Introduction
Bone regeneration is mediated by a wide range of
intracellular and extracellular events. In clinical settings,
platelet concentrates can be used to optimize the healing of hard
and soft tissues.
Platelet gel, which was first described in 1997, is
derived from collected autologous blood and formed by combining
platelet-rich plasma (PRP), thrombin, and calcium chloride
(1). PRP is an autologous source
of platelet-derived growth factor (PDGF) and transforming growth
factor β (TGF-β) that is obtained by sequestering and concentrating
platelets by gradient density centrifugation (2).
In 2001, platelet-rich fibrin (PRF) was discovered
in France by J. Choukroun using an innovative method that required
neither anticoagulants nor coagulation factors. PRF, which is
called the second-generation platelet concentrate, has been shown
to contain more growth factors than traditionally prepared PRP
(3).
Recently, numerous techniques using platelet
concentrates have been developed to obtain different ratios of
platelets, growth factors, leukocytes and fibrin matrices (4). Concentrated growth factors (CGFs) are
the PRF derivatives developed by Sacco in 2006 (5). CGFs are produced by centrifuging
blood samples with a special centrifuge device (Medifuge,
Silfradent Srl, Italy). Nevertheless, CGFs contain a much larger,
denser and richer growth factor fibrin matrix than PRF (5,6).
CGFs are now widely used to shorten the interval
between bone graft placement and implant insertion and increase the
success rate of bone grafting and implant therapy. Many articles
have been published on the application of CGFs in the dental and
maxillofacial field (7–9).
When using concentrated growth factors (CGFs), the
release of growth factors is excessively rapid. He et al
(10) showed that the
three-dimensional fiber network scaffold of CGFs can help release
growth factors slowly for at least 7–10 days. However, bone healing
is a long and complex process. Most of these studies focused on the
application of CGFs in the clinic. Extending the release cycle of
growth factors to match the cycle of bone remodeling is difficult,
and few reports have mentioned this issue.
Chitosan, also known as soluble chitin, is a natural
polysaccharide cellulose that is nontoxic, biocompatible,
biodegradable, and widely found in insect, crustacean shells and
fungal cell walls (11,12). Its preparation is simple, its
source is rich, and its hydrophilicity is strong. Chitosan can be
biodegradable by in vivo lysozyme, pepsin, and other
enzymes. The degradation products are nontoxic and can be
completely absorbed by the organisms (13). Chitosan has a good film-forming
property, and the film has good biological compatibility and
permeability. Thus, chitosan has important development and research
value for sustained-release drugs and targeted drug delivery, and
it is often used as the vehicle for the sustained release of drugs.
Sustained-release drugs are released slowly over a long period of
time to achieve sustained administration (14). Chitosan can be used to prepare
microspheres of different sizes. The combination of microspheres
and drugs can avoid the use of organic solvents and prevent the
denaturation of antigenic proteins (15). Chitosan has a unique polycationic
property.
Sodium alginate (SA), a negatively charged
biopolysaccharide, is extracted from brown seaweed. SA has also
been widely used for hemostatic applications because of its
excellent abilities to enhance the adhesion between composite
materials and wounds, to improve material plasticity through its
high water absorption rate and to easily form a viscous colloidal
solution (16,17).
However, while the positive charge of chitosan
promotes erythrocyte adhesion, fibrinogen adsorption, and platelet
adhesion/stimulation, it also inhibits the activation of the
contact system (18). Chitosan
interacts with sodium alginate (polyanion) by electrostatic
interaction, which can improve microcapsule stability and drug
loading and adjust the drug release rate (19).
Therefore, we aimed to preserve CGFs for storage by
freeze-drying without additives. Furthermore, we investigated
whether the freeze-dried CGFs in a chitosan-alginate composite gel
can release CGFs steadily to achieve effective osteogenesis.
Materials and methods
Blood sample centrifugation
Vein blood samples were collected from 10 healthy
volunteers (4 females and 6 males), who were nonsmokers between 20
and 30 years of age.
