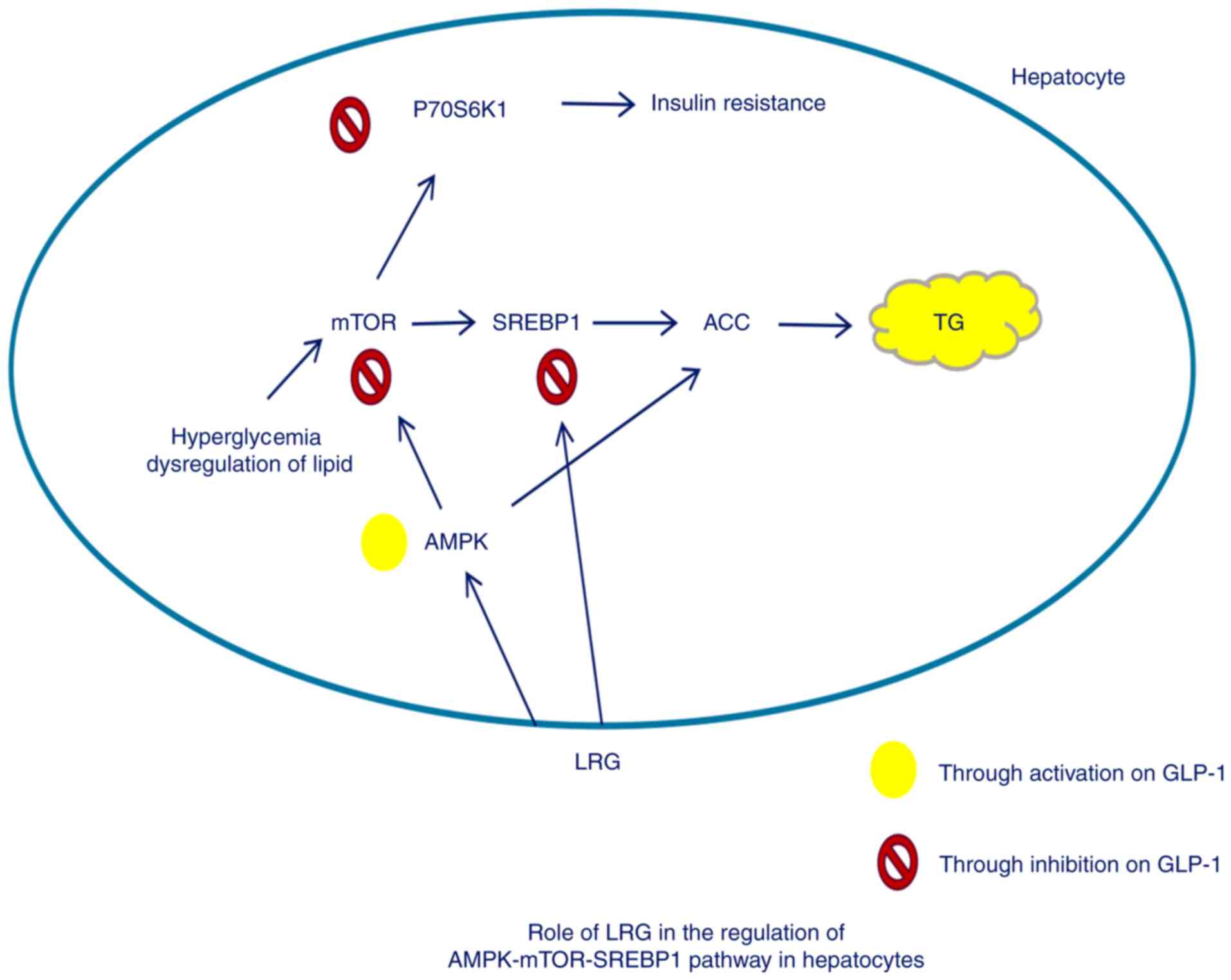
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most
widespread liver disease worldwide, and its incidence continues to
rise (1,2). It describes a number of livers
diseases including simple steatosis or NAFL with low inflammation,
and can progresses to non-alcoholic steatohepatitis (NASH). NASH is
the most severe form of NAFLD and is characterized by the presence
of an abnormal accumulation of fat in the liver which can progress
to liver cell injury (hepatocellular ballooning) and inflammation
(3). NASH results from aberrant
hepatic lipid accumulation, which is strongly associated with
high-fat diet (HFD)-induced metabolic abnormality. A continuous
intake of HFD contributes to the progression of NAFLD (4,5).
However, the exact molecular mechanisms underlying NASH remain
largely unknown, and currently there are no effective therapeutic
strategies for NASH apart from caloric restriction (CR) and regular
exercise (6,7). Therefore, further understanding of
the pathology of NASH is critical in order to develop effective
management strategies for NAFLD.
Sterol regulatory element-binding protein-1 (SREBP1)
mediates the expression of lipogenesis-associated triglyceride
synthesis and accumulation (8,9).
