Introduction
Spinal cord injury (SCI) is one of the most common
injuries that is identified in spine and neurosurgery departments,
frequently causing permanent disabilities, including paralysis,
loss of movement, sensation or autonomic control below the affected
region (1). Globally, ~23 cases
per million occur every year (2).
At present, SCI treatment is considered one of the greatest
challenges for clinical practice and basic science research
(3). Apoptosis is a primary
difficulty in SCI treatment, which has an important role in
physical and functional deficits (4,5).
Therefore, the development of a novel therapy is urgently required,
which suppresses apoptosis in the treatment of SCI.
Apoptosis, programmed cell death, has been
identified as a key process that influences the development of
neuronal tissue damage following SCI (6). It was previously identified that the
death receptor and mitochondrial pathways may induce apoptosis
(7). The B-cell lymphoma-2 (Bcl-2)
family members may mediate apoptotic signals through pro-apoptotic
proteins [apoptosis regulator BAX (Bax), Bcl-2 homologous
antagonist/killer and Bcl-2-associated agonist of cell death] and
anti-apoptotic proteins (Bcl-2 and B-cell lymphoma-extra large) in
the mitochondrial pathway (8). The
collapse of the mitochondrial membrane potential is defined as a
key process in the mitochondrial apoptotic pathway, which results
in the translocation of cytochrome c from the mitochondria
into the cytosol (9).
Subsequently, cytochrome c together with deoxyadenosine
triphosphate and apoptotic protease triggering factor-1 in the
cytosol, recruits and cleaves pro-caspase-9 into active caspase-9
(10). In turn, activated
caspase-9 cleaves effector caspases (caspase-3, −6 and −7)
(11). Therefore, cytochrome
c release is a crucial step for activating pro-caspase-9 in
apoptotic cell death.
MicroRNAs (miRNAs) are a class of small, non-coding,
single-stranded RNAs consisting of 21–23 nucleotides, which
modulate post-transcriptional regulation of target genes by
suppressing translation or inducing RNA degradation (12,13).
Previously, it was estimated that miRNAs regulate 60% of all genes
in the human genome (14). A
number of miRNAs were identified in the mammalian central nervous
system, including the brain and spinal cord, where they are
hypothesized to be key regulators of plasticity (15–17).
Additionally, a number of microRNAs have an important role in
neurodevelopment and are likely to be crucial mediators of cell
differentiation into specific tissues or organs (16). Previous studies demonstrated that
SCI may induce aberrant miRNA expression, which is involved in a
number of secondary injury responses, including inflammation,
apoptosis and oxidative stress, and regulates the expression of
their target genes (18,19). Recently, increasing evidence
suggested that numerous miRNAs regulate apoptosis by activating the
mitochondrial apoptotic pathway in various diseases (20–22).
Therefore, it was hypothesized that SCI-mediated miRNAs may promote
apoptosis by activating the mitochondrial apoptotic pathway.
In the present study, a rat SCI model was
established and microarray analysis was conducted to determine
miRNA expression profiles in spinal cord tissues. Subsequently, the
role of miR-124 in SCI-induced apoptosis was examined and the
underlying mechanisms in the mitochondrial apoptotic pathway were
investigated.
Materials and methods
Cell culture
The immortalized murine BV-2 cell line was obtained
from the Chinese Academy of Medical Science (Beijing, China) and
maintained in Dulbecco's modified Eagle's medium/F12 (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.), and 100 U/ml
penicillin and streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) in 25 cm2 culture flasks at 37°C in a
humidified atmosphere with 5% CO2.
Cell treatments
Cells were treated with different concentrations of
H2O2 (30% w/w solution; Sigma-Aldrich; Merck
KGaA) for 10 h to induce cell injury. H2O2
was administered to the cells at 50, 100, 200 and 400 µM solutions
in PBS.
Experimental animals
Adult female Sprague-Dawley rats (n=76; age, 6
weeks; weight, 200–250 g) were obtained from the Experimental
Animal Centre of Shandong University (Jinan, China). All
experimental procedures were approved by the Animal Care and Use
Committee of Shandong University. All animals were housed under
standard laboratory conditions, in a specific-pathogen-free
(22±1°C) room with relative humidity of 55–65%, under a normal
circadian cycle (12 h light/dark cycle), and had free access to
food and water. All efforts were made to minimize the number of
animals used and their suffering. Following adaptation to the novel
environment, rats were randomly assigned to four groups; sham
group, SCI group, agomir-124 group and agomir-negative control (NC)
group. For the sham group (n=6/group/time), the rats underwent a
T10 laminectomy without weight-drop injury. For the SCI group
(n=6/group/time), SCI was performed on the rats at the T10 spinal
segment impactor. For the agomir-124 group (n=6/group/time), SCI
was performed on the rats and they were treated intrathecally with
agomir-124 (1 µl/h; 20 nmol/ml) for 3 days. For the agomir-NC group
(n=6/group/time), rats were subjected to SCI and treated
intrathecally with NC agomir (1 µl/h; 20 nmol/ml). Agomir-124
(5′-CCGUAAGUGGCGCACGGAAU-3′) and NC agomir
(5′-UUCUCCGAACGUGUCACGUTT-3′) were designed and synthesized by
Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
Spinal cord injury model
Rats were intraperitoneally injected with 10%
chloral hydrate (350 mg/kg) anaesthesia, and a laminectomy was
conducted at the T9-T10 level, exposing the cord beneath without
disrupting the dura. No animals exhibited peritonitis as a result
of the intraperitoneal injection with 10% chloral hydrate.
Subsequently, the spinous processes of T8 and T11 were clamped to
stabilize the spine, and the exposed dorsal surface of the cord was
subjected to weight drop injury (10 g × 25 mm) using a New York
University (New York, NY, USA) impactor as described previously
(23). For the sham group, a T10
laminectomy without weight-drop injury was performed on the
animals. All experimental protocols and post-operative animal care
were approved by the Animal Care and Use Committee of Shandong
University.
miRNA microarray analysis
miRNA microarray analysis was conducted to evaluate
miRNA expression in the spine; rats (n=2/group) were anesthetized
at 14 days post-SCI, and a 10-mm long segment of spinal cord,
including the injury epicenter, was collected and fresh-frozen in
liquid nitrogen. Total RNA was isolated from spinal cord tissues
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and purified by the RNeasy MinElute Cleanup kit
(Qiagen GmbH, Hilden, Germany) according to the manufacturer's
protocol. Subsequent to measuring the quantity of RNA using a
NanoDrop™ ND-1000 spectrophotometer (Thermo Fisher Scientific,
Inc.), the miRNAs with Hy3 were isolated using the miRCURY™ array
labeling kit (Exiqon A/S, Vedbaek, Denmark) and hybridized on a
miRCURY™ LNA Array (version 18.0; Exiqon A/S). The Axon GenePix
4000B microarray scanner (Molecular Devices, LLC, Sunnyvale, CA,
USA) was used to scan the slides. The scanned images were analyzed
with the GenePix Pro6.0 program (Molecular Devices, LLC). The
miRNAs with intensities ≥50 in all samples were used to calculate a
normalization factor. Expressed data were normalized by median
normalization. Subsequently, the miRNAs were measured by Volcano
Plot filtering. Finally, hierarchical clustering was used to
determine the differences in the miRNA expression profiles using
MultiExperiment Viewer software (version 4.6; The Institute for
Genomic Research, Rockville, MA, USA) (24).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from spinal cord segments containing the
injury epicenter was isolated using TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. RT was performed using the TaqMan™ MicroRNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. PCR was
performed using the TaqMan™ MicroRNA Assay kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) on an Applied Biosystems 7500 Fast
Real-Time PCR system (Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 50°C for 2 min and 95°C
for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for
10 min. The following primer sequences were used: miR-124,
5′-TAAGGCACGCGGTGAATGCC-3′ (forward) and
5′-AATGATACGGCGACCACCGAACACTCTTTCCCTACACGACG-3′ (reverse); Bax,
5′-GGCTGGACACTGGACTTCCT-3′ (forward) and 5′-GGTGAGGACTCCAGCCACAA-3′
(reverse); and U6, 5′-CTCGCTTCGGCAGCACA-3′ (forward) and
5′-AACGCTTCACGAATTTGCGT-3′ (reverse). The relative expression of
miRNAs was normalized to U6. Data were analyzed using the
2−ΔΔCq method, as previously described (25). All reactions were performed in
triplicate.