CGFs were produced as follows: 5 ml of blood was
drawn from the arm vein in blood collection tubes without
anticoagulant solution, with 2 tubes used for each collection
between October 2017 and December 2018. These tubes were
immediately centrifuged in a special machine (Medifuge MF200,
Silfradent Srl, Italy). At the end of the process, there were three
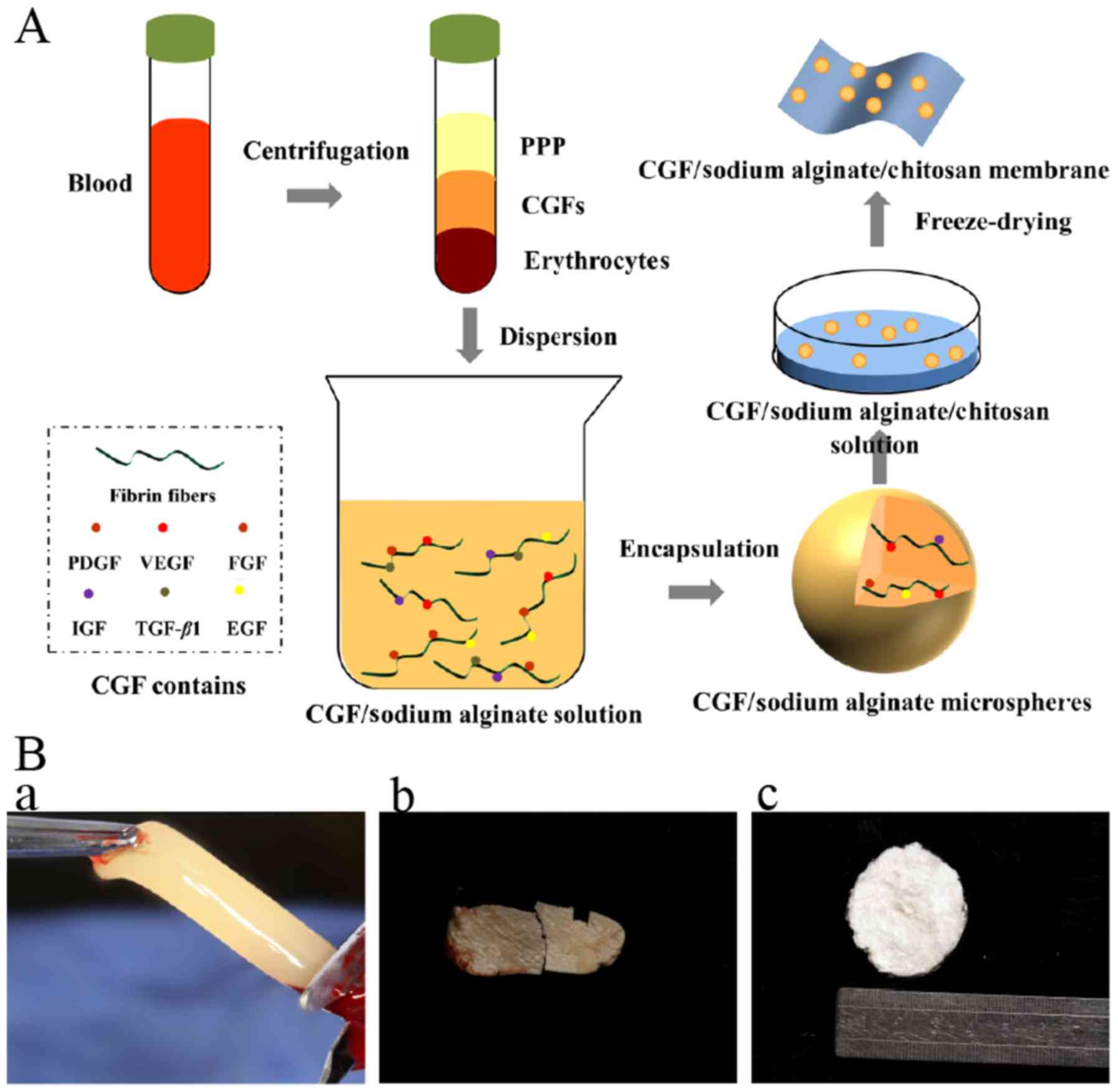
blood fractions. The middle part (CGF) as shown in Fig. 1A (fibrin-rich gel with aggregated
platelets and CGFs) was removed.
Lyophilization of CGFs
CGFs were pre-frozen at −80°C for 12 h and then
lyophilized for 24 h using a freeze dryer (Martin Christ Freeze
Dryers GmbH, Germany). After freeze-drying, samples were ground
into a powder, which we called freeze-dried CGF. Then, 0.02 g/ml
alginate was mixed with the CGF powder, and 0.02 g/ml chitosan was
mixed with the alginate-CGF powder composite hydrogels, then, the
composite CGF hydrogels were lyophilized again. Finally, the
chitosan-alginate composite CGF membrane, which was called
sustained release CGFs was obtained (Fig. 1). Both types of CGFs were stored at
−4°C for one month.
Cell culture
The murine-derived cell line MC3T3-E1 was used in
this study. MC3T3-E1 cell cultures were maintained in minimum
essential medium (MEM, Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher
Scientific, Inc.), and 1% (v/v) penicillin/streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a humidified
CO2 incubator. Cells at approximately 80% confluence,
were passaged by trypsin digestion and expanded through two
passages before being used for the study.
Material cytotoxicity test
Cell climbing of chitosan-alginate
composite hydrogels
The prepared chitosan-alginate composite gel was
spread on a cell slide, which was placed in a 6-well plate, air
dried, sterilized by ultraviolet light for 1 day, seeded with 5,000
cells per well, and fixed with paraformaldehyde after 3 days. After
climbing, air drying, and spraying with gold, the cell morphology
was observed under a scanning electron microscope (Hitachi S-3400N;
Hitachi, Ltd.).
Live and dead assays of
sustained-release CGFs
The sustained-release CGFs were spread on a 48-well
plate, which was seeded with 1×104 cells per well. Cell
viability was evaluated with confocal microscopy after staining
with calcein AM and propidium iodide (PI) (Invitrogen; Thermo
Fisher Scientific, Inc.) at days 3 and 5 after seeding. Samples
were incubated for 20 min at 37°C with the calcein solution in
culture medium (1 µl of calcein per ml of MEM), washed in
phosphate-buffered saline (PBS), exposed to the second staining
solution with PI in PBS (100 µl of PI pe ml of PBS), double washed
in PBS and immediately visualized with a confocal microscope (Leica
Microsystems CMS GmbH).