SREBP1 can cause excessive triglyceride accumulation in the liver,
thereby leading to NAFLD development (10). Mechanistic target of rapamycin
(mTOR) is a member of the phosphatidylinositide3-kinase-associated
family of kinases and forms two distinct complexes: mTORC1 and
mTORC2 (11). mTORC1 signaling
stimulates cell growth via multiple mechanisms, including promoting
lipid biosynthesis (12,13). In addition, mTORC1 enhances de
novo lipogenesis by enhancing the nuclear localization and
activity of SREBP1 (14–16). Therefore, agents targeting mTORC1
have therapeutic potential for NASH.
Glucagon-like peptide-1 (GLP-1), an incretin
hormone, as well as glucose-dependent insulinotropic polypeptide,
are responsible for mediating glucose-mediated insulin production
in pancreatic β-cells (17–19).
GLP-1 and its analogues also perform pleiotropic functions in
extra-pancreatic organs in mammals, including hepatic lipid
deposition alleviation, weight loss and appetite inhibition
(20,21). GLP-1 may regulate the expression of
genes associated with lipid metabolism in liver cells, thereby
preventing the development and progression of NAFLD (22). Therefore, the GLP-1 receptor
agonist liraglutide, may have potential for improving NASH outcomes
as a novel therapeutic agent by activating the adenosine
monophosphate-activated protein kinase (AMPK)/mTOR/SREBP1 signaling
pathway.
Materials and methods
Animals
A total of 32 male C57/BL6J mice (18–20 g), 8 weeks
of age, were supplied by the Laboratory Animal Center, West China
Hospital, Sichuan West China School of Medicine (Chengdu, China).
The mice were housed individually in cages at 20–25°C with a
constant humidity (55±5%) with a 12-h light/dark cycle. All animal
experiments were approved by the Ethics Committee of Sichuan
University (Sichuan, China) and were performed in accordance with
guidelines of the Institutional Animal Ethics Committee and
international guidelines (23,24).
Animal groups and treatments
C57BL/6J mice were randomly divided into 4 groups
(n=8/group) as follows: Standard-fat diet: i) Control (8 kcal %
fat, 42 kcal % protein and 50 kcal % carbohydrate); the HFD-fat
diet groups: ii) the ad libitum group [61 kcal % fat, 15
kcal % protein, and 25 kcal % carbohydrate, 0.2% cholesterol, high
glucose water (2% fructose plus 2.5% glucose) ad libitum],
iii) the ad libitum+ LRG (liraglutide, 0.6 mg/kg/day) and
iv) the CR group (calorically restricted to follow the food intake
trajectory of the ad libitum+ LRG group). Liraglutide (Novo
Nordisk, Oslo, Norway) was intraperitoneally injected into the mice
in the LRG group every day while saline was injected into the other
groups every day for 4 weeks. In all experiments, the body weight
and food intake of the mice were determined twice per week. Animals
were sacrificed at the end of week 12, and the liver tissues were
excised, immediately frozen in liquid nitrogen and stored at −80°C
for protein extraction.
Biochemical indices
Tail blood of mice was collected at 0, 3, 6, 9, 12
weeks and was measured using a blood glucose meter (Life Scan.
Inc., USA). After sacrificing the mice at the end of week 12, serum
was collected by centrifugation at 4,000 × g for 15 min at 4°C.
Serum levels of glutamic-oxaloacetic transaminase 1
(GOT1)/aspartate aminotransferase (AST), glutamic-pyruvate
(GPT)/alanine aminotransferase (ALT) and total cholesterol
(T-CHO) were assessed using an automatic biochemistry analyzer
(AU2700; Olympus Ltd., Japan). Serum triglyceride (TG) levels were
measured with ELISA kits (cat. no. BC0625; Solarbio Ltd., Beijing,
China) according to the manufacturer's instructions.
Hematoxylin and eosin (H&E) and
Oil red O staining
Following perfusion with phosphate-buffered saline
(PBS), the livers were removed for staining. For H&E staining,
the livers were fixed with 4% paraformaldehyde, embedded in
paraffin, cut into 8 µm sections, and then stained with H&E.
For Oil red O staining, the samples were embedded in optimal
cutting temperature medium (Leica Microsystems GmbH, Germany),
frozen and then cut into sections with a microtome. The frozen
sections were stained with Oil red O.
Western blot analysis
Protein expression and phosphorylation were assessed
by western blotting. The following antibodies from Cell Signaling
Technology, Inc. (Danvers, MA, USA) were used: AMPK (cat. no.
