Introduction
Myocardial ischemia/reperfusion (I/R) injury remains
a significant clinical problem with a lack of effective therapies,
and is a leading cause of morbidity and mortality in patients with
cardiovascular disease (1).
Previous studies have confirmed that multiple biological processes,
including myocardial cell injury, apoptosis, oxidative stress and
mitochondrial dysfunction, are involved in the pathophysiology of
myocardial I/R injury. However, the underlying molecular and
cellular events are complex and remain elusive (2,3).
Understanding these mechanisms would be beneficial towards the
development of effective interventions and strategies to prevent
myocardial I/R injury, and is therefore of great clinical
significance.
Puerarin
(7,4-dihydroxyisoflavone-8β-glucopyranoside) is a bioactive
isoflavone derived from the Kudzu (Pueraria montana var.
lobata) root, a well-known traditional Chinese medicine,
which is widely prescribed for patients with cardio- and
cerebrovascular diseases in China (4,5). In
recent years, a growing body of in vivo and in vitro
evidence has been reported, revealing the value of puerarin in the
treatment of cardiovascular diseases, including myocardial I/R
injury (6,7). Studies have demonstrated that the
therapeutic effect of this compound on I/R-induced myocardial
injury is closely associated with its anti-apoptotic role (8) and its regulation of mitochondrial
transmembrane pores and/or channels (9). Although puerarin is considered to be
a protective agent against myocardial I/R injury, the exact
cellular and molecular mechanisms by which it serves this
cardioprotective role are not well understood.
Endogenous 18–22 nucleotide microRNAs (miRNAs/miRs)
are considered to serve significant roles in cell differentiation,
apoptosis and oxidative stress (10). Recently, the function of miR-21 in
cardiovascular disease has received increasing attention (11,12).
miR-21 has been reported to be highly expressed in many types of
cardiovascular cells, including cardiomyocytes, and it functions in
myocardial I/R injury protection (13–15).
Studies have confirmed that overexpression of miR-21 via plasmid-
or adenovirus-mediated gene transfer suppresses cardiomyocyte
injury and apoptosis, providing a novel potential therapeutic
target for myocardial I/R injury (16–18).
Accordingly, inhibiting miR-21 cancels out the cardioprotective
effect of ischemic preconditioning (19). miR-21 has also been reported to be
involved in cardioprotection against myocardial I/R and
hypoxia/reoxygenation (H/R) injury in cardiomyocytes (20). However, whether miR-21 contributes
to puerarin-induced cardioprotection remains unknown.
The present study investigated the role of miR-21 on
the effect of puerarin on H/R-induced H9c2 cell injury, an in
vitro model of myocardial I/R injury. The results revealed that
miR-21 mediated the cardioprotective effects of puerarin against
H/R injury. Furthermore, apoptosis and oxidative stress was
inhibited, and the antioxidative defense system was enhanced. These
findings suggested a significant role for miR-21 in the
cardioprotective function of puerarin in H/R injury, indicating a
potential therapeutic target for treating myocardial I/R
injury.
Materials and methods
Cell culture
Embryonic rat myocardium-derived H9c2 cells were
obtained from the American Type Culture Collection (Manassas, VA,
USA) and cultured in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with
10% (v/v) fetal bovine serum and 1% (v/v) penicillin-streptomycin
(both Gibco; Thermo Fisher Scientific, Inc., Waltham MA, USA) at
37°C in a humidified incubator containing 95% air and 5%
CO2.
In vitro H/R model establishment
H9c2 cells were treated with H/R in order to mimic
myocardial I/R injury. Following culture to 70–80% confluence under
normal conditions as described above, cells were incubated with
serum-deficient DMEM in a hypoxic chamber supplied with 95%
N2 and 5% CO2 at 37°C for 6 h, and then moved
to an incubator with 95% air and 5% CO2 at 37°C for 12
h. The serum-deficient DMEM in the hypoxic conditions was replaced
by normal medium upon the initiation of reoxygenation. The control
cells were kept in normal culture medium with 95% air and 5%
CO2 at 37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total miRNA was extracted from the cultured H9c2
cells using the mirVana™ miRNA Isolation kit (cat. no. AM1561;
Thermo Fisher Scientific, Inc.). cDNA was generated from the total
miRNA (1 µg) using a TaqMan™ MicroRNA Reverse Transcription kit
(cat. no. 4366597; Thermo Fisher Scientific, Inc.). qPCR
amplification was performed on an ABI 7500 Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) using TaqMan™
Fast Advanced master mix (cat. no. 4444556; Thermo Fisher
Scientific, Inc.). All steps were performed according to the
protocols of the kit manufacturers. Following initial incubation at
45°C for 2 min and 95°C for 10 min, amplification was performed for
45 cycles of 95°C for 15 sec, 65°C for 20 sec and 72°C for 30 sec.
The relative expression was calculated using the 2–ΔΔCq
method (21). The results were
expressed as the ratios of target genes against the small nuclear
RNA U6 for miR-21. The primer sequences used in this study are as
follows: miR-21 forward, 5′-TGTACCACCTTGTCGGATAG-3′; and reverse,
5′-CTGCTGTTGCCATGAGAT-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′; and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′. The experiment was performed
in triplicate.
