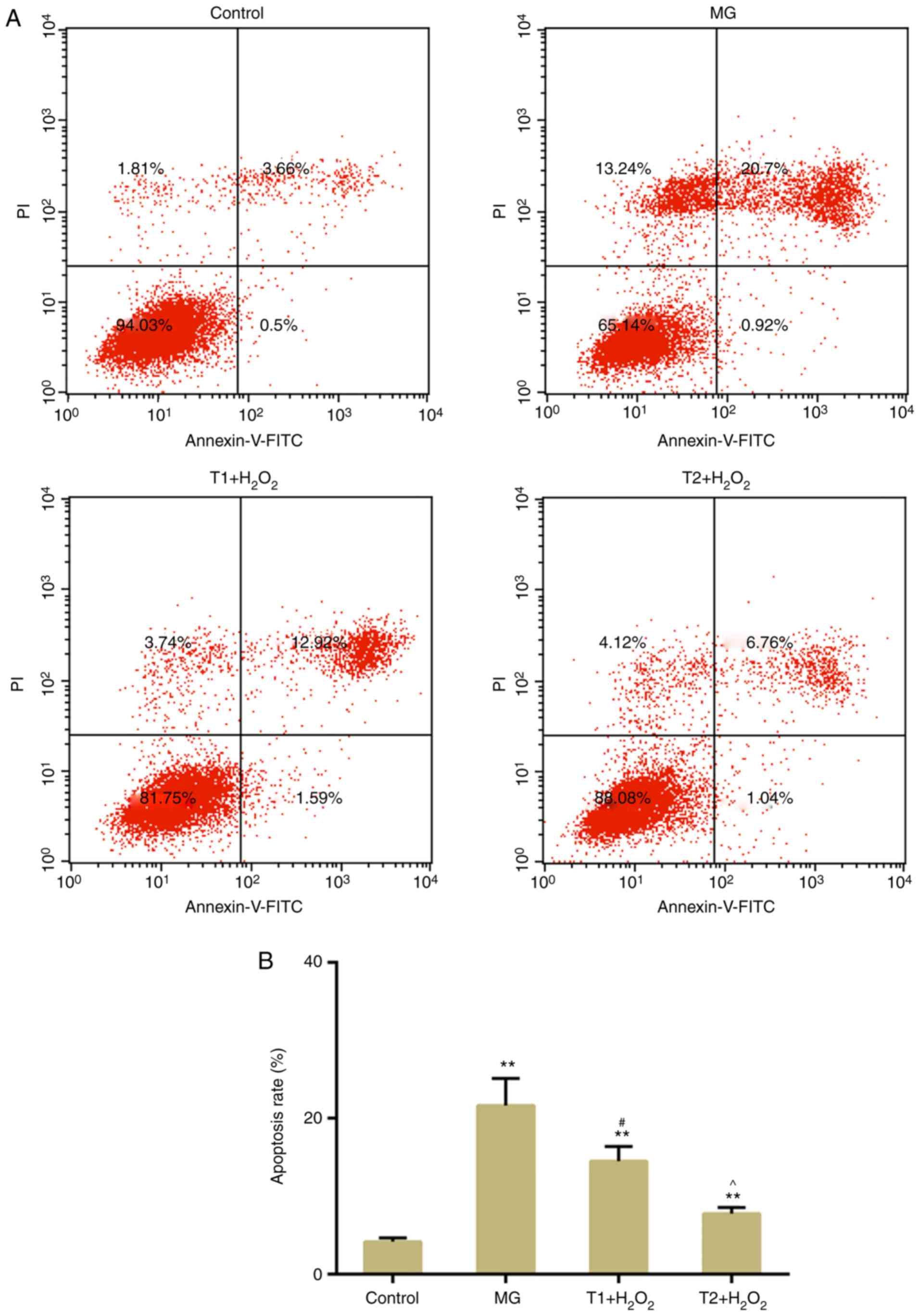
TFs suppress apoptosis and DNA damage
following oxidative stress
The results of flow cytometric analysis revealed
that H2O2-induced apoptosis was suppressed by
pretreatment with TFs (Fig. 3A and
B). DNA double-strand breaks (DSBs) are a type of detrimental
DNA damage, for which γH2AX is considered a surrogate marker. In
response to DSBs, H2AX is phosphorylated at Ser139 (γH2AX)
(25). The present results
demonstrated that TFs could decrease γH2AX expression compared with
in the model group (Fig. 4A and
B). In addition, western blotting confirmed that TFs inhibited
the expression levels of γH2AX (Fig.