#2795; dilution at 1:1,000), phosphorylated(p)-AMPKα (Thr172) (cat.
no. #8208; dilution at 1:1,000), mTOR (cat. no. #2983; dilution at
1:1,000), p-mTOR (Ser2448) (cat. no. #5536; dilution at 1:1,000),
S6 (cat. no. #2217 dilution at 1:1,000), p-S6 (Ser240/244) (cat.
no. #5364; dilution at 1:1,000), P70S6K1 (cat. no. #9234s; dilution
at 1:1,000), p-P70S6K1 (Thr389) (cat. no. #2708; dilution at
1:1,000), acetyl coenzyme A carboxylase (ACC; cat. no. #3662;
dilution at 1:1,000), and p-ACC (Ser79) (cat. no. #11818; dilution
at 1:1,000). The anti-SREBP1 (cat. no. #ab28481; dilution at 1:500)
antibody and anti-GAPDH (#ab9584; dilution at 1:10,000) were from
Abcam (Cambridge, MA, USA), and secondary HRP goat anti-rabbit IgG
antibodies (#511202; dilution at 1:5,000) was from Zen BioScience
(Chengdu, China).
Immunofluorescence assays
The liver sections were incubated with anti-SREBP-1
antibody (cat. no. #ab28481; Abcam; 5 µg/ml) and 1% goat serum
(cat. no. #ab7481; Abcam) at 4°C and 1:1,000 dilution overnight in
PBS. Following washing with PBS, the sections were incubated with
the secondary antibody (1:1,000; as aforementioned) at room
temperature for 1 h. Next, the sections were stained with
4′,6-diamidino-2- phenylindole (DAPI) for 5 min at room
temperature, fixed, washed extensively with PBS, and then imaged
under a fluorescence microscope (Axio Imager A2; Carl Zeiss, Jena,
Germany) at ×200 magnification.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6 software (GraphPad Software, Inc., La Jolla, CA, USA). The
results are expressed as the mean ± standard error. A Student's
t-test or paired Student's t-test were used to compare two groups.
Comparisons among multiple groups were analyzed using one-way
analysis of variance (ANOVA) followed by the Scheffe post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of LRG treatment on body
weight, energy intake and blood glucose in C57 mice
The body weight and energy changes as well as blood
fasting glucose in the different groups are presented in Fig. 1. To confirm the beneficial
metabolic effects of LRG treatment in vivo, the CR group was
designed in the present experiments to consistently match the
energy intake of the LRG-treated group, thereby avoiding the
effects of long-term LRG treatment on energy intake.
The body weights of the C57 mice fed the HFD both
treated with LRG (0.6 mg/kg/day) and the CR group (ad
libitum+LRG and CR groups) were significantly lower than that
of the ad libitum group (P<0.01 at 9 and 12 weeks);
whereas there were no significant differences in whole body weights
between the ad libitum+LRG and the CR group. The energy
intakes of mice treated with LRG were significantly decreased when
compared with the ad libitum (P<0.01 at 9 and 12 weeks;
Fig. 1B). No differences were
observed in the energy intakes between the CR and ad
libitum+LRG treatment groups. Furthermore, fasting blood
glucose levels were significantly lower throughout the experimental
period in the ad libitum+LRG group when compared with ad
libitum and CR mice (P<0.01 at 9 and 12 weeks; Fig. 1C).
Effects of LRG treatment alleviates
liver dysfunction and hepatic lipid accumulation in C57 mice
In comparison to the mice fed a standard diet
(control), the liver weight, adjusted by body weight, was increased
in the HFD-fed mice (ad libitum and CR groups; P<0.01)
compared to the control group; however, LRG treatment (ad
libitum+LRG group) significantly decreased the liver weight
when compared to the ad libitum group (P<0.01; Fig. 2A and B). In addition, liver
function profiling demonstrated that the GOT1 and GPT were
significantly decreased in the ad libitum+LRG group mice
when compared with those in the ad libitum group (P<0.01;
Fig. 2C and D); similar results
were found for the levels of total cholesterol and triglycerides
(Fig. 2E and F). Next, the present
study performed H&E and Oil red O staining (magnification, ×40)
of the liver sections from each group to visualize hepatic vacuole
steatosis and lipid accumulation. Extensive micro-vesicular
steatosis surrounding the perisinusoidal areas and lipid
accumulation were observed in the HFD-fed mice; however, there was
an apparent decrease in the amount of intracellular lipid droplets
in the livers of mice in the ad libitum+LRG group (Fig. 2G and H).