Cell transfection
miR-21 inhibitor and an inhibitor negative control
(miR-NC;both Thermo Fisher Scientific, Inc.) were transfected into
H9c2 cells using Lipofectamine® 3000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) at a final
concentration of 50 nM, according to the manufacturer's protocol.
Following transfection for 48 h, the transfection efficiency was
determined using RT-qPCR. The sequences were as follows: niR-21
inhibitor, 5′-UCAACAUCAGUCUGAUAAGCUA-3′; miR-NC,
5′-CAGUACUUUUGUGUAGUACAA-3′.
Assessment of cell viability
H9c2 cell viability was assessed using the MTT cell
proliferation and cytotoxicity assay kit (Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacturer's
protocol. In brief, H9c2 cells were seeded into 96-well plates at
4×103 cells/well. Following incubation with the
indicated reagents, MTT solution (5 mg/ml) was added to each well
and incubated for 4 h at 37°C. Subsequently, 150 µl
dimethylsulfoxide (Sigma-Aldrich; Merck KGaA) was added for 10–15
min to dissolve the precipitate. The absorbance was measured at 570
nm using a microplate reader (BioTek Instruments, Inc., Winooski,
VT, USA). The results are represented graphically as a percentage
of the control cells.
Measurement of lactate dehydrogenase
(LDH) activity in culture supernatants
Following the treatment with miR-21 inhibitors
and/or puerarin followed by H/R, cell damage was evaluated by
measuring the amount of LDH released into the culture supernatant
using a commercial LDH kit (cat. no. C0016; Beyotime Institute of
Biotechnology) at 490 nm, using a microplate reader. The results of
the LDH activity are expressed as a percentage of the activity in
the control group.
Analysis of cell apoptosis by flow
cytometry
The measurement of apoptosis was performed using an
Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide
(PI) apoptosis detection kit (cat. no. APOAF; Sigma-Aldrich; Merck
KGaA) according to the manufacturer's protocols. Briefly, H9c2
cells were collected, washed twice with cold PBS, and resuspended
in 1X binding buffer at 1×106 cells/ml. Subsequently,
the cells were stained with 5 µl Annexin V-FITC and 5 µl PI
solution for 10 min at room temperature in the dark. Following two
washes with PBS, the fluorescence was analyzed by flow cytometry
(BD Biosciences, Franklin Lakes, NJ, USA). The experiments were
performed in triplicate.
Determination of caspase-3
activity
Caspase-3 activity in H9c2 cells was assessed using
a caspase-3 colorimetric assay (cat. no. ab39401; Abcam, Cambridge,
UK), according to the manufacturer's protocols. The cell lysate was
collected and incubated with 150 µM DEVD-p-nitroaniline, a
fluorogenic substrate of caspase-3, at 37°C for 2 h. The
fluorescence was then measured using a Clariostar Monochromator
microplate reader (BMG Labtech GmbH, Ortenberg, Germany) with
excitation at 360 nm and emission at 460 nm.
Measurement of intracellular reactive
oxygen species (ROS) production
To evaluate the intracellular generation of ROS,
cells were stained with the fluorescent probe
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA, Sigma-Aldrich;
Merck KGaA). At the final stage of treatment with miR-21 inhibitors
and/or puerarin followed by H/R, the H9c2 cells were incubated with
10 µM DCFH-DA at 37°C for 20 min. Following two washes with PBS,
cell images were captured using a fluorescence microscope (Olympus
Corporation, Tokyo, Japan) and the DCF fluorescence was analyzed
using a FACScanto flow cytometer (BD Biosciences, San Jose, CA,
USA) using a 488 nm excitation filter and a 525 nm emission filter.
Finally, FlowJo version 7.6 (FlowJo LLC, Ashland, OR, USA) was used
to examine the production of intracellular ROS.
Measurement of malondialdehyde (MDA)
content, superoxide dismutase (SOD), catalase (CAT) and glutathione
peroxidase (GSH-Px) activities
Following treatment with miR-21 inhibitors and/or
puerarin followed by H/R, cells were ultrasonicated and centrifuged
at 12,000 × g at 4°C for 10 min. Protein concentration was
quantified using a bicinchoninic acid (BCA) protein assay (Beyotime
Institute of Biotechnology). MDA (cat. no. CEA597Ge) content and
SOD (cat. no. SES134Hu), CAT (cat. no. SEC418Hu) and GSH-Px (cat.
no. CEA294Ge) activities in the total cell lysate (10 µl) were
determined using commercially available kits (Uscn Life Science,
Inc., Wuhan, China), according to the manufacturer's protocols.
Optical density was measured using a microplate reader. Each
measurement was calculated compared with a standard curve and
normalized to the total protein concentration. Experiments were
repeated three times.
Western blot analysis
Following treatment with miR-21 inhibitors and/or
puerarin followed by H/R, the H9c2 cells were collected and lysed
in radioimmunoprecipitation assay lysis buffer (Beyotime Institute
of Biotechnology) supplemented with a protease inhibitor cocktail
(cat. no. 78425; Thermo Fisher Scientific, Inc.) on ice for 30 min.
The protein concentration was measured using the BCA protein assay.
Equal amounts of protein (30 µg/lane) were separated by SDS-PAGE
(12% gel; Beyotime Institute of Biotechnology) and transferred to a
polyvinylidene fluoride membrane membranes (Merck KGaA).