Behavior assessment
The Basso, Beattie and Bresnahan (BBB) score was
used to evaluate the locomotor activity at 1, 7, 14 and 28 days
post-SCI (26), which measured
locomotor ability for 4 min. Behavioral analyses were performed by
trained investigators, who were blind to the experimental
conditions. To test hind limb locomotor function, open-field
locomotion was assessed using the BBB locomotion scale, as
previously described (23,26). The final score from two
investigators was averaged for each rat.
Assessment of lesion volume
To measure the lesion volume following SCI or
treatment with agomir-124, the rats were intraperitoneally injected
with 10% chloral hydrate (350 mg/kg) for anaesthesia. No animals
experienced peritonitis as a result of the intraperitoneal
injection of 10% chloral hydrate. They were subsequently
transcardially perfused with 0.9% NaCl (250 ml; 4°C) followed by 4%
paraformaldehyde (PFA; 500 ml) in 0.1 M PBS (pH 7.4) at 4°C for 30
min. A 1 cm segment of spinal cord containing the injury epicenter
was removed and post-fixed in the same fixative at 4°C for 24 h.
The tissue blocks were embedded in paraffin following fixation.
Transverse sections (10 µm thickness) were taken through the width
of the spinal lesion site, and mounted onto Superfrost Plus Slides
(Thermo Fisher Scientific, Inc.). Samples (every 40th section of
the lesion site) were stained with 0.5% cresyl-violet acetate for 1
h at room temperature and imaged using an Olympus BH-2 microscope
(magnification, ×200; Olympus Corporation, Tokyo, Japan). The
lesion area and spared tissue area were outlined and quantified
using Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA) software. Spared tissue was reported as the remaining areas,
where normal spinal cord structure was preserved. The section with
the lowest percentage of spared tissue was defined as the injury
epicenter. Transverse sections, with intervals of 400 µm rostral
and caudal to this lesion epicenter, were analyzed up to a distance
of 1,600 µm away from the lesion epicenter for percentage tissue
sparing.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) staining
To detect apoptosis, serial spinal cord sections (10
µm thickness), obtained in the above experimental procedure, were
further subjected to TUNEL staining. The TUNEL Apoptosis Assay kit
(cat. no. 11684817910; Roche Diagnostics GmbH, Mannheim, Germany),
was used to detect apoptotic cells in spinal cord sections,
according to the manufacturer's protocol. The tissue sections were
immersed in the TUNEL reaction mixture for 1 h at 37°C.
Subsequently, nuclei were stained with 1 µg/ml DAPI at room
temperature for 10 min. Sections were mounted in Fluoromount™
aqueous mounting medium (Sigma-Aldrich; Merck KGaA). Quantification
was conducted by counting the number of positive cells in 10
randomly chosen fields within each slide with a Leica CM 1850
fluorescent microscope (magnification, ×200). Apoptosis was
determined by measuring the apoptotic cells and the total
cells.
Luciferase reporter assay
The potential binding site between Bax and miR-124
was searched in TargetScan (http://www.targetscan.org). The miR-124
mimics/inhibitor and corresponding NC were designed and synthesized
by Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The fragment of
the 3′-untranslated region (UTR) of Bax [wild-type (wt) or mutant
(mut)] was amplified and cloned into the pMIR-REPORT luciferase
vector (Ambion; Thermo Fisher Scientific, Inc.). Site-directed
mutagenesis of the Bax 3′-UTR at the putative miR-124 binding site
was performed using a QuikChange II Site-Directed Mutagenesis kit
(Agilent Technologies, Inc., Santa Clara, CA, USA). Subsequently,
BV-2 cells at a density of 2×105 cells/well were seeded
into 24-well plates and co-transfected with 0.8 µg pMIR-Bax-3′-UTR
or pMIR-Bax-mut-3′-UTR, 50 nM miR-124 mimics/inhibitor or
corresponding NC using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Renilla
luciferase was used to normalize the cell number 48 h after
transfection. Luciferase activity was measured using the Dual-Light
luminescent reporter gene assay (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Each assay was repeated three times.
Western blot analysis
Segments of spinal cord (1 cm) were isolated using
the lesion site as the epicenter, and protein was extracted from
spinal cord tissues or BV-2 cells using ice cold
radioimmunoprecipitation assay buffer (Roche Diagnostics GmbH) as
previously described (19).
Samples were sonicated using a W-385 sonicator (Qsonica LLC,
Newtown, CT, USA) at room temperature, 30 sec intervals and power
level six (20 kHz) for 15 min, then centrifuged at 20,000 × g at
4°C for 30 min. Supernatants were collected and stored at −80°C.
The protein expression level of the supernatant was determined
using a bicinchoninic acid (BCA) assay (Beyotime Institute of
Biotechnology, Haimen, China). Samples (30 µg protein) were
electrophoresed onto 12% SDS/PAGE (Sigma-Aldrich; Merck KGaA), and
transferred to polyvinylidene difluoride membranes (EMD Millipore,
Billerica, MA, USA). Subsequent to blocking with 5% non-fat milk at
4°C overnight, the membranes were incubated at 4°C overnight with
primary antibodies against Bax (1:1,000; cat. no. 2772; Cell
Signaling Technology, Inc., Danvers, MA, USA), Bcl-2 (1:1,000; cat.
no. sc-492; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
cleaved-caspase-9 (1:1,000; cat. no. 9509; Cell Signaling
Technology, Inc.), pro-caspase-9 (1:1,000; cat. no. sc-56073; Santa
Cruz Biotechnology, Inc.), cleaved-caspase-3 (1:1,000; cat. no.
9661; Cell Signaling Technology, Inc.) and pro-caspase-3 (1:1,000;
cat. no. 9662s; Cell Signaling Technology, Inc.). β-actin (1:1,000;
cat. no. A1978; Sigma-Aldrich; Merck KGaA) was used as an internal
control. The membrane was then incubated at 4°C for 90 min with
anti-mouse IgG horseradish peroxidase-conjugated secondary antibody
(1:200; cat. no. 7076; Cell Signaling Technology, Inc.). Protein
expression was detected using Amersham™ ECL™ Western Blotting
Detection Reagents (cat. no. RPN2106; GE Healthcare Life Sciences,
Little Chalfont, UK). The protein bands were quantified using a
PhosphorImager and ImageQuant 5.2 software (GE Healthcare Life
Sciences).