4C). The expression levels of apoptosis-associated factors,
including cleaved caspase-3 and Bax, were decreased in the TF
pretreatment groups compared with in the model group. The
expression levels of DNA damage-response genes, ATM and ATR, were
also decreased following TF pretreatment (Fig. 5A-C). However, the protein
expression levels of ATR were slightly, but not significantly,
increased in the T1 + H2O2 group.
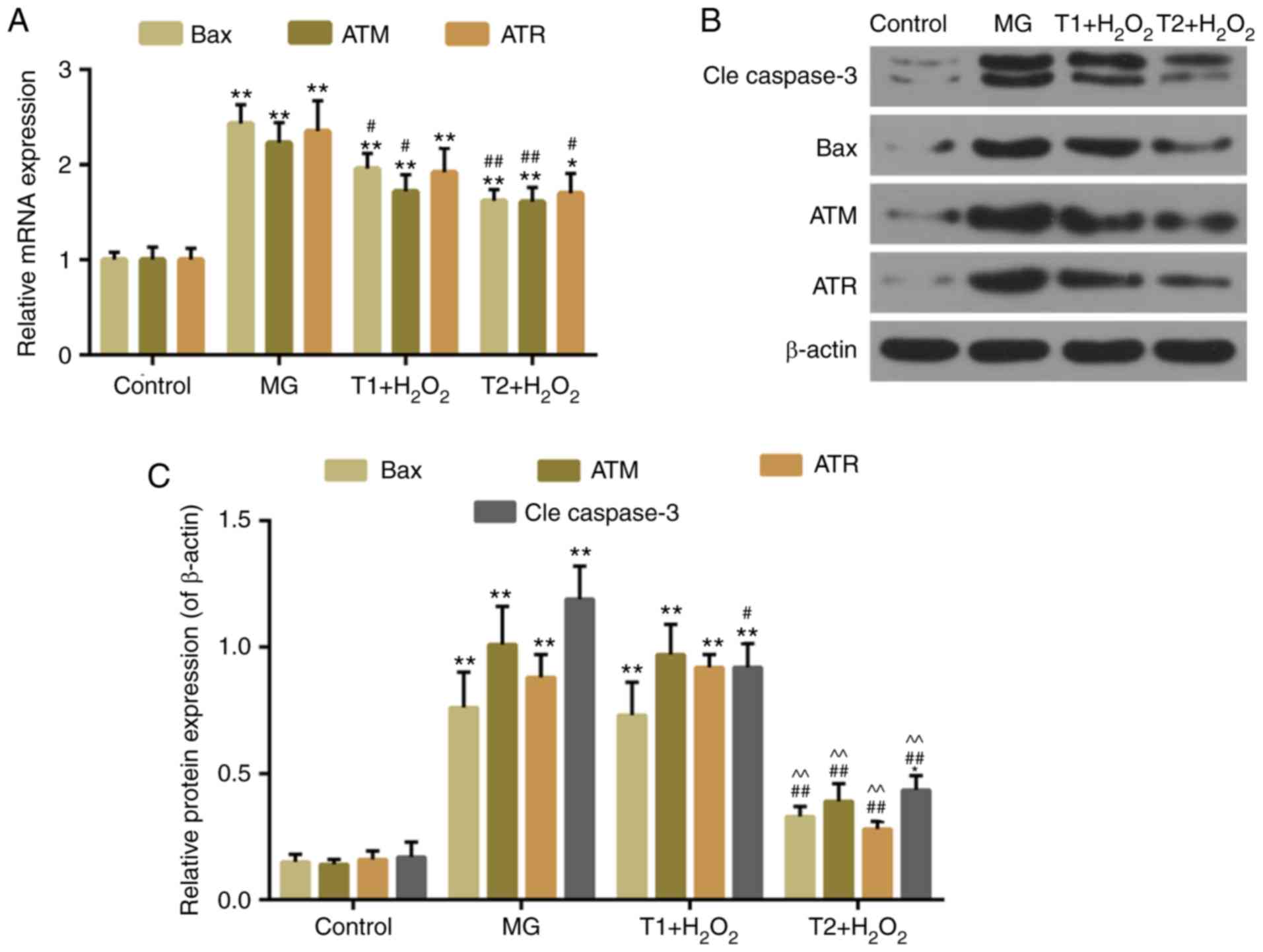 | Figure 5.(A) Quantitative analysis of Bax, ATR
and ATM mRNA expression. (B and C) Western blot analysis of cleaved
caspase-3, Bax, ATR and ATM. *P<0.05 and **P<0.01 vs.