Non-specific binding was blocked with 5% non-fat milk for 2 h at
room temperature, and then the membranes were incubated overnight
at 4°C with the following specific primary antibodies:
Anti-apoptosis regulator Bcl-2 (1:1,000, cat. no. 15071; Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-apoptosis
regulator Bax (1:1,000, cat. no. 2774; Cell Signaling Technology,
Inc.), cleaved caspase-3 (Asp175; 1:1,000, cat. no. 9661; Cell
Signaling Technology, Inc.), anti-NADPH oxidase 2 (NOX2; 1:1,000;
cat. no. ab129068; Abcam) and anti-GAPDH (1:2,000; cat. no. 5174;
Cell Signaling Technology, Inc.). Following three washes with
Tris-buffered saline with 0.1% (v/v) Tween-20, the membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies (goat anti-rabbit, 1:5,000; cat. no. 7074; Cell
Signaling Technology, Inc.) for 2 h at room temperature. Finally,
the protein bands were detected with a Super ECL Detection kit
(Beyotime Institute of Biotechnology). The band intensities were
quantified using Quantity One software version 4.62 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and normalized to GAPDH.
Statistical analysis
Data were expressed as the mean ± standard
deviation. Data were analyzed in SPSS version 13.0 (SPSS, Inc.,
Chicago, IL, USA) using one-way analysis of variance followed by
Bonferroni's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Puerarin attenuates cytotoxicity and
increases miR-21 levels in H/R treated H9c2 cells
Firstly, to investigate the cardioprotective effect
of puerarin in H/R-induced injury, H9c2 cells were pre-treated with
various doses of puerarin (50, 100 or 200 µM) for 1 h, followed by
H/R conditions. The results from the MTT and LDH activity assays
revealed that the H/R treatment alone significantly decreased the
H9c2 cell viability (Fig. 1A) and
increased LDH activity in the culture supernatant (Fig. 1B), whereas these effects were
blocked by the puerarin pretreatment in a dose-dependent manner.
Subsequently, the effect of puerarin on the expression of miR-21
under H/R conditions was investigated, and the results demonstrated
that the H/R conditions led to a decrease in the endogenous miR-21
levels in the H9c2 cells; this was also prevented by the puerarin
pretreatment in a concentration-dependent manner (Fig. 1C). The maximum protective effect
was achieved with 200 µM puerarin, which was the concentration
selected for subsequent experiments. These results suggested that
miR-21 upregulation may be associated with the cardioprotective
function of puerarin against H/R injury.
Inhibition of miR-21 eliminates the
puerarin-induced cardioprotection against H/R injury
To further confirm the role of miR-21 in
puerarin-induced protection against H/R injury, H9c2 cells were
transfected with miR-21 inhibitor to block miR-21 expression. As
demonstrated in Fig. 2A, cells
transfected with the miR-21 inhibitor exhibited a notable decrease
in miR-21 expression compared with those transfected with the
miR-NC. In the miR-21 inhibitor transfected group, no significant
difference in miR-21 expression was observed between the control
and H/R groups (Fig. 2A). In
addition, the inhibition of miR-21 further intensified the
H/R-induced decrease in cell viability (Fig. 2B) and increase in LDH activity
(Fig. 2C), and markedly reduced
the puerarin-induced protection against H/R-induced cytotoxicity in
H9c2 cells. These results indicated that miR-21 mediated the
cardioprotective function of puerarin against H/R injury.
Inhibition of miR-21 reverses the
puerarin-induced reduction of apoptosis in H/R-treated H9c2
cells
The effect of puerarin on apoptosis, and the role of
miR-21 in this process, was examined. The results revealed that the
H/R-induced apoptosis in H9c2 cells was enhanced following the
inhibition of miR-21 expression, but reduced following puerarin
treatment (Fig. 3A and B).
However, this effect of puerarin was blocked by miR-21 inhibition.
Furthermore, in H/R-treated cells, miR-21 inhibition increased the
activity of caspase-3 (Fig. 3C)
and the expression of cleaved caspase-3 (Fig. 3D), an apoptosis mediator in
intrinsic and extrinsic pathways (22), and reversed the puerarin-induced
repression. The western blot analysis results revealed that
puerarin alleviated the H/R-induced upregulation of the expression
of pro-apoptotic protein Bax, and downregulated that of
anti-apoptotic protein Bcl-2 (Fig.
3E and 3F). These effects were
blocked by miR-21 inhibition. In addition, transfection with miR-21
inhibitor intensified the H/R-induced changes in the expression of
Bax and Bcl-2. These results suggested that miR-21 contributed to
the protective function of puerarin against H/R-induced apoptosis,
likely in a mitochondrial-dependent manner.