Immunohistochemistry staining
The spinal cords were intracardially perfused with
0.9% NaCl followed by ice-cold 4% PFA in 0.1 M PBS (pH 7.4) at 4°C
for 30 min. A 10 mm segment of spinal cord encompassing the injury
site was subsequently harvested. Following fixation, the tissue
blocks were embedded in paraffin, and sectioned at 5 µm thickness.
Paraffin-embedded sections were deparaffinized with xylene at 50°C
for 3 min and hydrated through a graded alcohol series (100, 95,
80, 70 and 40%). Epitope unmasking was performed by microwave
irradiation in 10 mM citrate buffer (pH 6.0) twice for 5 min at 800
W prior to cooling for 30 min. Subsequently, endogenous peroxidase
was inactivated by incubation in 3% H2O2 for
15 min at room temperature. Subsequent to rinsing with 0.01 M PBS,
the sections were blocked with 10% fetal bovine serum (cat. no.
F4135; Sigma-Aldrich; Merck KGaA) in PBS at room temperature for 30
min and incubated overnight at 4°C with primary
anti-cleaved-caspase-3 antibodies (1:100; cat. no. 9661; Cell
Signaling Technology, Inc.). Subsequently, the sections were
incubated with anti-mouse IgG horseradish peroxidase-conjugated
secondary antibodies (1:100; cat. no. 7076; Cell Signaling
Technology, Inc.) at room temperature for 30 min. Finally, the
immunoreactivity was visualized by staining with
3,3′-diaminobenzidine at room temperature for 3 min, covered with a
coverslip. Images were photographed using an Olympus BX51 light
microscope (magnification, ×200; Olympus Corporation). Aperio
ImageScope version 9 was used to quantify immunohistochemistry
staining (Leica Biosystems Nussloch GmbH, Nussloch, Germany).
Apoptosis analysis by flow
cytometry
BV-2 cells (1×106) were harvested and
washed in ice-cold PBS, and fixed in 70% ice-cold ethanol in PBS
for 30 min. To measure apoptosis, the fluorescein isothiocyanate
(FITC) Annexin V Apoptosis Detection kit I (BD Bioscience, Franklin
Lakes, NJ, USA) was used according to the manufacturer's
instructions. Cells were washed twice in PBS, resuspended in
Annexin V binding buffer, and incubated with 5 µl Annexin V-FITC
and 1 µl propidium iodide (PI). The stained cells were analyzed
using a flow cytometer (EPICS XL-MCL FACScan; BD Biosciences,
Franklin Lakes, NJ, USA). The MultiCycle Software version 5.0
(Phoenix Flow Systems, San Diego, CA, USA) for Windows 7 (Microsoft
Corporation, Redmond, WA, USA) was used to analyze the experimental
data.
Caspase-3 activity
Caspase-3 activity was determined using a
colorimetric activity assay kit, according to the manufacturer's
protocol (BioVison, Inc., Milpitas, CA, USA). BV-2 cells were
harvested by centrifugation at 1,000 × g for 10 min at 4°C and
incubated in lysis buffer on ice for 15 min. Subsequently, the
lysate was centrifuged at 20,000 × g for 15 min at 4°C, the protein
concentration was measured using the BCA Protein Assay kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. The lysates (10 µl) were incubated with 10
µl 0.2 mM Ac-DEVD-pNA in 80 µl reaction buffer at 37°C for 2 h. The
samples were measured with a microplate reader (Model 680; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at an absorbance of 405
nm.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 18.0; SPSS, Inc., Chicago, IL, USA) or GraphPad
Prism (version 6.0; GraphPad Software, Inc., La Jolla, CA, USA).
Experiments were conducted in triplicate and data are presented as
the mean ± standard deviation. The correlation between Bax and
miR-124 expression was analyzed using Spearman's rank correlation
coefficient. Differences among multiple groups were analyzed by
one-way analysis of variance with Tukey's post hoc test, and
differences between two groups were analyzed by Student's t-test.
P<0.05 was used to indicate a statistically significant
difference.
Results
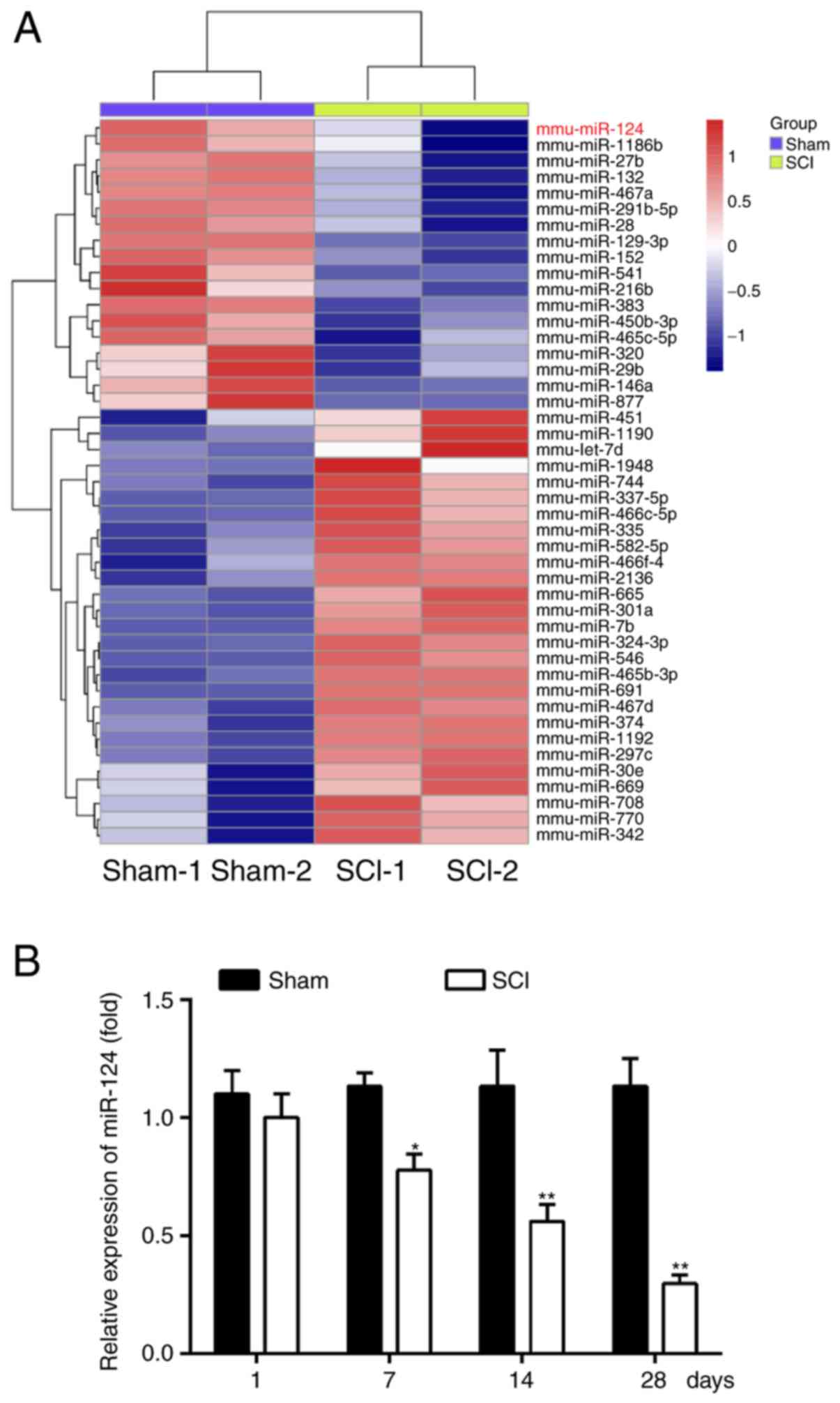
miRNA aberrant expression in rats
following SCI
To examine the potential involvement of miRNA in
SCI, a rat model of SCI was established and microarray analysis was
performed to determine miRNA expression levels in spinal cord
tissues. It was observed that a large number of miRNAs were altered
at 14 days post-SCI and that miR-124 was one of the miRNAs most
significantly downregulated compared with the sham group (Fig. 1A). A previous study observed that
miR-124 is relatively abundant in the spinal cord neurons, brain
and retina, and serves a critical role in neural development and
differentiation (27). Therefore,
RT-qPCR was performed to further verify the miR-124 expression
level in spinal cord tissues at 1, 7, 14 and 28 days post-SCI and
it was observed that it was additionally significantly
downregulated in the SCI group compared with the sham group between
7 and 28 days (P<0.05; Fig.