Cell proliferation and metabolic
activity of the two forms of CGFs
CGFs were placed in a 15-ml centrifuge tube, and 5
ml of culture medium was added. This sample was stored in a
refrigerator at 4°C for 24 h. The incubation solution was a
gradient series of concentrations (20, 40, 60, 80 and 100%).
MC3T3-E1 cells were seeded in a 96-well tissue culture plate at a
density of 3,000 cells per well. After 24 h of incubation, the
culture medium was removed and replaced with 200 µl of different
concentrations of CGF extract containing 10% FBS. At days 1, 3 and
5, Cell Counting Kit-8 (CCK-8) assay was applied to determine the
overall proliferation. The absorbance [optical density (OD) value
at 450 nm] was read by a microplate reader (Thermo Scientific,
Inc.). The most suitable concentrations of the CGFs were screened
based on the results of the MC3T3-E1 proliferation study and were
used in the following experiments.
A control group, a freeze-dried CGF group, a
sustained-release CGF group, and a blank chitosan-alginate
composite hydrogel group were used. MC3T3-E1 cells were seeded in a
96-well tissue culture plate at a density of 3,000 cells per well.
After 24 h of incubation, the culture medium was removed and
replaced with 200 µl of different extracts containing 10% FBS. On
days 1–6, a CCK-8 assay was applied to determine the overall
proliferation.
Alkaline phosphatase (ALP)
quantification
MC3T3-E1 cells were seeded in a 24-well tissue
culture plate at a density of 2.5×104 cells per well.
After 24 h, the corresponding reagents were added to the
experimental groups. The cells were lysed with Triton X-100 for 4,
7 and 14 days, and the total protein concentration was measured
using a BCA assay kit (Beyotime, China). The ALP activity was
measured using an ALP assay kit (Nanjing Jiancheng Biotech,
China).
Cells were seeded in a 48-well tissue culture plate
at a density of 2×104 cells per well. After 24 h, the
corresponding reagents were added to the experimental group. After
14 and 21 days, the cells were stained with a BCIP/NBT alkaline
phosphatase colorimetric kit (Beyotime).
Alizarin Red S staining
To identify the mineralization nodules, Alizarin Red
S (ARS; Sigma-Aldrich; Merck KGaA) staining was performed after the
MC3T3-E1 cells were seeded at a density of 2×104 cells
per well and grew for 21 days in conditioned or control media
without osteogenic supplements. After gently rinsing with
ddH2O, the cells were stained in a solution of 2% ARS at
pH 4.1 for 20 min and then washed with ddH2O. The
samples were air-dried, and images were captured under a light
microscope (magnification, ×6). Additionally, the bound dye was
dissolved with 10% cetylpyridinium chloride, and the ARS in the
samples was quantified by measuring the absorbance at 562 nm.
Bone-related gene expression
For the detection of bone-related genes (Table I), i.e., ALP, osteopontin
(OPN), osteoclastogenesis inhibitory factor (OPG),
collagen type I (COL1), osteocalcin (OG), Runx2 and
bone sialoprotein (BSP), MC3T3-E1 cells were plated at a
density of 1×104 cells per well in separate 6-well
plates. After 24 h of incubation, the culture medium was removed
and replaced with conditioned medium or control medium. Total RNA
from all groups was extracted using TRIzol reagent after 7, 14, 21
and 28 days of culture and analyzed by reverse
transcription-quantitative (RT-q) PCR.
 | Table I.Primer pairs used in reverse
transcription-quantitative quantitative PCR. |
Table I.
Primer pairs used in reverse
transcription-quantitative quantitative PCR.
| Genes | Primer
sequences |
|---|
| GAPDH | Forward:
5′-AAGAAGGTGGTGAAGCAGG-3′ |
|
| Reverse:
5′-GAAGGTGGAAGAGTGGGAGT-3′ |
| OPG | Forward:
5′-AGTTTTGGGAAAGTGGGATGT-3′ |
|
| Reverse:
5′-GCTACTCAGTTTATGGAGGATCA-3′ |
| OG | Forward:
5′-GTAACGAGTGTCATTAGCCTTG-3′ |
|
| Reverse:
5′-ATAACGACCTGGAATCTGTGC-3′ |
| Runx2 | Forward:
5′-TCAGCGTCAACACCATCATTC-3′ |
|
| Reverse:
5′-CCAGACCAGCAGCACTCCATA-3′ |
| BSP | Forward:
5′-CAAAAGTCTGTGCTTGGGGTG-3′ |
|
| Reverse:
5′-GGAAAACAATGAAGATTCTGAGGG-3′ |
| OPN | Forward:
5′-CCTTAGACTCACCGCTCTTCAT-3′ |
|
| Reverse:
5′-TTCACTCCAATCGTCCCTACA-3′ |
| ALP | Forward: 5′-
CAGTTCGTATTCCACATCAGTTC-3′ |
|
| Reverse:
5′-CAAGGACATCGCATATCAGCT-3′ |
| COL1A1 | Forward:
5′-AGAACAGCGTGGCCT-3′ |
|
| Reverse:
5′-TCCGGTGTGACTCGT-3′ |
ELISA quantification
The freeze-dried CGFs and sustained-release CGFs
were placed in a 15-ml centrifuge tube, and 5 ml of PBS was added.