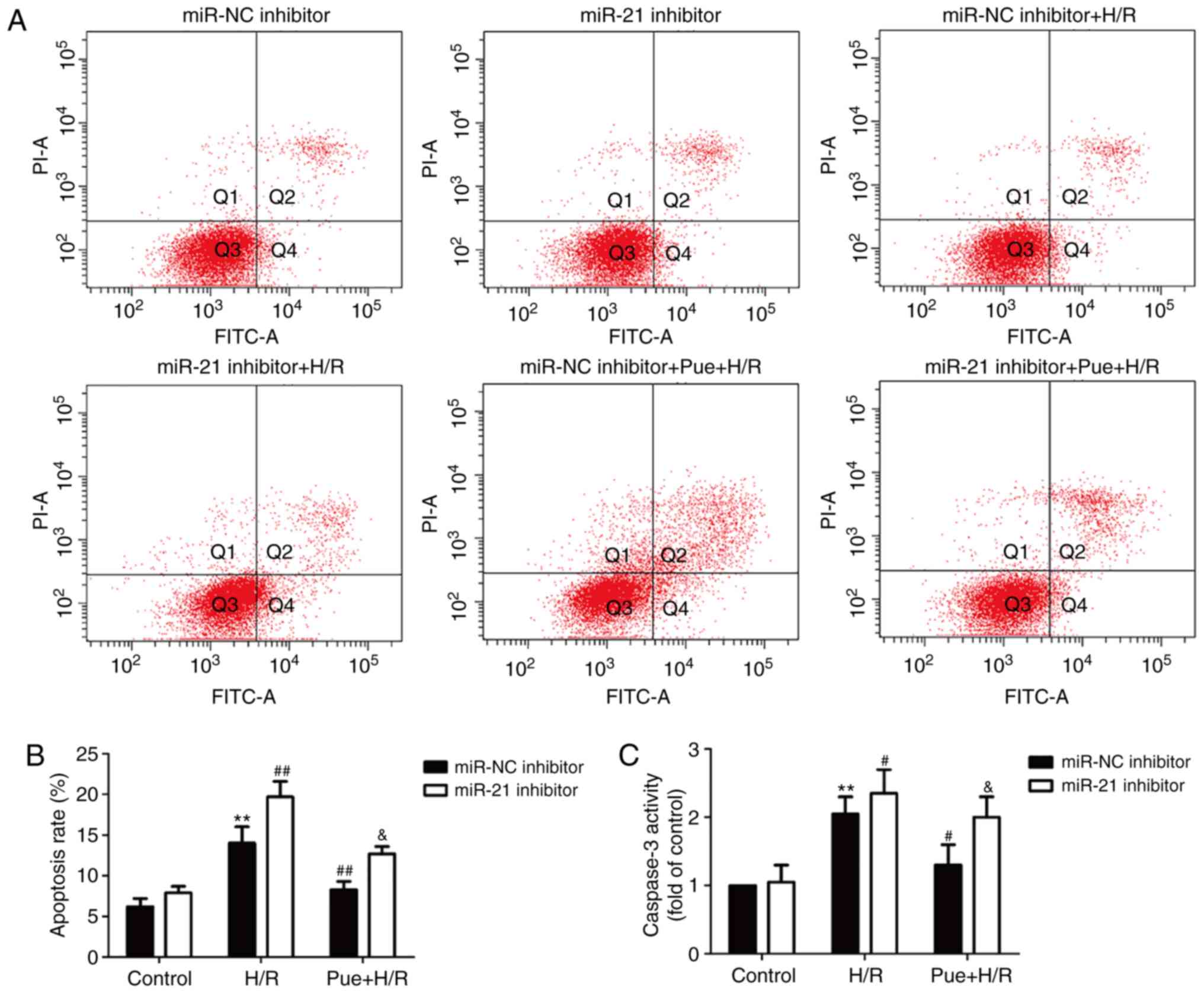 | Figure 3.Effects of miR-21 inhibition on
apoptosis under H/R conditions in the presence or absence of
puerarin in H9c2 cells. Cells were transfected with miR-21
inhibitor or miR-NC followed by treatment with 200 µM puerarin for
1 h prior to incubation under H/R conditions. (A) The apoptotic
rate was determined using an Annexin V-FITC/PI apoptosis detection
kit. (B) Quantitative analysis was performed following flow
cytometry. (C) Caspase-3 activity was detected by a colorimetric
assay. The expression of (D) cleaved caspase-3 and (E) Bax were
measured by western blot analysis. The bars represent the mean ±
standard deviation of ≥3 independent experiments. *P<0.05,
**P<0.01 vs. control + miR-NC inhibitor transfection group;
#P<0.05, ##P<0.01 vs. H/R + miR-NC
transfection group; &P<0.05,
&&P<0.01 vs. Pue + H/R + miR-NC transfection
group. Effects of miR-21 inhibition on apoptosis under H/R
conditions in the presence or absence of puerarin in H9c2 cells.
The expression of (F) Bcl-2 was measured by western blot analysis.