1B). These results suggested that SCI results in miRNA aberrant
expression in spinal cord tissues and miR-124 may regulate the
pathogenesis in rats with SCI.
Overexpression of miR-124 improves
functional recovery and inhibits apoptosis following SCI
To investigate the role of miR-124 in rats with SCI,
a rat SCI model was established and treated intrathecally with
agomir-124. The overexpression effect of agomir-124 in the spinal
cord was assessed using RT-qPCR. As demonstrated in Fig. 2A, the relative expression of
miR-124 was significantly upregulated at 7–28 days compared with
the agomir-NC (P<0.05) and was maximal at 14 days. Subsequently,
the BBB rating scale was used to evaluate motor function in the rat
SCI model following treatment intrathecally with agomir-124. The
results demonstrated that overexpression of miR-124 in the SCI +
agomir-124 group significantly improved motor function from 7 days
compared with the SCI group (P<0.05; Fig. 2B). Cresyl violet staining
demonstrated that the spared tissue in SCI + agomir-124 group was
significantly increased compared with the SCI group (P<0.05;
Fig. 2C), suggesting agomir-124
reduces lesion volume in spinal cord tissues following SCI. It was
additionally investigated whether miR-124 modulates
apoptosis-associated protein (cleaved-caspase-3) expression using
immunohistochemistry staining in spinal cord tissues following SCI.
As demonstrated in Fig. 2D, SCI
resulted in a significant upregulation of cleaved-caspase-3 in
spinal cord tissues compared with the shame group; whereas,
agomir-124 significantly inhibited the cleaved-caspase-3 expression
(P<0.01; Fig. 2D). Furthermore,
neuronal cell apoptosis was analyzed using TUNEL staining. It was
identified that the TUNEL-positive cells were significantly
increased in the SCI group compared with the sham group
(P<0.01); however, agomir-124 significantly decreased the number
of TUNEL-positive cells in the SCI + agomir-124 group compared with
the SCI group (P<0.01; Fig.
2E). These results suggested that agomir-124 improves
functional recovery, reduces lesion volume and suppresses apoptosis
in rats following SCI.
 | Figure 2.Agomir-124 alleviates SCI. (A)
Relative expression level of miR-124 was detected by reverse
transcription-quantitative polymerase chain reaction at 1, 7, 14
and 28 days in rats SCI model following intrathecal treatment with
agomir-124 (n=6/group/time). *P<0.05, **P<0.01 vs. respective
agomir-NC. (B) BBB score was used to evaluate locomotor activity at
1, 3, 7, 14 and 28 days post-SCI following agomir-124 treatment
(n=6/group/time). (C) Lesion analysis at the rostral (−) and caudal
(+) margins from the lesion epicenter was performed using cresyl
violet staining 14 days after the SCI model was established
(n=6/group/time). *P<0.05, **P<0.01 vs. SCI + agomir-124
group. (D) Immunohistochemistry staining was used to detect
cleaved-caspase-3 expression in spinal cord tissues following SCI
(magnification, ×200). *P<0.05, **P<0.01 vs. sham group;
##P<0.01. (E) Terminal deoxynucleotidyl transferase
dUTP nick end labeling staining was used to analyze neuronal
apoptosis at 14 days post-SCI (n=6/group/time) (magnification,
×200). Data are presented as the mean ± standard deviation of three
individual experiments. SCI, spinal cord injury; miR, microRNA; NC,
negative control; BBB, Basso, Beattie and Bresnahan. |
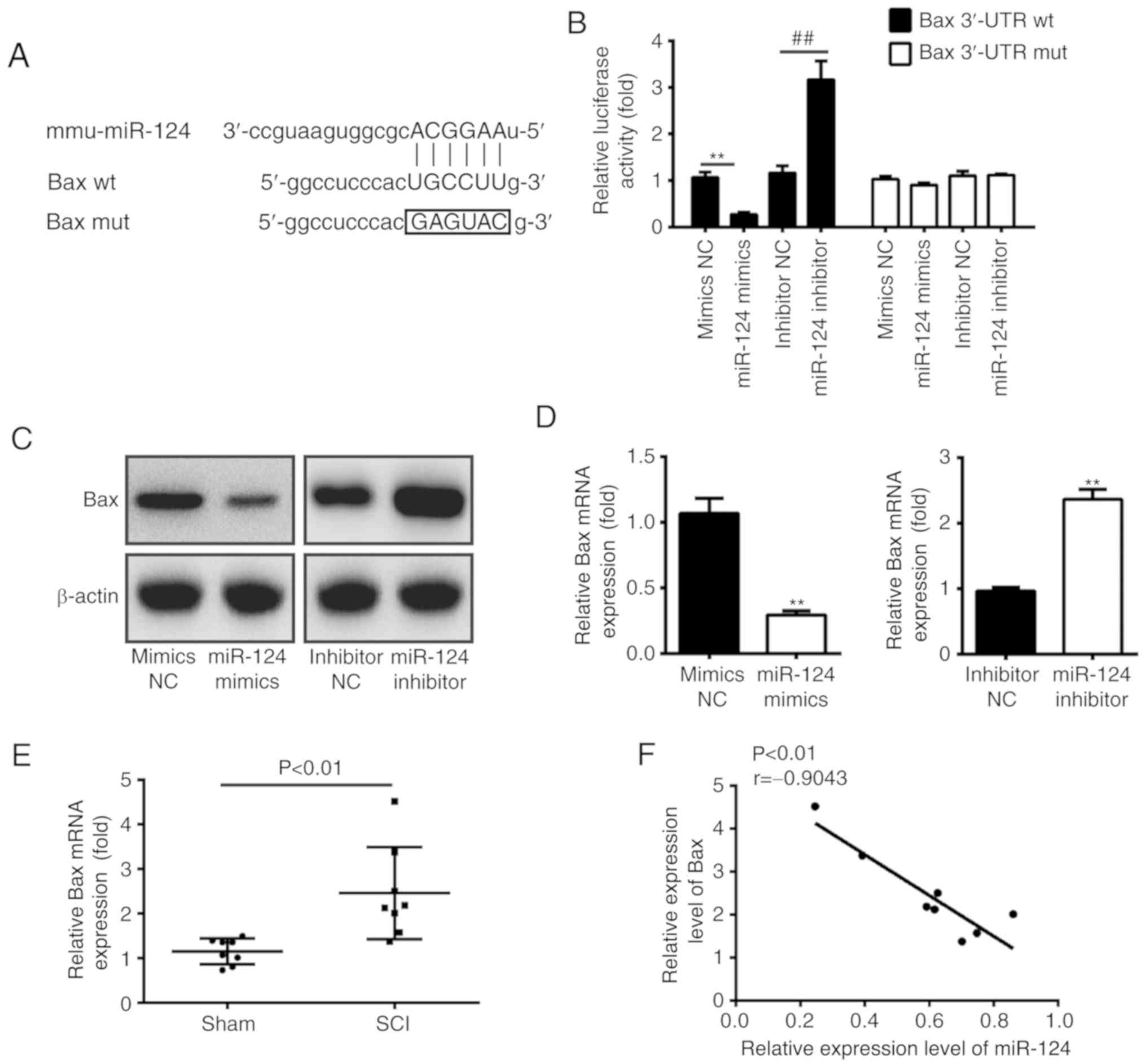
miR-124 suppresses Bax expression by
targeting its 3′-UTR in BV-2 cells
A previous study suggested that miR-124 exerts
protective effects against neuron apoptosis in rats with thyroid
hypofunction via downregulation of pro-apoptosis proteins Bax and
caspase-3 (28). Therefore, it was
hypothesized that miR-124 inhibits neuronal cell apoptosis in rats
with SCI via downregulation of Bax. Bioinformatics analysis was
conducted to predict the putative targets of miR-124, and it was