 | Figure 2.Effects of LRG treatment on hepatic
function and hepatic lipid accumulation in HFD-fed mice. (A and B)
LRG treatment significantly decreased the liver weight and
liver-to-body weight ratio, (C and D) the GOT1 and GPT, and (E and
F) the triglyceride and total cholesterol levels, which were
increased in the HFD-fed mice. (G and H) H&E (magnification,
×40) and oil red O (magnification, ×40) staining in hepatic
sections from the different groups revealed that hepatic vacuole
steatosis and lipid accumulation were significantly increased in
the HFD-fed mice and were decreased in the LRG-treated mice,
indicating that LRG treatment alleviates the hepatic damage induced
by the HFD. Values are expressed as the mean ± standard error (n=8
animals/group). *P<0.05 and **P<0.01 vs. the control group;
#P<0.05 and ##P<0.01 vs. the ad
libitum group. LRG, liraglutide; GOT1, glutamic-oxaloacetic
transaminase 1; GPT, glutamic-pyruvate transaminase; HFD, high-fat
diet; H&E, hematoxylin and eosin. |
Effects of LRG treatment on
AMPK/mTOR/SREBP1 signaling in the liver of C57 mice
AMPK is a metabolic fuel gauge that regulates lipid
metabolism by sensing changes in the intracellular AMP/adenosine
triphosphate ratio, especially in the liver as reported by Liu
et al (25). A canonical
downstream mediator of AMPK is mTORC1. As shown in Fig. 3B, the level of phosphorylated
(p)-mTOR in the ad libitum+LRG mice was significantly lower
when compared with the ad libitum group (P<0.01), whereas
was insignificant different compared with the CR group. As shown in
Fig. 3A, LRG treatment
significantly decreased the levels of p-S6 and p-p70S6K1, two
conserved downstream targets of mTORC1, in the mice of the ad
libitum and CR groups (P<0.01). When compared with the
control, hepatic expression of p-AMPK was observed to be
downregulated in the ad libitum and CR mice, while these
levels were significantly restored in the ad libitum+LRG
mice (P<0.01; Fig. 3C). ACC, a
downstream target of AMPK, is a key enzyme in fatty acid
metabolism. Activated AMPK phosphorylates and inhibits ACC