The bars represent the mean ± standard deviation of ≥3 independent
experiments. *P<0.05 vs. control + miR-NC inhibitor transfection
group; #P<0.05 vs. H/R + miR-NC transfection group;
&P<0.05 vs. Pue + H/R + miR-NC transfection
group. miR, microRNA; miR-NC, miR-21 inhibitor negative control;
H/R, hypoxia/reoxygenation; Bax, apoptosis regulator Bax; Bcl-2,
apoptosis regulator Bcl-2; Pue, puerarin; FITC, fluorescein
isothiocyanate; PI, propidium iodide. |
Inhibition of miR-21 reverses the
puerarin-induced inhibition of oxidative stress in H/R-treated H9c2
cells
Oxidative stress serves a fundamental role in the
physiological process of myocardial I/R injury (23). Therefore, to examine the effects of
miR-21 in puerarin-induced cardioprotection against this factor,
oxidative stress parameters, including ROS production, MDA content
and NOX2 expression, were measured. As shown in Fig. 4, puerarin treatment led to a marked
decrease in endogenous ROS production induced by H/R, whereas this
effect was reversed by the miR-21 inhibitor (Fig. 4A and B). In the H/R-treated group,
the miR-21 inhibitor led to the intensification of the H/R-induced
increase in ROS. In addition, the miR-21 inhibition led to the
inhibition of the puerarin-induced decrease in MDA content in the
H/R-treated group, and aggravated the H/R-induced increase
(Fig. 4C). Furthermore, H/R
treatment resulted in an increase in NOX2 expression, which was
mitigated by puerarin pretreatment, and enhanced by miR-21
inhibition (Fig. 4D and E).
Notably, miR-21 inhibition eradicated the effects of puerarin.
These results indicated that puerarin inhibits H/R-induced
oxidative stress by enhancing miR-21 expression.
 | Figure 4.Effects of miR-21 inhibition on
oxidative stress in the presence or absence of puerarin in
H/R-treated H9c2 cells. Cells were transfected with miR-21
inhibitor or miR-NC followed by treatment with 200 µM puerarin for
1 h prior to incubation under H/R conditions. (A) Endogenous ROS
production was determined by DCFH-DA staining. Scale bar, 100 µm.
(B) Quantitative analysis of ROS production was processed by flow
cytometry analysis. (C) MDA content was measured using a
commercially available kit, calculated based on a standard curve
and normalized to the total protein concentration. (D) NOX2
expression was detected by western blot analysis. (E) Quantitative
analysis of NOX2 expression. Bars represent the mean ± standard
deviation from ≥3 independent experiments. *P<0.05, **P<0.01
vs. control + miR-NC transfection group; #P<0.05,
##P<0.01 vs. H/R + miR-NC transfection group;
&P<0.05 vs. Pue + H/R + miR-NC transfection
group. miR, microRNA; H/R, hypoxia/reoxygenation; miR-NC, miR-21
inhibitor negative control; DCFH-DA,
2′,7′-dichlorodihydrofluorescein diacetate; ROS, reactive oxygen
species; MDA, malondialdehyde; NOX2, NADPH oxidase 2; Pue,
puerarin. |
Inhibition of miR-21 halts the
puerarin-induced improvement of the antioxidant defense system in
H/R-treated H9c2 cells
The endogenous antioxidant system provides defense
against oxidative stress-induced cytotoxicity, through enzymatic
and/or non-enzymatic mechanisms (24). In the present study, the effects of
puerarin on the antioxidant enzymes SOD, CAT and GSH-Px in H9c2
cells were evaluated. The results revealed that H/R leads to a
decrease in SOD activity and CAT and GSH-Px levels (Fig. 5A-C). These effects were enhanced by
miR-21 inhibition and abated by puerarin pretreatment. Furthermore,
miR-21 inhibition reversed the puerarin-induced upregulation of SOD
activity and CAT and GSH-Px levels in the H/R-treated group
(Fig. 5A-C). These results
demonstrated that miR-21 caused the puerarin-induced enhancement of
the antioxidant defense system under H/R conditions.
 | Figure 5.Effects of miR-21 inhibition on SOD
activity, as well as levels of CAT and GSH-Px in the presence or
absence of puerarin in H/R-treated H9c2 cells. Cells were
transfected with miR-21 inhibitor or miR-NC followed by treatment
with 200 µM puerarin for 1 h prior to incubation under H/R
conditions. (A) SOD activity, (B) CAT levels and (C) GHS-Px levels
were determined using commercially available kits. Data are
presented relative to the miR-NC transfection group. Bars represent
the mean ± standard deviation from ≥3 independent experiments.
*P<0.05, **P<0.01 vs. control + miR-NC transfection group;
#P<0.05, ##P<0.01 vs. H/R + miR-NC
transfection group; &P<0.05,
&&P<0.01 vs. Pue + H/R + miR-NC transfection
group. miR, microRNA; SOD, superoxide dismutase; CAT, catalase;
GSH-Px, glutathione peroxidase; H/R, hypoxia/reoxygenation; miR-NC,
miR-21 inhibitor negative control; Pue, puerarin. |
Discussion
The present study was the first to demonstrate that
miR-21 mediated the cardioprotective effect of puerarin against
H/R-induced injury in H9c2 cells by inhibiting apoptosis and
oxidative stress.
Puerarin is a major bioactive ingredient of the
Pueraria lobata (Willd.) Ohwi. root, a traditional Chinese
medicine widely used in the treatment of cardiovascular diseases,
including myocardial I/R injury (4,8,25).
miR-21 has been reported to be involved in the regulation of I/R
injury and the associated processes (14,15,26).
However, its role in the cardioprotective effect of puerarin has
not been reported to date. To the best of our knowledge, the
present study was the first to demonstrate that puerarin reduced
the H/R-induced downregulation of miR-21 in H9c2 cells. Notably,
the inhibition of miR-21 in H/R-treated H9c2 cells blocked the
puerarin-induced increase in cell viability and decrease in LDH
activity. These results indicated that miR-21 mediated the
puerarin-induced cardioprotection against H/R injury.