The samples were stored in a refrigerator at 4°C. We took 1 ml
extract out to perform the ELISA and added 1 ml fresh PBS to the
tube to maintain a total volume of 5 ml at each time point. When
all samples were collected, quantifications of transforming growth
factor β1 (TGF-β1), insulin-like growth factor-1 (IGF-1),
platelet-derived growth factor-AB (PDGF-AB), vascular endothelial
growth factor (VEGF), and thrombospondin-1 (TSP-1) were performed
using commercially available ELISA kits (Cusabio, Biotech Co, Ltd.,
Wuhan, China). For each molecule and each experimental period, the
means and standard deviations were calculated. Finally, for each
tested molecule, the total released amounts were calculated, and
these results were then compared to the initial amount forcibly
extracted from the freeze-dried CGFs and the sustained-release
CGFs.
Statistical analysis
All analyses were performed using SPSS 25 software
(IBM Corp., Armonk, NY, USA). All experiments were performed at
least in three independent repeats. All data are shown as the mean
and standard deviation (SD) and were analyzed using one-way ANOVA
or a nonparametric test followed by the least significant
difference post hoc test. The levels of significance were set at
*P<0.05, **P<0.01 and ***P<0.001 (as indicated in the
figures and legends with the corresponding symbols).
Results
Characterization of the three types of
CGFs
The architecture and morphology of the CGFs,
freeze-dried CGFs, and sustained-release CGFs are shown in Fig. 1B. The surface morphology of
freeze-dried CGFs and powdered freeze-dried CGFs were examined via
scanning electron microscopy (SEM) (Fig. 2A). The freeze-dried CGF powder,
hereafter called freeze-dried CGF, was used for the following in
vitro studies.
Evaluation of cell bioactivity with
the sustained-release CGFs
The cell climbing results with the chitosan-alginate
composite hydrogels are presented in Fig. 2B. There were many cell nuclei
highlighting the hydrogels (Fig.
2B-b). At the junction of the gel, MC3T3-E1 cells (Fig. 2B-c) could be observed, which
suggested that the chitosan-alginate composite hydrogels had
excellent biocompatibility and was non-toxic to the MC3T3-E1
cells.
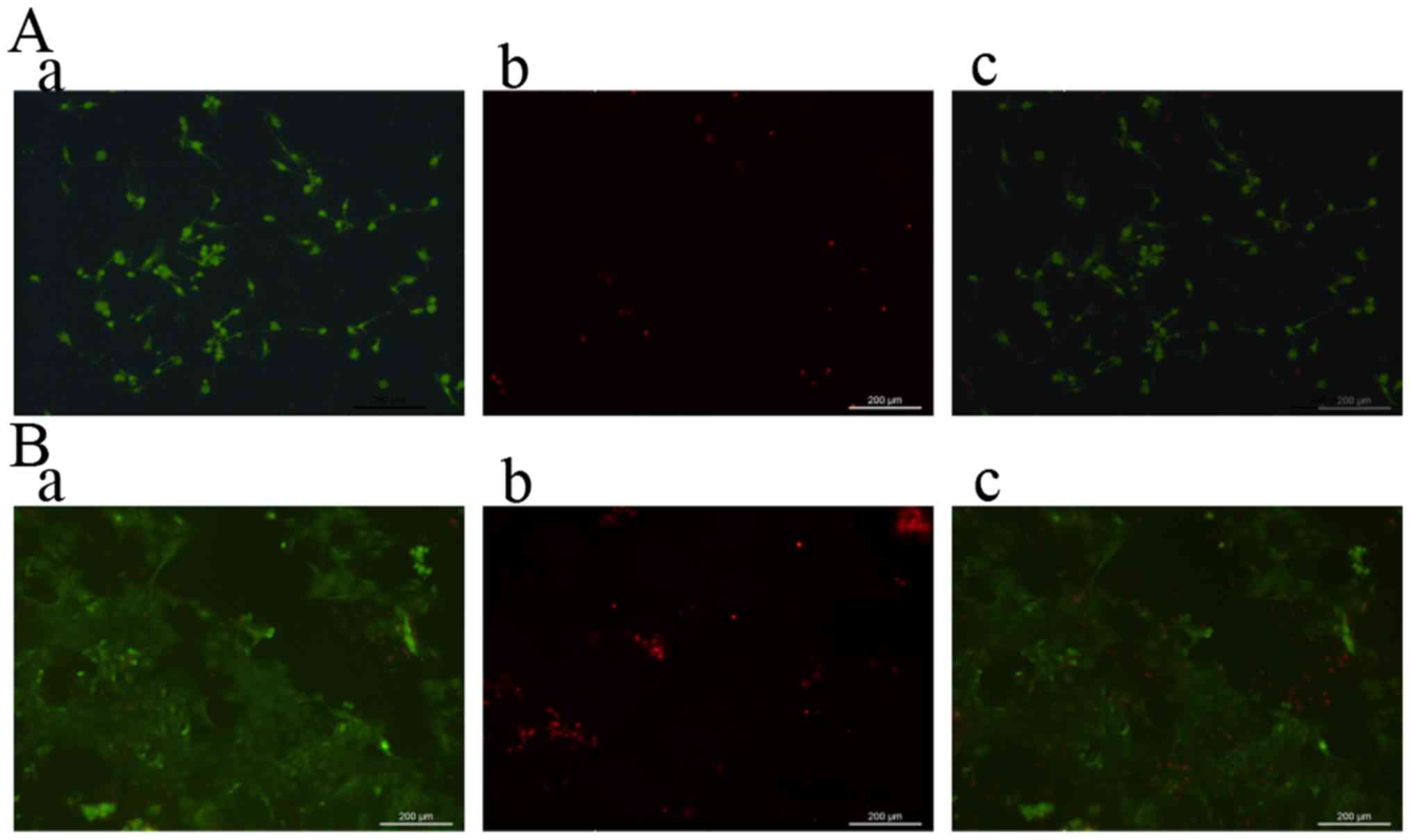
The live and dead assays of the sustained-release
CGFs and blank chitosan-alginate gel on day 5 are shown in Fig. 3. For this assay, we observed a much
greater abundance of living MC3T3-E1 cells in the sustained-release
CGF group than that in the blank gel group. Compared to the cells
in the blank gel group, the MC3T3-E1 cells in the sustained-release
CGF group adhered more tightly. This finding suggested that the
sustained-release CGFs had an obvious ability to promote cell
proliferation and adhesion.