activity, leading to the inhibition of fat synthesis and increased
oxidation (Fig. 4). The present
study demonstrated that LRG treatment upregulated the
phosphorylation of ACC and AMPK in the mice treated with HFD
(Fig. 3A and C). SREBP1c promotes
the synthesis of fatty acids and triglycerides (Fig. 4). As shown in Fig. 3B, the HFD-fed mice (ad
libitum and CR groups) had significantly increased SREBP1
activity (P<0.01) when compared with the control group, yet this
increase was significantly attenuated in the ad libitum+LRG
mice when compared with the ad libitum group
(P<0.05).
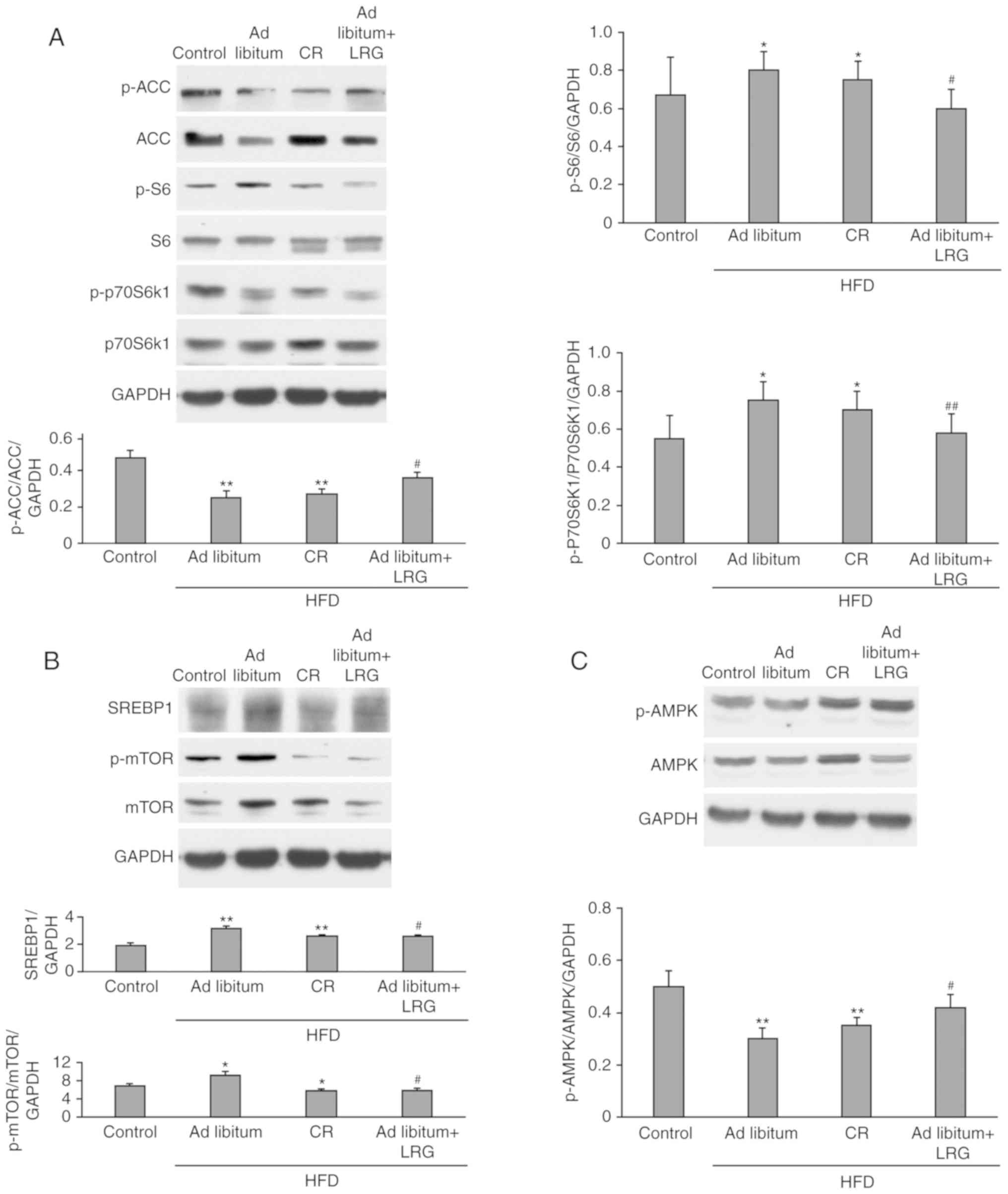 | Figure 3.Effects of LRG treatment on the
protein expression of AMPK/mTOR/SREBP1 in HFD-fed mice. C57 mice
were fed a HFD for 12 weeks, and their liver tissues were then
obtained. The protein expression levels of factors associated with
lipogenesis [(A) ACC, p-ACC, S6, p-S6, p70S6k1, p-p70S6k1; (B)
SREBP1, mTOR, p-mTOR; (C) AMPK, p-AMPK] were determined and
compared between groups. Representative images of the western
blotting assays of indicators associated with hepatic tissues are
presented. Values are expressed as the mean ± standard error
(n=8/group). *P<0.05 and **P<0.01 vs. the control group;
#P<0.05 and ##P<0.01 vs. the ad
libitum group. LRG, liraglutide; AMPK, adenosine
monophosphate-activated protein kinase; mTOR, mechanistic target of
rapamycin; SREBP1, sterol regulatory element-binding protein 1;
HFD, high-fat diet. |
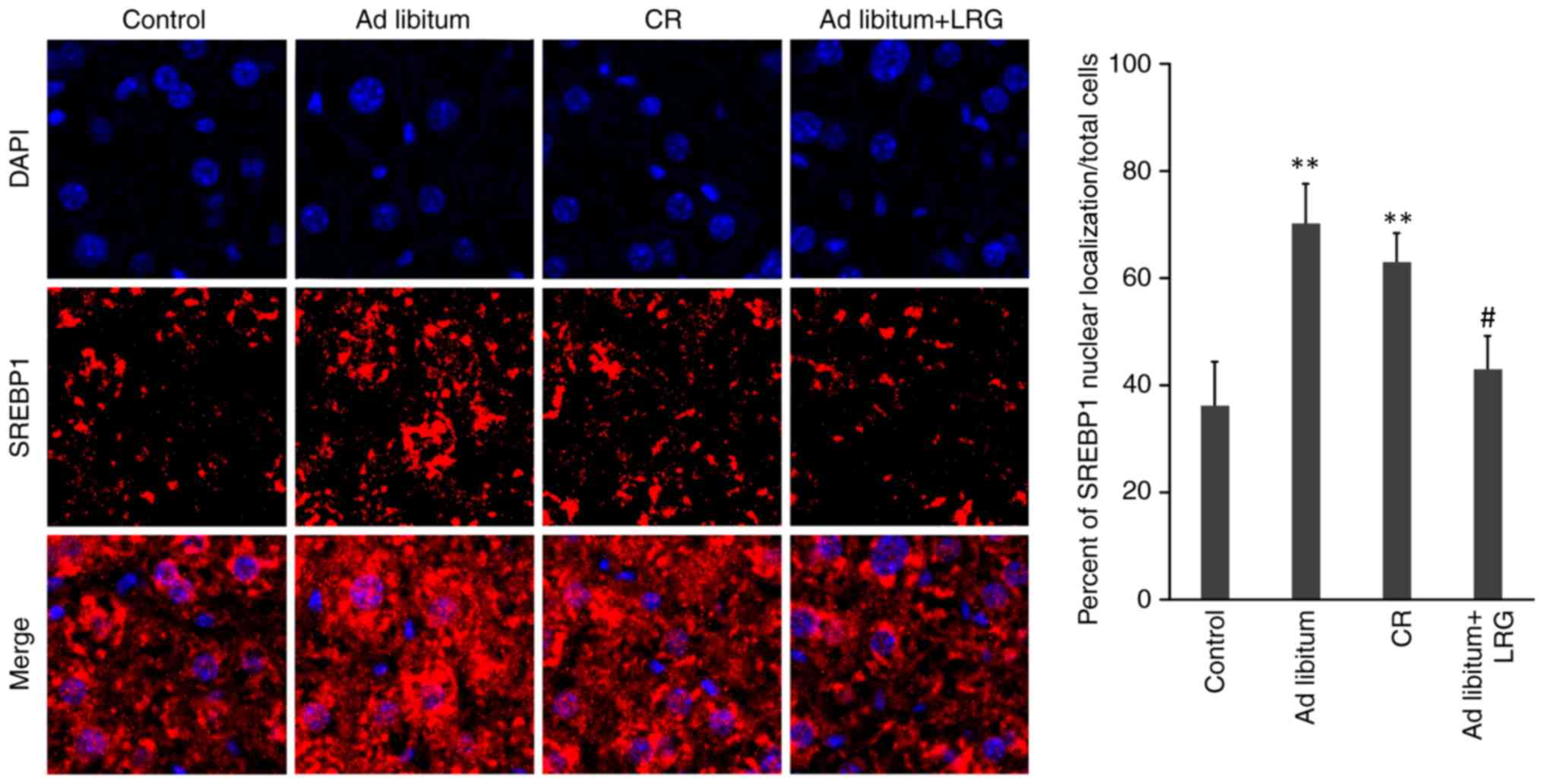
Effects of LRG treatment on the
nuclear translocation of SREBP1 in the livers of mice
The present study further determined SREBP-1c
expression and intracellular localization by conducting
immunofluorescence staining of the liver tissues from each group
(magnification, ×200). There were significantly fewer hepatocytes
positively stained with SREBP-1c in the ad libitum+LRG group
compared with that observed in the mice in the ad libitum
and CR mice (P<0.05). In addition, HFD led to the nuclear
accumulation of SREBP1, which was inhibited by LRG treatment
(Fig. 5).