Apoptosis is a form of cardiomyocyte damage in the
early stages of myocardial I/R injury, and is crucial in its
pathophysiological processes (27,28).
Studies have demonstrated that puerarin exhibits protective effects
against I/R injury in cardiac tissues (29–31).
The potential underlying mechanisms involve its anti-apoptotic
functions. Guo et al (8)
reported that puerarin decreases I/R-induced myocardial injury in
diabetic rats via the suppression of apoptosis. In line with these
studies, the present study demonstrated that puerarin attenuated
the H/R-induced increase in apoptosis, as evidenced by the increase
in apoptotic rate and caspase-3 activity. Notably, miR-21 is
pro-survival and anti-apoptotic, and a number of studies have
confirmed the protective function of miR-21 in ischemic heart
diseases (16,32,33).
The present results further revealed that the inhibition of miR-21
in H/R-treated H9c2 cells enhanced the apoptotic rate, and reversed
the puerarin-induced downregulation of apoptosis and caspase-3
activity, indicating that miR-21 mediated the protective effect of
puerarin against H/R-induced apoptosis.
The Bcl-2 family proteins are also major regulators
of the apoptotic process, and one of the major processes regulated
by these factors is mitochondria-dependent apoptosis (34). The present results revealed that
the inhibition of miR-21 in H/R-treated H9c2 cells mitigated the
puerarin-induced decrease in pro-apoptotic protein Bax expression
and increase in anti-apoptotic protein Bcl-2 expression. Existing
studies have confirmed that puerarin inhibits apoptosis via
modulation of caspase-3 activity and Bcl-2/Bax expression,
resulting in the restoration of mitochondrial function, and that
miR-21 eliminates mitochondrial apoptosis (35–37).
The present results further demonstrated that miR-21 inhibition in
H/R-treated H9c2 cells enhanced the H/R-induced changes in Bax and
Bcl-2 expression, and reversed the puerarin-induced downregulation
of Bax and upregulation of Bcl-2. These results suggested that
miR-21 mediated the protection of puerarin against apoptosis, and
this process may be mitochondria-dependent.
Oxidative stress is a major contributor to
myocardial I/R injury (23). In
vivo and in vitro experiments have demonstrated that
puerarin exhibits an anti-oxidative function (38–40).
Gang et al (41)
demonstrated that puerarin suppresses angiotensin II-induced
cardiac hypertrophy by inhibiting NOX2 activation and oxidative
stress. However, the effect of puerarin on oxidative stress in
myocardial I/R injury remains unclear. In the present study,
puerarin significantly attenuated H/R-induced oxidative stress in
H9c2 cells, as evidenced by the decrease in endogenous ROS
production, MDA content and NOX2 expression. Furthermore, it has
been reported that miR-21 overexpression alleviates cardiomyocyte
apoptosis induced by oxidative stress (42). The results of the study by Wu et
al (37) revealed that miR-21
mimic transfection inhibits ROS-activated mitochondrial apoptosis.
In line with these findings, the present study demonstrated that
miR-21 inhibition exacerbated H/R-induced oxidative stress and
reduced the protective effect of puerarin in H9c2 cells. These
results suggested that miR-21 contributed to the protective effects
of puerarin against H/R-induced cell damage and apoptosis by
inhibiting oxidative stress processes.
Oxidative stress is caused by the imbalance between
ROS production and their neutralization and removal by the
antioxidant defense system (43).
Studies have suggested that myocardial I/R injury can lead to a
decrease in the levels of endogenous antioxidants, including SOD,
CAT and GSH-Px, and abolish antioxidant defenses, resulting in
cellular oxidative stress (43,44).
In addition, puerarin attenuates anoxia/reoxygenation-induced ROS
production, and SOD and GSH-Px inhibition in rat primary
cardiomyocytes (45). The present
findings confirmed that puerarin pre-treatment notably increased
SOD activity, and CAT and GSH-Px levels in H/R treated cells,
indicating the effects of puerarin on the promotion of the
anti-oxidative defense system in H/R injury. Furthermore, miR-21
inhibition worsened the H/R-induced loss of the anti-oxidative
system and reversed the puerarin-induced enhancement of antioxidant
levels under H/R conditions. These results showed that puerarin
protected against H/R-induced oxidative stress by enhancing the
anti-oxidative defense system, in a miR-21-dependent manner.
In summary, the results of the present study
indicated that miR-21 served an important role in the
puerarin-induced protection against myocardial H/R injury.
Enhancement of endogenous miR-21 expression, induced by puerarin,
alleviated H/R-induced cardiomyocyte injury, and the underlying
mechanism potentially involved the inhibition of apoptosis and
oxidative stress. The present study demonstrated that the use of
puerarin may be promising in the management of ischemic heart
diseases, which was involved in regulating miR-21.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HXX analyzed the data and wrote the manuscript. WP
and JFQ performed the experiments and analyzed of data. FL and HQD
made substantial contributions to the acquisition and analysis of
the data. QJL designed the study and was involved in drafting the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Turer AT and Hill JA: Pathogenesis of
myocardial ischemia-reperfusion injury and rationale for therapy.