observed that Bax may be a target gene of miR-124 with the target
site located in the 3′-UTR (Fig.
3A). To validate this bioinformatics predication, the wild-type
(wt) or mutant (mut) type of Bax-3′-UTR was constructed, which was
inserted into the firefly luciferase expressing the vector
pMIR-REPORT. To investigate the pathologic factors following SCI,
the BV-2 cell line was used as it is reported to share various
characteristics with primary microglia (29). The reporter plasmids were
co-transfected with either miR-124 mimics/inhibitor or
mimics/inhibitor NC in BV-2 cells, and the luciferase activity was
measured. Compared with the mimics NC, the miR-124 mimics
significantly inhibited the luciferase activity in the presence of
the wt 3′-UTR; whereas, the miR-124 inhibitor significantly
increased the luciferase activity compared with the inhibitor NC
(P<0.01; Fig. 3B).
Additionally, miR-124 did not suppress the luciferase activity of
the reporter vector containing 3′-UTR of Bax with mutations in the
miR-124-binding site (Fig. 3B). To
further verify that Bax is negatively regulated by miR-124, western
blotting and RT-qPCR analysis was performed to detect the protein
and mRNA expression level for Bax, respectively. It was observed
that overexpression of miR-124 decreased the expression of Bax at
the mRNA and protein expression level in BV-2 cells (Fig. 3C and D). Conversely, downregulation
of miR-124 increased the Bax expression at the mRNA and protein
expression level (Fig. 3C and D).
Furthermore, RT-qPCR analysis was used to determine the Bax mRNA
expression level in spinal cord tissues (n=8). It was demonstrated
that the Bax mRNA expression level was significantly increased in
the SCI group compared with the sham group (P<0.01; Fig. 3E). The correlation analysis
revealed a strong negative correlation between Bax and miR-124
expression in spinal cord tissues (r=−0.9043; P<0.01; Fig. 3F). Collectively, these results
suggested that miR-124 suppressed Bax expression by directly
targeting its 3′-UTR in BV-2 cells, suggesting that Bax may be a
target of miR-124 in spinal cord tissues.
Overexpression of Bax inhibits the
protective effect of miR-124 on H2O2-treated
BV-2 cells
A previous study demonstrated that reactive oxygen
species have key roles in SCI as they may activate various pathways
of apoptosis, and H2O2-treated BV-2 cells
widely serve as a cellular model of SCI to examine the pathologic
factors following SCI (30). In
the present study, murine BV-2 cells were treated with
H2O2 (50–400 µM) for 10 h, and the miR-124
expression was measured using RT-qPCR analysis. As demonstrated in
Fig. 4A, treatment with
H2O2 results in the significant
downregulation of miR-124 in BV-2 cells, and miR-124 downregulation
was dose-dependent at H2O2 concentrations of
50–200 µM (P<0.05). Furthermore, RT-qPCR and western blot
analysis was performed to evaluate the overexpression efficiency of
miR-124 or Bax, respectively. The results demonstrated that miR-124
and Bax was upregulated in BV-2 cells treated with miR-124 mimics
and pc-DNA-Bax, respectively (Fig. 4B
and C). In addition, it was observed that overexpression of
miR-124 decreased the Bax protein expression level in
H2O2-treated BV-2 cells; whereas, the
restoration of Bax by pc-DNA-Bax inhibited the effect of
miR-124-reduced Bax on H2O2-treated BV-2
cells (Fig. 4D). Notably, the
present results demonstrated that overexpression of miR-124
significantly decreased the portion of apoptotic cells in
H2O2-treated BV-2 cells compared with the
control; however, the protective effect of miR-124 on BV-2 cells
was significantly decreased with Bax overexpression (P<0.01;
Fig. 4E). Furthermore,
upregulation of Bax inhibited the effect of miR-124-inhibited
caspase-3 activity in H2O2-treated BV-2 cells
(P<0.01; Fig. 4F). Taken
together, these results suggested that miR-124 suppresses cell
apoptosis by inhibiting Bax in H2O2-treated
BV-2 cells.