Effect of the two forms of CGFs on
osteoblast proliferation
The proliferation of MC3T3-E1 cells under different
concentrations of CGFs was assessed using CCK-8 assay. The analysis
showed that the metabolic activity of MC3T3-E1 cells at different
concentrations of CGFs had significant differences (Fig. 4). The medium with incubation
solution extracts (20%) was used for the following in vitro
studies.
MC3T3-E1 cells grew well in both the culture medium
and the medium with a series of material extracts. The CCK-8
analysis showed that the overall metabolic activity of most groups
with 20% material extracts increased in a time-dependent manner
(Fig. 5). Compared with that of
the control group, the cell proliferation rates in the freeze-dried
CGF group and the sustained-release CGF group were significantly
increased on day 6 (P<0.001). In contrast, the material group
did not exhibit a difference in cell proliferation throughout the
time period. This finding suggested that the simple
chitosan-alginate composite hydrogels could not promote MC3T3-E1
cell proliferation and that only sustained-release CGFs, which
combined chitosan-alginate composite hydrogels and CGFs, could
promote cell proliferation.
Effect of the two forms of CGFs on ALP
activity
ALP is central to osteogenesis. The level and
activity of ALP are considered early osteogenic markers,
particularly in in vitro experiments, as predictors of bone
maturation and mineralization (20). The results of ALP staining
(Fig. 6A) in the (a) control
group, (b) freeze-dried CGF group and (c) sustained-release CGF
group are shown on day 14 of culture. The ALP activity in the
MC3T3-E1 cells at day 14 of culture for the different groups is
shown in Fig. 6A-d. ALP production
increased with incubation time in all groups. At 14 days of
incubation, the freeze-dried CGF group had significantly higher ALP
activity than the other groups (Fig.
6A-d).
The results of ALP staining of MC3T3-E1 cells at 14
days are shown in Fig. 6A. At 21
days (Fig. 6C, first row), the ALP
staining of the MC3T3-E1 cells in the sustained-release CGF group
(Fig. 6C-c-1) was clearer than
that in the other groups (Fig. 6C-a-1
and -b-1). This finding suggested that, between 14 and 21 days,
the expression of ALP in the sustained-release CGF group gradually
increased, possibly due to the slow release of growth factors in
the sustained release CGFs.
Effect of the two forms of CGFs on
mineralization capability
The effects of the two forms of CGFs on MC3T3-E1
cell mineralization capability were assessed using ARS staining. As
shown in Fig. 6B [(a) control
group, (b) freeze-dried CGF group and (c) sustained-release CGF
group], the amount of mineralization nodules in the
sustained-release CGF group was obviously higher than that in the
other group (Fig. 6B-d). When we
performed ALP staining, we found that, in the location with ALP
staining, there was a translucent circular plaque in the middle,
which we suspected was a mineralized nodule. To understand the
relationship between ALP and mineralization, we performed ALP
staining and ARS staining in the same cell frame (Fig. 6C) (Fig. 6C-a-2 to c-2; second row). The
double staining results suggested that the sustained-release CGFs
(Fig. 6C-c-2) had an obvious
ability to promote ALP expression and osteogenic mineralization
under long-term observation.
Assessment of osteogenesis-associated
genes
The expression of genes associated with MC3T3-E1
were evaluated by RT-qPCR on days 7, 14, 21 and 28. As shown in
Fig. 7, BSP (Fig. 7A), OG (Fig. 7B), OPG (Fig. 7C), and OPN (Fig. 7D) showed more significant
upregulation on day 7 in the freeze-dried CGFs group when compared
with the other groups. (Fig. 7E) A
significant upregulation of the gene expression of COL1A1 occurred
on day 14 in the freeze-dried CGF group. (Fig. 7G) ALP was found to be significantly
upregulated on day 28 in the sustained-release CGFs. (Fig. 7F) The gene expression of Runx2
showed significant upregulation on day 21.