Discussion
The pathology of steatohepatitis (NASH) is largely
unknown, and there are currently no effective treatments available
for NASH N apart from diet and physical activity. In the present
study, we investigated the therapeutic effects of the GLP-1
receptor agonist liraglutide (LRG) on NASH and the underlying
mechanisms. The results revealed that C57 mice with HFD-induced
NASH exhibited increased body weight and increased levels of
hepatic fat. In addition, LRG treatment significantly reduced the
body weight, improved hepatic lipid accumulation, and suppressed
the elevated levels of total cholesterol and low-density
lipoprotein cholesterol in the serum of HFD-fed mice.
Mechanistically, it was revealed that LRG improves NASH through the
AMPK/mTOR/SREBP1 signaling pathway.
AMPK is a sensor of intracellular energy status and
negatively regulates mTORC1 (26–28).
Recent studies have reported that AMPK agonists (such as metformin
and adiponectin) improve NASH by inhibiting lipid synthesis via
mTORC1/SREBP-1c signaling (12,29–31).
In addition, ACC is a downstream target of AMPK and a rate-limiting
enzyme involved in the synthesis of fatty acids. Activated liver
AMPK inhibits fatty acid synthesis by increasing the
phosphorylation and inactivation of ACC in order to reduce the
production of malonyl coenzyme A (32,33).
In addition, mTORC1 triggers hepatic de novo lipogenesis to
promote lipid synthesis by activating SREBP-1c (26,33,34).
Furthermore, mTORC1 regulates SREBP-1 activation by stimulating
SREBP-1 mRNA expression, and promoting the nuclear localization and
activity of SREBP-1 (30–32). Düvel et al (35) also demonstrated that p70S6K1 is
required for the mTORC1-mediated increase and activation of SREBP1
(36–38). In the present study, LRG treatment
reduced lipid accumulation in the liver by activating AMPK, thereby
suppressing the mTORC1/SREBP1 signaling pathway.
The present results demonstrated that HFD inhibited
the phosphorylation of AMPK in the mouse liver and significantly
enhanced the phosphorylation of mTOR and 70S6K1 as well as the
expression of SREBP1. However, LRG treatment activated AMPK and
suppressed the mTOR-mediated activation of SREBP1, thereby blocking
the transcription of target lipogenic genes involved in the liver
steatosis of HFD-fed C57 mice. In addition, LRG treatment inhibited
HFD-induced nuclear SREBP1 activation, thereby inhibiting SREBP1
translocation into the nucleus and the subsequent changes in liver
triglyceride accumulation.
The GLP-1 receptor is widely expressed in various
organs of the body and is responsible for improving islet function,
suppressing appetite and reducing body weight (39–41).
We hypothesized that drug-induced GLP-1 activation may reduce the
body weight of HFD-fed mice. Indeed, the body weight changes
between the CR group and the group fed HFD ad libitum
following week 9 were significantly different; however, the
biochemical index, molecular expression level and histological
features were not significantly different between the two groups.
By contrast, the above indicators were significantly different
between the CR and LRG groups, indicating that the activation of
GLP-1 by LRG directly exerts a pharmacological action on NAFLD in
HFD-fed mice.
In conclusion, the GLP-1 receptor agonist LRG
reduced the accumulation of hepatic lipids by regulating the
AMPK/mTOR/SREBP1 signaling pathway. The present results identified
a mechanism by which LRG alleviates hepatic lipid accumulation.
Thus, the results of the present study may be used to develop novel
therapeutic strategies for steatohepatitis.