control; #P<0.05 and ##P<0.01 vs. MG;
^^P<0.01 vs. T1 + H2O2. ATM,
ATM serine/threonine kinase; ATR, ATR serine/threonine kinase; Bax,
B-cell lymphoma 2-associated X protein; MG, model group; T1,
pretreatment with 10 µg/ml TFs; T2, pre-treatment with 20 µg/ml
TFs; TFs, theaflavins. |
TFs decrease the activity of AKT,
FOXO3a, Gpx1 and CAT
Emerging evidences have demonstrated that FOXO
proteins are important mediators of oxidative stress (15,16).
Compared with in the model group, the protein expression levels of
p-AKT and p-FOXO3a were mitigated by TF pretreatment, whereas the
expression levels of Gpx1 and CAT were enhanced (Fig. 6A and B).
 | Figure 6.(A and B) Western blot analysis of
AKT, p-AKT, FOXO3a, p-FOXO3a, Gpx1 and CAT. **P<0.01 vs.
control; #P<0.05 and ##P<0.01 vs. MG;
^^P<0.01 vs. T1 + H2O2. CAT,
catalase; FOXO3a, Forkhead box O3a; MG, model group; Gpx,
glutathione peroxidase 1; p-, phosphorylated; T1, pretreatment with
10 µg/ml TFs; T2, pre-treatment with 20 µg/ml TFs; TFs,
theaflavins. |
AKT activity is necessary for the
protective effects of TFs
To further confirm the role of AKT in the present
study, apoptosis and DNA damage were detected following treatment
with the AKT activator, IGF-I. The results demonstrated that TF
pretreatment did not significantly reverse
H2O2-induced apoptosis following persistent
activation of AKT (Fig. 7A and B).
In addition, TF-induced inhibition of DNA damage was reversed
following persistent activation of AKT (Fig. 8A-C).
Discussion
Tea is one of the most widely consumed beverages
worldwide (26). The potential
health benefits of tea have been widely reported, particularly with
regards to the prevention of cardiovascular disorders and cancer.
Phenols and polyphenols are the primary bioactive substances in tea
that exert health effects (27);
therefore, attention has been paid to the antioxidative effects of
tea polyphenols (28). Cartilage
degeneration is a serious complication of osteoarthritis, which is
mainly caused by oxidative stress (29). A previous study demonstrated the
positive function of tea polyphenols in maintaining bone
homeostasis (2). TFs are the
primary active content of tea phenols (30); however, little is currently known
about the effects of TFs on cartilage degeneration.
Studies have revealed the connection between
structural degeneration and biochemical markers (31–34).
Several biochemical markers, including MMP-13 (32), IL-1β (33) and Cgp-39 (34), are used to diagnose patients with a
high risk of joint degeneration. The present study demonstrated
that TFs inhibited H2O2-mediated cartilage
degeneration by decreasing the levels of MMP-13, IL-1β and Cgp-39.
This study also aimed to illustrate the molecular mechanisms
underlying the effects of TFs on the cartilage cells. The results
demonstrated that ROS production was markedly increased in the
model group, whereas pretreatment with TFs significantly decreased
ROS levels. Furthermore, pretreatment with TFs reduced cell
apoptosis and DNA damage caused by H2O2, and
decreased the expression levels of cleaved caspase-3 and Bax, which
are closely associated with cell apoptosis (35,36).