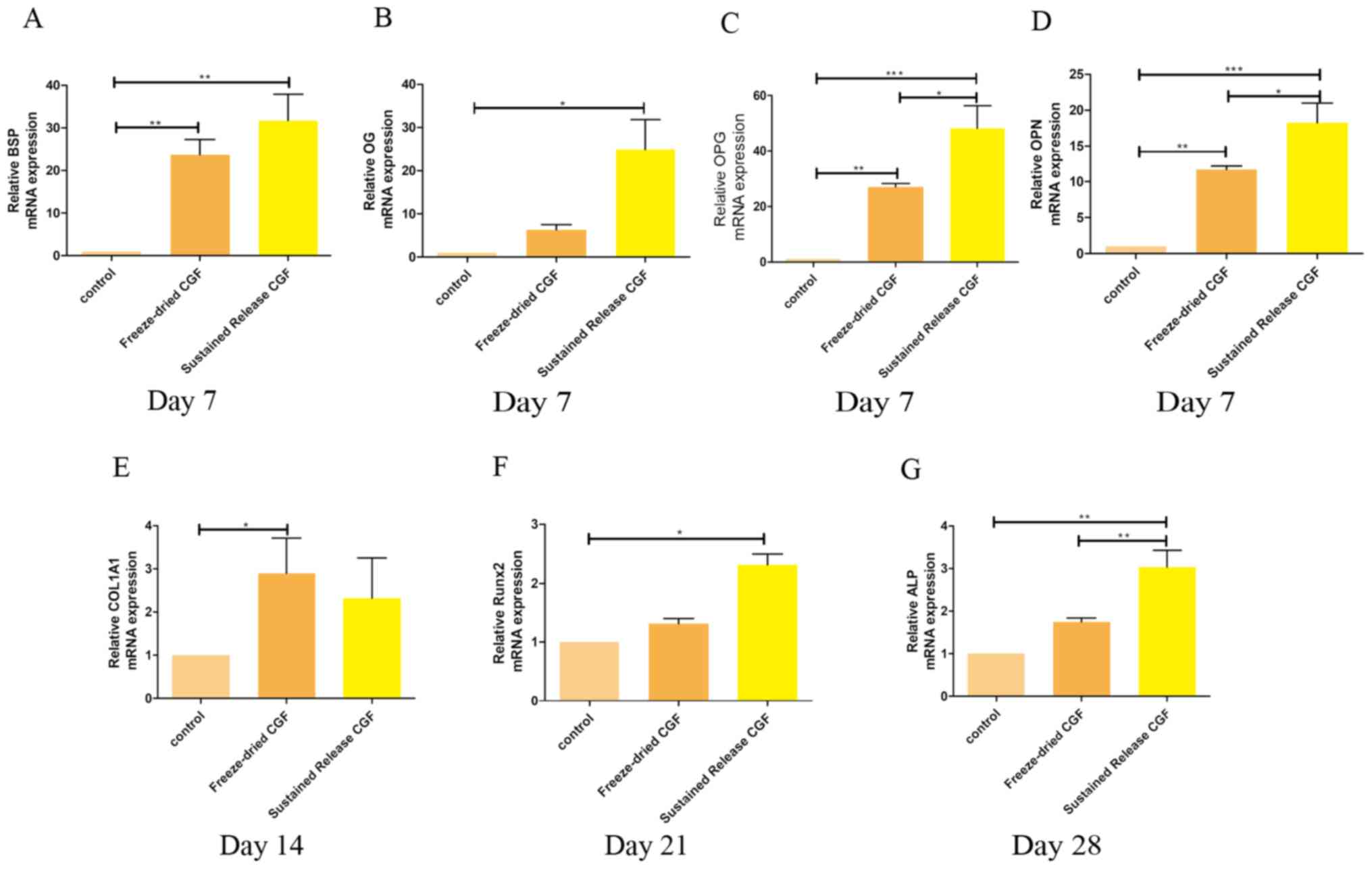 | Figure 7.Expression of the
osteogenesis-associated genes BSP, OG, OPG, OPN, COL1A1,
Runx2 and ALP were evaluated by RT-qPCR on days 7, 14,
21, 28 (data not shown for all times). Addition of freeze-dried CGF
and sustained-release CGF to the culture media significantly
increased expression of (A) BSP, (B) OG, (C)
OPG and (D) OPN in the MC3T3-E1 cells on day 7, (E)
COL1A1 on day 14 and (F) Runx2 on day 21. Significant
upregulation of (G) ALP was found in the MC3T3-E1 cells
cultured with the freeze-dried CGFs and sustained-release CGFs
compared to that in the control group on day 28. *P<0.05,
**P<0.01, ***P<0.001. CGFs, concentrated growth factors;
OPG, osteoprotegerin; OG, osteocalcin; Runx2,
runt-related transcription factor 2; BSP, bone sialoprotein;
OPN, osteopontin; ALP, alkaline phosphatase; COL1A1,
collagen τype I α1. |
Growth factor release analysis
Significant amounts of each growth factors were
found at each experimental time point, even 30 days after the
production of the freeze-dried CGFs and the sustained-release CGFs
(Fig. 8). Moreover, these growth
factors and TSP-1 resulted in a significantly rapid declined in the
freeze-dried group before day 7. This finding could also explain
the very high expression of osteogenic genes (BSP, OG, OPN,
OPG) on day 7 for the the sustained release CGF group. The
sustained-release group maintained the release of growth factors at
the same concentration.