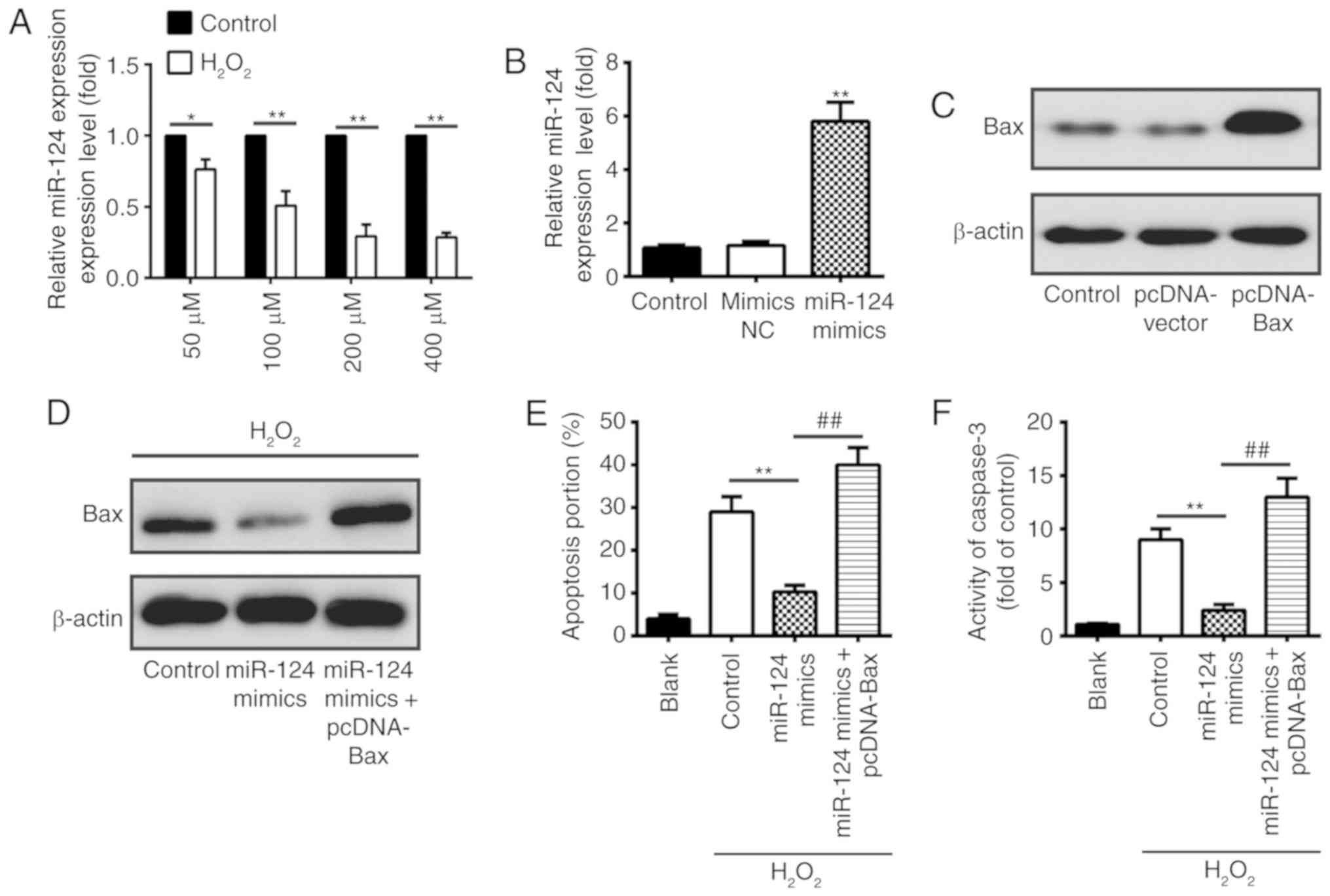 | Figure 4.Restoration of Bax inhibits the
protective effect of miR-124 in H2O2-treated
BV-2 cells. (A) BV-2 cells were treated with
H2O2 (50–400 µM) for 10 h, and the miR-124
expression was measured using RT-qPCR analysis. *P<0.05,
**P<0.01 vs. respective control group. (B) RT-qPCR analysis was
used to measure the miR-124 expression level in BV-2 cells
transfected with miR-124 mimics or mimics NC. **P<0.01 vs.
mimics NC group. (C) BV-2 cells were transfected with pc-DNA-Bax or
pc-DNA-vector, and Bax expression was determined by western
blotting. (D) Western blotting was used to detect Bax expression in
H2O2-treated BV-2 cells transfected with
miR-124 mimics or co-transfected with miR-124 mimics and
pc-DNA-Bax. Following treatment with H2O2,
cells were transfected with miR-124 mimics or co-transfected with
miR-124 mimics and pc-DNA-Bax, and (E) apoptotic cells and (F)
caspase-3 activity were measured using flow cytometry and a
colorimetric activity assay, respectively. **P<0.01,
##P<0.01. Data are presented as the mean ± standard
deviation of three individual experiments. Bax, apoptosis regulator
BAX; miR, microRNA; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; NC, negative control. |
Overexpression of miR-124 blocks the
mitochondrial apoptotic pathway
Bax, is a Bcl-2 family protein (31), which has been identified to possess
a pro-apoptotic effect and causes the release of cytochrome
c (32,33). In the apoptotic process, the
upregulation of Bax may promote apoptosis by inhibiting the
anti-apoptotic protein Bcl-2 (34). The mitochondrial apoptotic pathway
and death receptor apoptotic pathway are two important apoptotic
pathways (35,36). Bax is a key molecule in the
regulation of the mitochondrial apoptotic pathway, which is able to
form homodimers on the mitochondrial membrane and open the
permeability transition pore on the mitochondrial membrane to
promote the release of cytochrome c from mitochondria into
the cytoplasm (37). In the
mitochondrial apoptotic pathway, cytochrome c may active the
caspase-9-mediated cascade amplification reaction, which in turn
processes pro-caspase-3 to generate active caspase-3 (38). To investigate whether miR-124 may
regulate the mitochondrial apoptotic pathway by suppressing
apoptotic-associated protein expression in rats following SCI,
western blotting was used to detect the Bax, Bcl-2,
cleaved-caspase-9, pro-caspase-9, cleaved-caspase-3 and
pro-caspase-3 expression levels in spinal cord tissues. It was
observed that Bax, cleaved-caspase-9 and cleaved-caspase-3 were
significantly upregulated, and Bcl-2, pro-caspase-9 and
pro-caspase-3 were significantly downregulated in the SCI +
agomir-NC group compared with the sham group. However,
overexpression of miR-124 significantly decreased the Bax,
cleaved-caspase-9 and cleaved-caspase-3 expression levels and
significantly increased the Bcl-2, pro-caspase-9 and pro-caspase-3
expression levels in the SCI + agomir-124 group compared with the
SCI + agomir-NC group (P<0.01; Fig.
5). These results suggested that overexpression of miR-124 may
block the mitochondrial apoptotic pathway by suppressing Bax
expression in rats following SCI.
 | Figure 5.MicroRNA-124 blocks the mitochondrial
apoptotic pathway. Rats were subjected to SCI and treated
intrathecally with agomir-124 or agomir-NC, and western blot
analysis was conducted to determine the Bax, Bcl-2,
cleaved-caspase-9, pro-caspase-9, cleaved-caspase-3 and
pro-caspase-3 expression levels in spinal cord tissues. β-actin was
used as an internal control. Data are presented as the mean ±
standard deviation of three individual experiments. **P<0.01 vs.