Am J Cardiol. 106:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thind GS, Agrawal PR, Hirsh B, Saravolatz
L, Chen-Scarabelli C, Narula J and Scarabelli TM: Mechanisms of
myocardial ischemia-reperfusion injury and the cytoprotective role
of minocycline: Scope and limitations. Future Cardiol. 11:61–76.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Q, He GW, Underwood MJ and Yu CM:
Cellular and molecular mechanisms of endothelial
ischemia/reperfusion injury: Perspectives and implications for
postischemic myocardial protection. Am J Transl Res. 8:765–777.
2016.PubMed/NCBI
|
|
4
|
Liu B, Tan Y, Wang D and Liu M: Puerarin
for ischaemic stroke. Cochrane Database Syst Rev.
2:CD0049552016.PubMed/NCBI
|
|
5
|
Wei SY: Progress on cardiovascular
protections and mechanism research of puerarin. Zhongguo Zhong Yao
Za Zhi. 40:2278–2284. 2015.(In Chinese). PubMed/NCBI
|
|
6
|
Tang H, Song X, Ling Y, Wang X, Yang P,
Luo T and Chen A: Puerarin attenuates myocardial
hypoxia/reoxygenation injury by inhibiting autophagy via the Akt
signaling pathway. Mol Med Rep. 15:3747–3754. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wenjun H, Jing W, Tao L, Ling M, Yan Y,
Xiaorong Z and Rui Z: The protective effect of puerarin on
myocardial infarction reperfusion injury (MIRI): A meta-analysis of
randomized studies in rat models. Med Sci Monit. 21:1700–1706.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo BQ, Xu JB, Xiao M, Ding M and Duan LJ:
Puerarin reduces ischemia/reperfusion-induced myocardial injury in
diabetic rats via upregulation of vascular endothelial growth
factor A/angiotensin-1 and suppression of apoptosis. Mol Med Rep.
17:7421–7427. 2018.PubMed/NCBI
|
|
9
|
Gao Q, Pan HY, Bruce IC and Xia Q:
Improvement of ventricular mechanical properties by puerarin
involves mitochondrial permeability transition in isolated rat
heart during ischemia and reperfusion. Conf Proc IEEE Eng Med Biol
Soc. 6:5591–5594. 2005.PubMed/NCBI
|
|
10
|
Ambros V: MicroRNA pathways in flies and
worms: Growth, death, fat, stress, and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koroleva IA, Nazarenko MS and Kucher AN:
Role of microRNA in development of instability of atherosclerotic
plaques. Biochemistry (Mosc). 82:1380–1390. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pordzik J, Pisarz K, De Rosa S, Jones AD,
Eyileten C, Indolfi C, Malek L and Postula M: The potential role of
platelet-related microRNAs in the development of cardiovascular
events in high-risk populations, including diabetic patients: A
review. Front Endocrinol (Lausanne). 9:742018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Q, Yang K and Li A: microRNA-21
protects against ischemia-reperfusion and
hypoxia-reperfusion-induced cardiocyte apoptosis via the
phosphatase and tensin homolog/Akt-dependent mechanism. Mol Med
Rep. 9:2213–2220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu H and Fan GC: Role of microRNAs in the
reperfused myocardium towards post-infarct remodelling. Cardiovasc
Res. 94:284–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gu GL, Xu XL, Sun XT, Zhang J, Guo CF,
Wang CS, Sun B, Guo GL, Ma K, Huang YY, et al: Cardioprotective
effect of MicroRNA-21 in murine myocardial infarction. Cardiovasc
Ther. 33:109–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han Q, Zhang HY, Zhong BL, Zhang B and
Chen H: Antiapoptotic effect of recombinant HMGB1 A-box protein via
regulation of microRNA-21 in myocardial ischemia-reperfusion injury
model in rats. DNA Cell Biol. 35:192–202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin Y, Yu Y, Dong H, Bian X, Guo X and
Dong S: MicroRNA 21 inhibits left ventricular remodeling in the
early phase of rat model with ischemia-reperfusion injury by
suppressing cell apoptosis. Int J Med Sci. 9:413–423. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Q, Yang K, Li A and Tan W:
Anti-apoptosis and expression of microRNA-21 in rat myocardium
during early ischemia-reperfusion injury. Zhong Nan Da Xue Xue Bao
Yi Xue Ban. 38:483–489. 2013.(In Chinese). PubMed/NCBI
|
|
19
|
Cheng Y and Zhang C: MicroRNA-21 in
cardiovascular disease. J Cardiovasc Transl Res. 3:251–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng Y, Zhu P, Yang J, Liu X, Dong S,
Wang X, Chun B, Zhuang J and Zhang C: Ischaemic
preconditioning-regulated miR-21 protects heart against
ischaemia/reperfusion injury via anti-apoptosis through its target
PDCD4. Cardiovasc Res. 87:431–439. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mirzayans R, Andrais B, Kumar P and Murray
D: The growing complexity of cancer cell response to DNA-damaging
agents: Caspase 3 mediates cell death or survival? Int J Mol Sci.
17:E7082016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sinning C, Westermann D and Clemmensen P:
Oxidative stress in ischemia and reperfusion: Current concepts,
novel ideas and future perspectives. Biomark Med. 11:11031–11040.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Becker BF, Massoudy P, Permanetter B,
Raschke P and Zahler S: Possible significance of free oxygen
radicals for reperfusion injury. Z Kardiol. 5 (Suppl 82):49–58.