 | Figure 8.The release of (A) IGF-1, (B) TSP-1,
(C) PDGF-AB, (D) TGF-β1 and (E) VEGF from the freeze-dried CGFs and
the sustained-release CGFs for 30 days. Values are expressed as the
cumulative mean quantity of molecules at 1, 6, 24 h, 3, 5, 7, 10,
13, 16, 20, 25 and 30 days. IGF-1, insulin-like growth factor-1;
TSP-1, thrombospondin-1; PDGF-AB, platelet-derived growth
factor-AB; TGF-β1, transforming growth factor β1; VEGF, vascular
endothelial growth factor; CGFs, concentrated growth factors. |
Discussion
CGFs have recently received a great deal of interest
as the growth factors in CGFs can be locally released into tissue
to enhance wound healing.
Seventy percent of the growth factors in PRP are
released within 10 min, and almost 100% are released within 1 h
after activation (21,22). Dohan Ehrenfest et al
(23) found that platelet-rich
fibrin (PRF) can release growth factors slowly for at least one
week, which is attributed to its fiber network scaffold. The study
also suggested that the release rate is dependent on the
environment surrounding the PRF (24). CGFs can release growth factors
slowly for at least 7–10 days. The new bone formation (NBF) rate
around an implant treated with CGFs is higher than that treated
with PRF (25).
Currently, the major applications of CGFs in damaged
tissue utilize its gelatin form. We lyophilized and ground the CGFs
into a powder. Then, we integrated chitosan-alginate composite gel,
freeze-dried CGFs into a membrane and evaluated the physical and
osteogenic capacities of this membrane. In addition, through the
quantitative detection of growth factors released from freeze-dried
CGFs and sustained release CGFs, we explored the feasibility of
using this composite gel as a carrier to achieve long-term
sustained-release of CGFs.
We first assessed the biosafety of the
chitosan-alginate composite gel via cell climbing and cell
proliferation assays (Figs. 2 and
5). The data indicated that the
gel was biocompatibility and had low cytotoxicity and that the
cells could maintain normal growth on the gel. Under scanning
electron microscope, it was observed that the gel possessed high
porosity to provide suitable space for cell adhesion and
proliferation and to allow the exchange of nutrients and waste
(Fig. 2). When the gel and the
CGFs powder were integrated, the sustained-release CGFs were
obtained. The osteogenesis of the sustained-release CGFs compared
to the freeze-dried CGFs was verified. It was found that the
freeze-dried CGF group showed a stronger ability to boost the
proliferation of MC3T3-E1 cells during the first 6 days (Fig. 5), and in the first 14 days, ALP
protein expression increased. Afterward, the sustained-release CGF
group achieved better results (Fig.
6). On day 21, the ALP staining and mineralized nodule staining
results of the sustained-release CGF group were significantly
better than those of the freeze-dried CGF group (Fig. 6). The expression of OPG, OPN,
Runx2 and ALP was also increased (Fig. 7).
To evaluate whether chitosan-alginate composite gel
could be used as a carrier for growth factors, we investigated the
concentration of growth factors released from sustained-release CGF
and freeze-dried CGF in vitro were investigated. Our data
revealed that different growth factors showed different
time-dependencies.
The thrombospondin-1 protein (TSP-1) has been shown
to affect platelet aggregation, angiogenesis, and tissue remodeling
(26,27). TSP-1 is a major regulator of
transforming growth factor β-1 (TGF-β1) activation but also has
TGF-β-independent functions in hemostasis, cell adhesion,
migration, and growth factor (EGF, VEGF, FGF) regulation (28). TGF-β1 performs many cellular
functions, including the control of cell growth, cell
proliferation, cell differentiation, and apoptosis (29). TGF-β1 increases the synthesis of
collagen types I, III and IV and the deposition of fibronectin,
proteoglycans and tenascin (30).
IGF-I is an important factor that regulates bone cell function and
metabolism. It can reduce collagen degradation, increase bone
deposition, and promote osteoblast differentiation, maturation and
supplementation (31).
Platelet-derived growth factor-AB (PDGF-AB) promotes the secretion
of collagen and glycoproteins by osteoblasts to synthesize the bone
matrix through the action of osteoblasts and participates in bone
matrix calcification (32).
Vascular endothelial growth factor (VEGF) influences skeletal
development and postnatal bone repair. Modulation of VEGF levels in
bones represents a potential strategy for treating compromised bone
repair and improving bone regeneration (33).