Acknowledgements
The authors would like to thank the Experimental
Center of West China Hospital of Sichuan University, West China
Animal Research Center and the West China Hospital Department of
Endocrinology Experimental Center.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HYC carried out the molecular studies, participated
in the immunoassays and drafted the manuscript. SSW carried out the
immunoassays. HMT and TH conceived and participated in the design
of the study, performed the statistical analysis and helped to
draft the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Ethics
Committee of Sichuan University (Sichuan, China) and were performed
in accordance with guidelines of the Institutional Animal Ethics
Committee and international guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
Glossary
Abbreviations
Abbreviations:
|
ACC
|
acetyl-CoA carboxylase
|
|
AMPK
|
AMP-activated protein kinase
|
|
GLP-1
|
glucagon-like peptide-1
|
|
H&E
|
hematoxylin and eosin
|
|
HFD
|
high-fat diet
|
|
mTOR
|
mechanistic target of rapamycin
|
|
NAFLD
|
nonalcoholic fatty liver disease
|
|
SREBP1
|
sterol regulatory element-binding
transcription factor 1
|
References
|
1
|
Vernon G, Baranova A and Younossi ZM:
Systematic review: The epidemiology and natural history of
non-alcoholic fatty liver disease and non-alcoholic steatohepatitis
in adults. Aliment Pharmacol Ther. 34:274–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thorn SR, Baquero KC, Newsom SA, El Kasmi
KC, Bergman BC, Shulman GI, Grove KL and Friedman JE: Early life
exposure to maternal insulin resistance has persistent effects on
hepatic NAFLD in juvenile nonhuman primates. Diabetes.
63:2702–2713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chackelevicius CM, Gambaro SE, Tiribelli C
and Rosso N: Th17 involvement in nonalcoholic fatty liver disease
progression to non-alcoholic steatohepatitis. World J
Gastroenterol. 22:9096–9103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clarke JD, Dzierlenga AL, Nelson NR, Li H,
Werts S, Goedken MJ and Cherrington NJ: Mechanism of altered
metformin distribution in nonalcoholic steatohepatitis. Diabetes.
64:3305–3313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Little TJ, Doran S, Meyer JH, Smout AJ,
O'Donovan DG, Wu KL, Jones KL, Wishart J, Rayner CK, Horowitz M and
Feinle-Bisset C: The release of GLP-1 and ghrelin, but not GIP and
CCK, by glucose is dependent upon the length of small intestine
exposed. Am J Physiol Endocrinol Metab. 291:E647–E655. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kitade H, Chen G, Ni Y and Ota T:
nonalcoholic fatty liver disease and insulin resistance: New
insights and potential new treatments. Nutrients. 9(pii): E3872017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Byrne CD and Targher G: NAFLD: A
multisystem disease. J Hepatol. 62 (Suppl):S47–S64. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamazaki S, Satoh H and Watanabe T:
Liraglutide enhances insulin sensitivity by activating
AMP-activated protein kinase in male Wistar rats. Endocrinology.
155:3288–3301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bakan I and Laplante M: Connecting mTORC1
signaling to SREBP-1 activation. Curr Opin Lipidol. 23:226–234.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li S, Ogawa W, Emi A, Hayashi K, Senga Y,
Nomura K, Hara K, Yu D and Kasuga M: Role of S6K1 in regulation of
SREBP1c expression in the liver. Biochem Biophys Res Commun.
412:197–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim LC, Cook RS and Chen J: mTORC1 and
mTORC2 in cancer and the tumor microenvironment. Oncogene.
36:2191–2201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song YM, Lee YH, Kim JW, Ham DS, Kang ES,
Cha BS, Lee HC and Lee BW: Metformin alleviates hepatosteatosis by
restoring SIRT1-mediated autophagy induction via an AMP-activated
protein kinase-independent pathway. Autophagy. 11:46–59. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He Q, Sha S, Sun L, Zhang J and Dong M:
GLP-1 analogue improves hepatic lipid accumulation by inducing
autophagy via AMPK/mTOR pathway. Biochem Biophys Res Commun.
476:196–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soliman GA: The integral role of mTOR in
lipid metabolism. Cell Cycle. 10:861–862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Quan HY, Kim DY, Kim SJ, Jo HK, Kim GW and
Chung SH: Betulinic acid alleviates non-alcoholic fatty liver by
inhibiting SREBP1 activity via the AMPK-mTOR-SREBP signaling
pathway. Biochem Pharmacol. 85:1330–1340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Li X, Guo H, Yuan Z, Wang T, Zhang
L and Jiang Z: Emodin alleviates hepatic steatosis by inhibiting
SREBP1 activity via the CaMKK-AMPK-mTOR-p70S6K signaling pathway:
Emodin alleviates NAFLD by CaMKK-AMPK-mTOR-SREBP1. Hepatol Res.
47:2016.
|
|
17
|
Jones B, Bloom SR, Buenaventura T, Tomas A
and Rutter GA: Control of insulin secretion by GLP-1. Peptides.
100:75–84. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ayala JE, Bracy DP, James FD, Julien BM,
Wasserman DH and Drucker DJ: The glucagon-like peptide-1 receptor
regulates endogenous glucose production and muscle glucose uptake
independent of its incretin action. Endocrinology. 150:1155–1164.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee J, Hong SW, Rhee EJ and Lee WY: GLP-1
receptor agonist and non-alcoholic fatty liver disease. Diabetes
Metab J. 36:262–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Butler PC: Glucagon-like peptide 1 drugs
as second-line therapy for type 2 diabetes. JAMA Intern Med.