SCI + agomir-NC group; #P<0.05,
##P<0.01 vs. sham group. SCI, spinal cord injury; NC,
negative control; Bax, apoptosis regulator BAX; Bcl-2, B-cell
lymphoma-2. |
Discussion
SCI induces widespread molecular and biochemical
alterations, which are characterized by the production of free
radicals, inflammatory activation, axonal plasticity and neuronal
cell death (39,40). Previously, certain studies have
clarified that SCI may induce miRNA aberrant expression and the
dysregulated miRNAs may influence SCI pathophysiology and
functional outcome (18,41). However, the functional significance
of the unique role of miRNAs has yet to be elucidated in SCI. In
the present study, a rat SCI model was established and a miRNA
microarray analysis was performed to determine miRNA expression
profiles at different times post-SCI. It was observed that SCI
induces dysregulated miRNA expression in rats with SCI and miR-124
was one of the most significantly downregulated miRNAs in spinal
cord tissues. Furthermore, the present results demonstrated that
overexpression of miR-124 alleviates SCI by improving functional
recovery, reducing lesion volume and suppressing apoptosis. In
addition, it was demonstrated that miR-124 inhibits Bax expression
by directly targeting its 3′-UTR in BV-2 cells. The overexpression
of Bax inhibits the protective effect of miR-124 on BV-2 cells
treated with H2O2. Notably, the present
results demonstrated that miR-124 may exert its protective effect
on SCI by blocking the mitochondrial apoptotic pathway.
miR-124 is one of the most abundantly expressed
miRNAs in the nervous system, including in the brain and spinal
cord (42–44). Mammalian miR-124 was first detected
in differentiating neurons and persists in mature neurons,
suggesting that miR-124 serves key roles in neural development
(43,45). Previous studies demonstrated that
the expression level of miR-124 was decreased in the brain and
spinal cord tissues of rats following SCI, and its expression may
affect the severity of SCI (46,47).
Consistent with these previous studies, the present results
demonstrated that SCI alters miRNA expression and miR-124 was one
of the most significantly downregulated miRNAs in the SCI group. A
previous study reported that miR-124 exerts protective effects on
SCI via regulation of neural stem cells (48). In the present study, the present
results demonstrated that overexpression of miR-124 may improve
functional recovery, reduce lesion volume and suppress apoptosis in
rats, following SCI. These results suggested that miR-124 exerted a
protective effect on rats with SCI and is a potential candidate
target for SCI therapy. However, the molecular mechanism requires
further clarification.
Mitochondria serve an important role in the
apoptotic process by releasing apoptogenic molecules, including
cytochrome c (49,50). Bax has been identified to have a
pro-apoptotic effect, which may open the permeability transition
pore on the mitochondrial membrane to trigger the release of
cytochrome c from mitochondria into the cytoplasm (37). Cytochrome c may trigger the
caspase-9-molulated cascade amplification reaction in the
mitochondrial apoptotic pathway, which in turn processes
pro-caspase-3 to generate active caspase-3 (38). In a previous study, it was
identified that miR-124 exhibited a protective effect against
neuron apoptosis in rats with thyroid hypofunction by decreasing
Bax expression (28). In the
present study, it was demonstrated that miR-124 suppresses Bax
expression by targeting its 3′-UTR in BV-2 cells. Furthermore, the
correlation analysis demonstrated a negative correlation between
Bax and miR-124 expression in the spinal cord tissues of rats with
SCI, suggesting that Bax may be a target of miR-124 in vivo.
Therefore, it was hypothesized that miR-124 may regulate the
mitochondrial apoptotic pathway by inhibiting Bax expression in
rats with SCI. The present results suggested that upregulation of
miR-124 decreased the Bax, cleaved-caspase-9 and cleaved-caspase-3
expression levels, and increased the Bcl-2, pro-caspase-9 and
pro-caspase-3 expression levels in the spinal cord tissues of rats
with SCI. Collectively, these results suggested that miR-124 may
exert its therapeutic effects on SCI by blocking the mitochondrial
apoptotic pathway.
However, there are some limitations in the present
study. For example, only miR-124 was explored, whereas other miRNAs
may also be relevant for the pathogenesis of SCI. Additionally, the
number of experimental animals was limited. In the future, further
systematic and in-depth studies investigating the pathogenesis of
SCI will be conducted.
In conclusion, the present results demonstrated that
SCI induces miRNA aberrant expression in a rat SCI model and
miR-124 was one of the most significantly downregulated miRNAs in
spinal cord tissues. In addition, it was observed that
overexpression of miR-124 is able to improve functional recovery,
reduce lesion volume and suppress neuronal cell apoptosis in rats
following SCI. Notably, the present results demonstrated that
miR-124 is able to target Bax in BV-2 cells and may exert its
protective effect on SCI by blocking the mitochondrial apoptotic
pathway, suggesting that miR-124 may serve as a promising novel
therapeutic target for the treatment of SCI.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Shandong Province (grant no.
ZR2015YL034).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YZ conceived the study and provided experimental
materials. ZX and KZ performed the experiments and wrote the paper.
ZX, KZ, QW and YZ analyzed the data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Animal Care and Use Committee of Shandong University (Jinan,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen Y, Cao S, Xu P, Han W, Shan T, Pan J,
Lin W, Chen X and Wang X: Changes in the expression of miR-34a and
its target genes following spinal cord injury in rats. Med Sci
Monit. 22:3981–3993. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu W, Wang H, Liu Z, Liu Y, Wang R, Luo X
and Huang Y: Neuroprotective effects of lycopene in spinal cord
injury in rats via antioxidative and anti-apoptotic pathway.
Neurosci Lett. 642:107–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thuret S, Moon LD and Gage FH: Therapeutic
interventions after spinal cord injury. Nat Rev Neurosci.
7:628–643. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blight AR: Miracles and molecules-progress
in spinal cord repair. Nat Neurosci. 5 (Suppl 1):S1051–S1054. 2002.
View Article : Google Scholar
|
|
5
|
Rabchevsky AG, Patel SP and Springer JE:
Pharmacological interventions for spinal cord injury: Where do we
stand? How might we step forward? Pharmacol Ther. 132:15–29.
2011.PubMed/NCBI
|
|
6
|
Kawabata H, Setoguchi T, Yone K, Souda M,
Yoshida H, Kawahara K, Maruyama I and Komiya S: High mobility group
box 1 is upregulated after spinal cord injury and is associated
with neuronal cell apoptosis. Spine (Phila Pa 1976). 35:1109–1115.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Huang C-Y, Zheng RL, Cui KR and Li
JF: Hydrogen peroxide induces apoptosis in human hepatoma cells and
alters cell redox status. Cell Biol Int. 24:9–23. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reuter S, Eifes S, Dicato M, Aggarwal BB
and Diederich M: Modulation of anti-apoptotic and survival pathways
by curcumin as a strategy to induce apoptosis in cancer cells.
Biochem Pharmacol. 76:1340–1351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balaban RS, Nemoto S and Finkel T:
Mitochondria, oxidants, and aging. Cell. 120:483–495. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li P, Nijhawan D and Wang X: Mitochondrial
activation of apoptosis. Cell 116 (2 Suppl). S57–S61. 2004.
|
|
11
|
Nicholson DW and Thornberry NA: Caspases:
Killer proteases. Trends Biochem Sci. 22:299–306. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krichevsky AM: MicroRNA profiling: From
dark matter to white matter, or identifying new players in
neurobiology. ScientificWorldJournal. 7:155–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kosik KS: The neuronal microRNA system.
Nat Rev Neurosci. 7:911–920. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bak M, Silahtaroglu A, Møller M,
Christensen M, Rath MF, Skryabin B, Tommerup N and Kauppinen S:
MicroRNA expression in the adult mouse central nervous system. RNA.
14:432–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu NK, Wang XF, Lu QB and Xu XM: Altered
microRNA expression following traumatic spinal cord injury. Exp
Neurol. 219:424–429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu G, Keeler BE, Zhukareva V and Houlé
JD: Cycling exercise affects the expression of apoptosis-associated
microRNAs after spinal cord injury in rats. Exp Neurol.