The expression levels of ATR and ATM, which is the master kinase
that controls the DNA damage check point (37,38),
were decreased in the TF pretreatment groups compared with in the
model group. These findings indicated that TFs may prevent
cartilage matrix degeneration by inhibiting DNA damage and
apoptosis. The inhibitory effects of TFs on apoptosis were
supported by a recent study in PC12 neural cells (39). In addition, DNA damage can be
modified by TFs in human lymphocytes (40). However, numerous studies have
demonstrated that TFs inhibit proliferation and induce apoptosis in
cancer cells (41–43). These contradictory results may be
due to the distinct cell types used in each study model.
To explore the possible underlying mechanisms, the
effects of TFs on the activity of AKT/FOXO3 signaling were
investigated. It was noted that TFs mitigated the expression of
p-AKT and p-FOXO3a, and enhanced Gpx1 and CAT activities compared
with in the model group. It has previously been demonstrated that
the reduced activity of AKT/FOXOs mitigates cell dysfunction in
diabetic kidney disease (44).
Notably, the present study revealed that TF-induced inhibition of
apoptosis and DNA damage was reversed following persistent
activation of AKT. Therefore, it may be hypothesized that the
effects of TFs on cartilage cells may be tightly linked to AKT/FOXO
signaling. Since phosphorylation of FOXO3 results in its
inactivation, TFs may reduce inactivation of FOXO3 by suppressing
AKT. However, this speculation was not validated in the present
study. The protective effect of FOXO3 inactivation on cartilage
still requires further investigation. In addition, the activity of
FOXOs can be regulated by other signals (45); however, the regulation is rather
complex, and parts of it are contradictory. For example, FOXO3 can
be activated by the phosphorylation of 5′AMP-activated protein
kinase, c-Jun N-terminal kinase and macrophage-stimulating 1
(46). Therefore, it would be
useful to investigate how the upstream signals co-regulate FOXO3
signaling in future studies.
In conclusion, the present study demonstrated that
TFs inhibited the ROS burst in cartilage destruction. TFs
suppressed apoptosis and DNA damage by reducing the expression
levels of cleaved caspase-3, Bax, ATR and ATM. Furthermore, TFs
enhanced the activity of Gpx1 and CAT, and decreased the expression
levels of p-AKT and p-FOXO3a. Notably, AKT signaling was necessary
for the effects of TFs on apoptosis and DNA damage. The results of
the present study demonstrated that TFs may be a potential
candidate drug for the prevention of cartilage degeneration.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL and JZ designed the study, performed the
experiments and performed the data analysis. JL wrote the
manuscript. JL and JZ revised the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Graham HN: Tea: The plant and its
manufacture; chemistry and consumption of the beverage. Prog Clin
Biol Res. 158:29–74. 1984.PubMed/NCBI
|
|
2
|
Oka Y, Iwai S, Amano H, Irie Y, Yatomi K,
Ryu K, Yamada S, Inagaki K and Oguchi K: Tea polyphenols inhibit
rat osteoclast formation and differentiation. J Pharm Sci.
118:55–64. 2012. View Article : Google Scholar
|
|
3
|
Liu S, Lu H, Zhao Q, He Y, Niu J, Debnath
AK, Wu S and Jiang S: Theaflavin derivatives in black tea and
catechin derivatives in green tea inhibit HIV-1 entry by targeting
gp41. Biochim Biophys Acta. 1723:270–281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maron DJ, Lu GP, Cai NS, Wu ZG, Li YH,
Chen H, Zhu JQ, Jin XJ, Wouters BC and Zhao J: Cholesterol-lowering
effect of a theaflavin-enriched green tea extract: A randomized
controlled trial. Arch Intern Med. 163:1448–1453. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin JK, Chen PC, Ho CT and Lin-Shiau SY:
Inhibition of xanthine oxidase and suppression of intracellular
reactive oxygen species in HL-60 cells by
theaflavin-3,3′-digallate, (−)-epigallocatechin-3-gallate, and
propyl gallate. J Agric Food Chem. 48:2736–2743. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pap T and Korb-Pap A: Cartilage damage in
osteoarthritis and rheumatoid arthritis-two unequal siblings. Nat
Rev Rheumatol. 11:606–615. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hosseinzadeh A, Kamrava SK, Joghataei MT,
Darabi R, Shakeri-Zadeh A, Shahriari M, Reiter RJ, Ghaznavi H and
Mehrzadi S: Apoptosis signaling pathways in osteoarthritis and
possible protective role of melatonin. J Pineal Res. 61:411–425.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lepetsos P and Papavassiliou AG:
ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys
Acta. 1862:576–591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu SM and Kim SJ: Withaferin A-caused
production of intracellular reactive oxygen species modulates
apoptosis via PI3K/Akt and JNKinase in rabbit articular
chondrocytes. J Korean Med Sci. 29:1042–1053. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen AF, Davies CM, De Lin M and Fermor B:
Oxidative DNA damage in osteoarthritic porcine articular cartilage.