1993.(In German).
|
|
25
|
Xie B, Wang Q, Zhou C, Wu J and Xu D:
Efficacy and safety of the injection of the traditional chinese
medicine puerarin for the treatment of diabetic peripheral
neuropathy: A systematic review and meta-analysis of 53 randomized
controlled trials. Evid Based Complement Alternat Med.
2018:28346502018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye Y, Perez-Polo JR, Qian J and Birnbaum
Y: The role of microRNA in modulating myocardial
ischemia-reperfusion injury. Physiol Genomics. 43:534–542. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fliss H and Gattinger D: Apoptosis in
ischemic and reperfused rat myocardium. Circ Res. 79:949–956. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li W, Lu M, Zhang Y, Xia D, Chen Z, Wang
L, Yin N and Wang Z: Puerarin attenuates the daunorubicin-induced
apoptosis of H9c2 cells by activating the PI3K/Akt signaling
pathway via the inhibition of Ca2+ influx. Int J Mol Med.
40:1889–1894. 2017.PubMed/NCBI
|
|
30
|
Ling C, Liang J, Zhang C, Li R, Mou Q, Qin
J, Li X and Wang J: Synergistic effects of salvianolic acid B and
puerarin on cerebral ischemia reperfusion injury. Molecules.
23:E5642018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Tang Q, Shao S, Chen Y, Chen W and
Xu X: Lyophilized powder of catalpol and puerarin protected
cerebral vessels from ischemia by its anti-apoptosis on endothelial
cells. Int J Biol Sci. 13:327–338. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang Z, Li Z, Huang P, Luo J, Liu P, Wang
Y, Xia T and Zhou Y: Remote ischemic preconditioning upregulates
microRNA-21 to protect the kidney in children with congenital heart
disease undergoing cardiopulmonary bypass. Pediatr Nephrol.
33:911–919. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Richart A, Loyer X, Neri T, Howangyin K,
Guérin CL, Ngkelo A, Bakker W, Zlatanova I, Rouanet M, Vilar J, et
al: MicroRNA-21 coordinates human multipotent cardiovascular
progenitors therapeutic potential. Stem Cells. 32:2908–2922. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gross A: BCL-2 family proteins as
regulators of mitochondria metabolism. Biochim Biophys Acta.
1857:1243–1246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song XB, Liu G, Wang ZY and Wang L:
Puerarin protects against cadmium-induced proximal tubular cell
apoptosis by restoring mitochondrial function. Chem Biol Interact.
260:219–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Y, Ren L, Liu W and Xiao Z: MiR-21
regulates the apoptosis of keloid fibroblasts by caspase-8 and
mitochondrial-mediated apoptotic signaling pathway via targeting
fasL. Biochem Cell Biol. 96:548–555. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu H, Wang J, Ma H, Xiao Z and Dong X:
MicroRNA-21 inhibits mitochondria-mediated apoptosis in keloid.
Oncotarget. 8:92914–92925. 2017.PubMed/NCBI
|
|
38
|
Li X, Cai W, Lee K, Liu B, Deng Y, Chen Y,
Zhang X, He JC and Zhong Y: Puerarin attenuates diabetic kidney
injury through the suppression of NOX4 expression in podocytes. Sci
Rep. 7:146032017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang T, Liu Y, Huang C, Mansai HAA, Wei W,
Zhang X, Li X, Liu S and Yang S: Puerarin promotes MIN6 cell
survival by reducing cellular reactive oxygen species. Mol Med Rep.
17:7281–7286. 2018.PubMed/NCBI
|
|
40
|
Zhou X, Bai C, Sun X, Gong X, Yang Y, Chen
C, Shan G and Yao Q: Puerarin attenuates renal fibrosis by reducing
oxidative stress induced-epithelial cell apoptosis via MAPK signal
pathways in vivo and in vitro. Ren Fail. 39:423–431. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gang C, Qiang C, Xiangli C, Shifen P,
Chong S and Lihong L: Puerarin suppresses angiotensin II-induced
cardiac hypertrophy by inhibiting NADPH oxidase activation and
oxidative stress-triggered AP-1 signaling pathways. J Pharm Pharm
Sci. 18:235–248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wei C, Li L, Kim IK, Sun P and Gupta S:
NF-κB mediated miR-21 regulation in cardiomyocytes apoptosis under
oxidative stress. Free Radic Res. 48:282–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zuo L, Zhou T, Pannell BK, Ziegler AC and
Best TM: Biological and physiological role of reactive oxygen
species - the good, the bad and the ugly. Acta Physiol (Oxf).
214:329–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhou T, Prather ER, Garrison DE and Zuo L:
Interplay between ROS and antioxidants during ischemia-reperfusion
injuries in cardiac and skeletal muscle. Int J Mol Sci.
19:E4172018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma Y, Gai Y, Yan J, Li J and Zhang Y:
Puerarin attenuates anoxia/reoxygenation injury through enhancing
Bcl-2 associated athanogene 3 expression, a modulator of apoptosis
and autophagy. Med Sci Monit. 22:977–983. 2016. View Article : Google Scholar : PubMed/NCBI
|



