226:200–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun Y, Su Q, Li L, Wang X, Lu Y and Liang
J: MiR-486 regulates cardiomyocyte apoptosis by p53-mediated BCL-2
associated mitochondrial apoptotic pathway. BMC Cardiovasc Disord.
17:1192017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Makhdoumi P, Roohbakhsh A and Karimi G:
MicroRNAs regulate mitochondrial apoptotic pathway in myocardial
ischemia-reperfusion-injury. Biomed Pharmacother. 84:1635–1644.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Jiao Y, Cui L and Jiang L: miR-30
functions as an oncomiR in gastric cancer cells through regulation
of P53-mediated mitochondrial apoptotic pathway. Biosci Biotechnol
Biochem. 81:119–126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yune TY, Lee JY, Jung GY, Kim SJ, Jiang
MH, Kim YC, Oh YJ, Markelonis GJ and Oh TH: Minocycline alleviates
death of oligodendrocytes by inhibiting pro-nerve growth factor
production in microglia after spinal cord injury. J Neurosci.
27:7751–7761. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saeed AI, Sharov V, White J, Li J, Liang
W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et
al: TM4: A free, open-source system for microarray data management
and analysis. Biotechniques. 34:374–378. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu JY, Chung KH, Deo M, Thompson RC and
Turner DL: MicroRNA miR-124 regulates neurite outgrowth during
neuronal differentiation. Exp Cell Res. 314:2618–2633. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shao Q, Jiang W and Jin Y: MiR-124 effect
in neurons apoptosis in newborn rat with thyroid hypofunction. Int
J Clin Exp Pathol. 8:14465–14471. 2015.PubMed/NCBI
|
|
29
|
Yu DS, Lv G, Mei XF, Cao Y, Wang YF, Wang
YS and Bi YL: MiR-200c regulates ROS-induced apoptosis in murine
BV-2 cells by targeting FAP-1. Spinal Cord. Dec 2–2014.(Epub ahead
of print).
|
|
30
|
Hu F, Min J, Cao X, Liu L, Ge Z, Hu J and
Li X: MiR-363-3p inhibits the epithelial-to-mesenchymal transition
and suppresses metastasis in colorectal cancer by targeting Sox4.
Biochem Biophys Res Commun. 474:35–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nishimura R, Tabata K, Arakawa M, Ito Y,
Kimura Y, Akihisa T, Nagai H, Sakuma A, Kohno H and Suzuki T:
Isobavachalcone, a chalcone constituent of Angelica keiskei,
induces apoptosis in neuroblastoma. Biol Pharm Bull. 30:1878–1883.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He J, Xiao Y, Casiano CA and Zhang L: Role
of mitochondrial cytochrome c in cocaine-induced apoptosis
in coronary artery endothelial cells. J Pharmacol Experimental
Ther. 295:896–903. 2000.
|
|
33
|
Liu H, Qin CK, Han GQ, Xu HW, Ren WH and
Qin CY: Synthetic chenodeoxycholic acid derivative, HS-1200,
induces apoptosis of human hepatoma cells via a mitochondrial
pathway. Cancer Lett. 270:242–249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Noguchi K, Kitanaka C, Yamana H, Kokubu A,
Mochizuki T and Kuchino Y: Regulation of c-Myc through
phosphorylation at Ser-62 and Ser-71 by c-Jun N-terminal kinase. J
Biol Chem. 274:32580–32587. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Klapsinou E, Argyri E, Panotopoulou E,
Daskalopoulou D, Patsouris E, Nonni A, Lazaris AC and Thomopoulou
GH: Bax and Bak expression in cervical smears of women with low-and
high-risk HPV types: A study of 120 cases. J Cytol. 32:223–229.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Su CC, Lee KI, Chen MK, Kuo CY, Tang CH
and Liu SH: Cantharidin induced oral squamous cell carcinoma cell
apoptosis via the JNK-regulated mitochondria and endoplasmic
reticulum stress-related signaling pathways. PLoS One.
11:e01680952016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu Z, Chen H, Zheng XM and Chen ML:
Experimental study on the apoptosis of cervical cancer Hela cells
induced by juglone through c-Jun N-terminal kinase/c-Jun pathway.
Asian Pac J Trop Med. 10:572–575. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zou H, Li Y, Liu X and Wang X: An
APAF-1.cytochrome c multimeric complex is a functional
apoptosome that activates procaspase-9. J Biol Chem.
274:11549–11556. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Giovanni SD, Knoblach SM, Brandoli C, Aden
SA, Hoffman EP and Faden AI: Gene profiling in spinal cord injury
shows role of cell cycle neuronal death. Ann Neurol. 53:454–468.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
De Biase A, Knoblach SM, Di Giovanni S,
Fan C, Molon A, Hoffman EP and Faden AI: Gene expression profiling
of experimental traumatic spinal cord injury as a function of
distance from impact site and injury severity. Physiol Genomics.
22:368–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu JZ, Huang JH, Zeng L, Wang G, Cao M and
Lu HB: Anti-apoptotic effect of microRNA-21 after contusion spinal
cord injury in rats. J Neurotrauma. 30:1349–1360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lagos-Quintana M, Rauhut R, Yalcin A,
Meyer J, Lendeckel W and Tuschl T: Identification of
tissue-specific MicroRNAs from mouse. Curr Biol. 12:735–739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Krichevsky AM, King KS, Donahue CP,
Khrapko K and Kosik KS: A microRNA array reveals extensive
regulation of microRNAs during brain development. RNA. 9:1274–1281.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sempere LF, Freemantle S, Pitha-Rowe I,
Moss E, Dmitrovsky E and Ambros V: Expression profiling of
mammalian microRNAs uncovers a subset of brain-expressed microRNAs
with possible roles in murine and human neuronal differentiation.
Genome Biol. 5:R132004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Miska EA, Alvarez-Saavedra E, Townsend M,
Yoshii A, Sestan N, Rakic P, Constantine-Paton M and Horvitz HR:
Microarray analysis of microRNA expression in the developing
mammalian brain. Genome Biol. 5:R682004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhao Y, Zhang H, Zhang D, Yu CY, Zhao XH,
Liu FF, Bian GL, Ju G and Wang J: Loss of microRNA-124 expression
in neurons in the peri-lesion area in mice with spinal cord injury.
Neural Regen Res. 10:1147–1152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Song JL, Zheng W, Chen W, Qian Y, Ouyang
YM and Fan CY: Lentivirus-mediated microRNA-124 gene-modified bone
marrow mesenchymal stem cell transplantation promotes the repair of
spinal cord injury in rats. Exp Mol Med. 49:e3322017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu W, Wang X, Li P, Qin K and Jiang X:
miR-124 regulates neural stem cells in the treatment of spinal cord
injury. Neurosci Lett. 529:12–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Malhotra R, Lin Z, Vincenz C and Brosius
FC III: Hypoxia induces apoptosis via two independent pathways in
Jurkat cells: Differential regulation by glucose. Am J Physiol Cell
Physiol. 281:C1596–C1603. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Susin SA, Lorenzo HK, Zamzami N, Marzo I,
Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler
M, et al: Molecular characterization of mitochondrial
apoptosis-inducing factor. Nature. 397:441–446. 1999. View Article : Google Scholar : PubMed/NCBI
|