J Cell Physiol. 217:828–833. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Davies CM, Guilak F, Weinberg JB and
Fermor B: Reactive nitrogen and oxygen species in
interleukin-1-mediated DNA damage associated with osteoarthritis.
Osteoarthritis Cartilage. 16:624–630. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hashimoto S, Ochs RL, Komiya S and Lotz M:
Linkage of chondrocyte apoptosis and cartilage degradation in human
osteoarthritis. Arthritis Rheum. 41:1632–1638. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim HA and Blanco FJ: Cell death and
apoptosis in osteoarthritic cartilage. Curr Drug Targets.
8:333–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calnan DR and Brunet A: The FoxO code.
Oncogene. 27:2276–2288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van der Horst A and Burgering BMT:
Stressing the role of FoxO proteins in lifespan and disease. Nat
Rev Mol Cell Biol. 8:440–450. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brigelius-Flohé R and Maiorino M:
Glutathione peroxidases. Biochim Biophys Acta. 1830:3289–3303.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
García Z, Kumar A, Marqués M, Cortés I and
Carrera AC: Phosphoinositide 3-kinase controls early and late
events in mammalian cell division. EMBO J. 25:655–661. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W, Sun Y, Liu J, Wang J, Li Y, Li H
and Zhang W: Protective effect of theaflavins on
homocysteine-induced injury in HUVEC cells in vitro. J Cardiovasc
Pharmacol. 59:434–440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lahiry L, Saha B, Chakraborty J,
Bhattacharyya S, Chattopadhyay S, Banerjee S, Choudhuri T, Mandal
D, Bhattacharyya A, Sa G and Das T: Contribution of p53-mediated
Bax transactivation in theaflavin-induced mammary epithelial
carcinoma cell apoptosis. Apoptosis. 13:771–781. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu CJ, O'Rourke DM, Feng GS, Johnson GR,
Wang Q and Greene MI: The tyrosine phosphatase SHP-2 is required
for mediating phosphatidylinositol 3-kinase/Akt activation by
growth factors. Oncogene. 20:6018–6025. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamada T, Takeuchi S, Fujita N, Nakamura
A, Wang W, Li Q, Oda M, Mitsudomi T, Yatabe Y, Sekido Y, et al: Akt
kinase-interacting protein1, a novel therapeutic target for lung
cancer with EGFR-activating and gatekeeper mutations. Oncogene.
32:4427–4435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fani S, Kamalidehghan B, Lo KM, Nigjeh SE,
Keong YS, Dehghan F, Soori R, Abdulla MA, Chow KM, Ali HM, et al:
Anticancer activity of a monobenzyltin complex C1 against
MDA-MB-231 cells through induction of Apoptosis and inhibition of
breast cancer stem cells. Sci Rep. 6:389922016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Guo YY, Wu W, Bai JL, Xuan ZQ, Yang
J and Wang J: Detecting DNA damage of human lymphocytes exposed to
1,2-DCE with γH2AX identified antibody using flow cytometer assay.
Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 29:16–19. 2011.(In
Chinese). PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu YK, Lu Y, Yu Y and Yang J: γH2AX: A
biomarker for DNA double-stranded breaks. Chin J Pharm Tox.
19:237–240. 2005.
|
|
26
|
Wang D, Gao Q, Wang T, Qian F and Wang Y:
Theanine: the unique amino acid in the tea plant as an oral
hepatoprotective agent. Asia Pac J Clin Nutr. 26:384–391.
2017.PubMed/NCBI
|
|
27
|
Schneider C and Segre T: Green tea:
Potential health benefits. Am Fam Physician. 79:591–594.