176:1–3. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang XC, Gusdon AM, Liu H and Qu S:
Effects of glucagon-like peptide-1 receptor agonists on
non-alcoholic fatty liver disease and inflammation. World J
Gastroenterol. 20:14821–14830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong Y, Lv Q, Li S, Wu Y, Li L, Li J,
Zhang F, Sun X and Tong N: Efficacy and safety of glucagon-like
peptide-1 receptor agonists in non-alcoholic fatty liver disease: A
systematic review and meta-analysis. Clin Res Hepatol
Gastroenterol. 41:284–295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hansen BC, Gografe S, Pritt S, Jen KC,
McWhirter CA, Barman SM, Comuzzie A, Greene M, McNulty JA, Michele
DE, et al: Ensuring due process in the IACUC and animal welfare
setting: Considerations in developing noncompliance policies and
procedures for institutional animal care and use committees and
institutional officials. FASEB J. 31:4216–4225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheluvappa R, Scowen P and Eri R: Ethics
of animal research in human disease remediation, its institutional
teaching; and alternatives to animal experimentation. Pharmacol Res
Perspect. 5:2017. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu J, Wang G, Jia Y and Xu Y: GLP-1
receptor agonists: Effects on the progression of non-alcoholic
fatty liver disease. Diabetes Metab Res Rev. 31:329–335. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peterson TR, Sengupta SS, Harris TE,
Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter
AE, Finck BN and Sabatini DM: mTOR complex 1 regulates lipin 1
localization to control the SREBP pathway. Cell. 146:408–420. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lempiäinen H, Uotila A, Urban J, Dohnal I,
Ammerer G, Loewith R and Shore D: Sfp1 interaction with TORC1 and
Mrs6 reveals feedback regulation on TOR signaling. Mol Cell.
33:704–716. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gowans GJ, Hawley SA, Ross FA and Hardie
DG: AMP is a true physiological regulator of AMP-activated protein
kinase by both allosteric activation and enhancing net
phosphorylation. Cell Metab. 18:556–566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bojic LA, Telford DE, Fullerton MD, Ford
RJ, Sutherland BG, Edwards JY, Sawyez CG, Gros R, Kemp BE,
Steinberg GR and Huff MW: PPARδ activation attenuates hepatic
steatosis in Ldlr-/- mice by enhanced fat oxidation, reduced
lipogenesis, and improved insulin sensitivity. J Lipid Res.
55:1254–1266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Laplante M and Sabatini DM: An emerging
role of mTOR in lipid biosynthesis. Curr Biol. 19:R1046–R1052.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Melnik BC: Linking diet to acne
metabolomics, inflammation, and comedogenesis: An update. Clin
Cosmet Investig Dermatol. 8:371–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carling D and Viollet B: Beyond energy
homeostasis: The expanding role of AMP-activated protein kinase in
regulating metabolism. Cell Metab. 21:799–804. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jeong KJ, Kim GW and Chung SH:
AMP-activated protein kinase: An emerging target for ginseng. J
Ginseng Res. 38:83–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Horton JD, Goldstein JL and Brown MS:
SREBPs: Activators of the complete program of cholesterol and fatty
acid synthesis in the liver. J Clin Invest. 109:1125–1131. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Düvel K, Yecies JL, Menon S, Raman P,
Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S,
et al: Activation of a metabolic gene regulatory network downstream
of mTOR complex 1. Mol Cell. 39:171–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kelleher AR, Kimball SR, Dennis MD,
Schilder RJ and Jefferson LS: The mTORC1 signaling repressors
REDD1/2 are rapidly induced and activation of p70S6K1 by leucine is
defective in skeletal muscle of an immobilized rat hindlimb. Am J
Physiol Endocrinol Metab. 304:E229–E236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ricoult SJ, Yecies JL, Ben-Sahra I and
Manning BD: Oncogenic PI3K and K-Ras stimulate de novo lipid
synthesis through mTORC1 and SREBP. Oncogene. 35:1250–1260. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Laplante M and Sabatini DM: Regulation of
mTORC1 and its impact on gene expression at a glance. J Cell Sci.
126:1713–1719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Katsurada K and Yada T: Neural effects of
gut- and brain-derived glucagon-like peptide-1 and its receptor
agonist. J Diabetes Investig. 7 (Suppl 1):S64–S69. 2016. View Article : Google Scholar
|
|
40
|
Cariou B: Pleiotropic effects of insulin
and GLP-1 receptor agonists: Potential benefits of the association.
Diabetes Metab 41 (6 Suppl 1). 6S28–6S35. 2015. View Article : Google Scholar
|
|
41
|
Seufert J and Gallwitz B: The
extra-pancreatic effects of GLP-1 receptor agonists: A focus on the
cardiovascular, gastrointestinal and central nervous systems.
Diabetes Obes Metab. 16:673–688. 2014. View Article : Google Scholar : PubMed/NCBI
|