2009.PubMed/NCBI
|
|
28
|
Tipoe GL, Leung TM, Hung MW and Fung ML:
Green tea polyphenols as an anti-oxidant and anti-inflammatory
agent for cardiovascular protection. Cardiovasc Hematol Disord Drug
Targets. 7:135–144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li YS, Xiao WF and Luo W: Cellular aging
towards osteoarthritis. Mech Ageing Dev. 162:80–84. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morinobu A, Biao W, Tanaka S, Horiuchi M,
Jun L, Tsuji G, Sakai Y, Kurosaka M and Kumagai S:
(−)-Epigallocatechin-3-gallate suppresses osteoclast
differentiation and ameliorates experimental arthritis in mice.
Arthritis Rheum. 58:2012–2018. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bruyere O, Collette JH, Ethgen O, Rovati
LC, Giacovelli G, Henrotin YE, Seidel L and Reginster JY:
Biochemical markers of bone and cartilage remodeling in prediction
of longterm progression of knee osteoarthritis. J Rheumatol.
30:1043–1050. 2003.PubMed/NCBI
|
|
32
|
Attur M, Yang Q, Kirsch T and Abramson SB:
Role of periostin and discoidin domain receptor-1 (DDR1) in the
regulation of cartilage degeneration and expression of MMP-13.
Osteoarthritis Cartilage. 24:S1562016. View Article : Google Scholar
|
|
33
|
Goldring SR: Pathogenesis of bone and
cartilage destruction in rheumatoid arthritis. Rheumatology
(Oxford). 42 Suppl 2:ii11–ii16. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zivanović S, Rackov LP, Vojvodić D and
Vucetić D: Human cartilage glycoprotein 39-biomarker of joint
damage in knee osteoarthritis. Int Orthop. 33:1165–1170. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee JH and Paull TT: Activation and
regulation of ATM kinase activity in response to DNA double-strand
breaks. Oncogene. 26:7741–7748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K
and Linn S: Molecular mechanisms of mammalian DNA repair and the
DNA damage checkpoints. Annu Rev Biochem. 73:39–85. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang J, Cai S, Li J, Xiong L, Tian L, Liu
J, Huang J and Liu Z: Neuroprotective effects of theaflavins
against oxidative stress-induced apoptosis in PC12 cells. Neurochem
Res. 41:3364–3372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alotaibi A, Bhatnagar P, Najafzadeh M,
Gupta KC and Anderson D: Tea phenols in bulk and nanoparticle form
modify DNA damage in human lymphocytes from colon cancer patients
and healthy individuals treated in vitro with platinum-based
chemotherapeutic drugs. Nanomedicine (Lond). 8:389–401. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Adhikary A, Mohanty S, Lahiry L, Hossain
DM, Chakraborty S and Das T: Theaflavins retard human breast cancer
cell migration by inhibiting NF-kappaB via p53-ROS cross-talk. FEBS
Lett. 584:7–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bhattacharya U, Halder B, Mukhopadhyay S
and Giri AK: Role of oxidation-triggered activation of JNK and p38
MAPK in black tea polyphenols induced apoptotic death of A375
cells. Cancer Sci. 100:1971–1978. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schuck AG, Ausubel MB, Zuckerbraun HL and
Babich H: Theaflavin-3,3′-digallate, a component of black tea: An
inducer of oxidative stress and apoptosis. Toxicol In Vitro.
22:598–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kato M, Yuan H, Xu ZG, Lanting L, Li SL,
Wang M, Hu MC, Reddy MA and Natarajan R: Role of the Akt/FoxO3a
pathway in TGF-beta1-mediated mesangial cell dysfunction: A novel
mechanism related to diabetic kidney disease. J Am Soci Nephrol.
17:3325–3335. 2006. View Article : Google Scholar
|
|
45
|
Zhao Y, Wang Y and Zhu WG: Applications of
post-translational modifications of FoxO family proteins in
biological functions. J Mol Cell Biol. 3:276–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wilk A, Urbanska K, Yang S, Wang JY, Amini
S, Del Valle L, Peruzzi F, Meggs L and Reiss K: Insulin-like growth
factor-I-forkhead box O transcription factor 3a counteracts high
glucose/tumor necrosis factor-α-mediated neuronal damage:
Implications for human immunodeficiency virus encephalitis. J
Neurosci Res. 89:183–198. 2011. View Article : Google Scholar : PubMed/NCBI
|