In the present study, at the beginning of the
experiment, more IGF-1, VEGF, TGF-β1 and PDGF-AB were released by
the freeze-dried CGFs than by the sustained-release CGFs. The
release rate of these growth factors then decreased rapidly in the
freeze-dried CGF group, while the release rate of the sustained
release CGF group increased slowly. On day 7, the concentrations of
IGF-1 and VEGF in the two groups were almost the same. On day 14,
the concentrations of TGF-β1 and PDGF-AB in the two groups were
almost the same (Fig. 8). This
result may be relevant for the different growth factor release
patterns of freeze-dried CGFs and sustained-release CGFs. In the
freeze-dried CGF group, the growth factors were in a state of free
diffusion in the extract, and they were completely exposed to the
solution. Over time, the growth factors slowly inactivated; then,
the concentration of the growth factors in the solution decreased.
In the sustained-release CGF group, the gel carrier enveloped the
growth factors so that they were slowly released in the solution,
and the concentration of the growth factors was maintained for a
long period. However, the pore structure of the gel helped the
cells adhere, which benefits cell proliferation and
osteogenesis.
After day 14, the concentration of growth factors in
the freeze-dried group decreased sharply from that at the
beginning, while the sustained-release group maintained a stable
concentration, which made the osteogenic expression ability of the
sustained-release group significantly higher than that of the
freeze-dried group. In the present study, a significant phenomenon
was noted. The medium used in the design of this experiment was not
supplemented with osteogenic mineralization-inducing solution.
However, in both experimental groups, obvious mineralized nodules
were observed, which suggests that the composite growth factors in
CGFs may have the ability to promote osteoblast
self-mineralization. However, this possibility requires
verification through further experiments.
Numerous drug delivery systems based on the
association of chitosan have been reported in the literature
(34). For example, layered
composite hydrogels have been used as cell culture carriers and
matrices for the control release (35), and microparticles based on
alginate/chitosan, alginate-chitosan beads or chitosan-coated
alginate beads have been used as sustained release drug delivery
systems (36). The results of the
present study verified that the use of chitosan-alginate gel
carrier can achieve the slow release of growth factors in CGFs and
that the sustained-release CGFs can achieve superior osteogenic
effects.
In conclusion, the freeze-dried CGFs demonstrated
superior osteogenic performance than the sustained-release CGFs in
the early stages. Over time, the sustained-release CGFs had
significant advantages over the freeze-dried CGFs in terms of
promoting osteogenic mineralization. The present study revealed
that lyophilization and the chitosan-alginate carrier enabled the
growth factors in CGFs to maintain a stable release concentration
and to achieve a superior osteogenesis-promotive effect.
Acknowledgements
Professor Yin Xiao from the Institute of Health and
Biomedical Innovation of the Queensland University of Technology of
Australia is kindly thanked for providing assistance with the
experiments.
Funding
The study was supported by the Liwan District
Science and Technology Project (grant no. 2016080065).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW and MW made equal contributions to this article
and should be regarded as co-first authors. LW conceived and
designed the study. MW and ZL performed the experiments. MW wrote
the initial manuscript. NZ and DL were responsible for data and
statistical analyses. LG reviewed the findings and LG checked the
acquired data, contributed in the analysis and interpretation of
data and edited the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The collection and use of blood samples were
performed with informed consent from the volunteers and all
protocol consistently adhered to the privacy rights of the human
volunteers. This study was approved by the Ethics Committee of
Stomatology Hospital, Guangzhou Medical University (KY2018013).
Patient consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest
associated with the present study.
References
|
1
|
Whitman DH, Berry RL and Green DM:
Platelet gel: An autologous alternative to fibrin glue with
applications in oral and maxillofacial surgery. J Oral Maxillofac
Surg. 55:1294–1299. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Vos RJ, Weir A, van Schie HT,
Bierma-Zeinstra SM, Verhaar JA, Weinans H and Tol JL: Platelet-rich
plasma injection for chronic Achilles tendinopathy: A randomized
controlled trial. JAMA. 303:144–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dohan DM, Choukroun J, Diss A, Dohan SL,
Dohan AJJ, Mouhyi J and Gogly B: Platelet-rich fibrin (PRF): A
second-generation platelet concentrate. Part III: Leucocyte
activation: A new feature for platelet concentrates? Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 101:e51–e55. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anitua E, Aguirre JJ, Algorta J, Ayerdi E,
Cabezas AI, Orive G and Andia I: Effectiveness of autologous
preparation rich in growth factors for the treatment of chronic
cutaneous ulcers. J Biomed Mater Res B Appl Biomater. 84:415–421.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rodella LF, Favero G, Boninsegna R,
Buffoli B, Labanca M, Scarì G, Sacco L, Batani T and Rezzani R:
Growth factors, CD34 positive cells, and fibrin network analysis in
concentrated growth factors fraction. Microsc Res Tech. 74:772–777.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawase T and Tanaka T: An updated proposal
for terminology and classification of platelet-rich fibrin. Regen
Ther. 7:80–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sohn DS, Moon JW, Moon YS, Park JS and
Jung HS: The use of concentrated growth factor (CGF) for sinus
augmentation. J Oral Implant. 38:25–38. 2009.
|
|
8
|
Sohn DS, Heo JU, Kwak DH, Kim DE, Kim JM,
Moon JW, Lee JH and Park IS: Bone regeneration in the maxillary
sinus using an autologous fibrin-rich block with concentrated
growth factors alone. Implant Dent. 20:389–395. 2011.PubMed/NCBI
|
|
9
|
Yu B and Wang Z: Effect of concentrated
growth factors on beagle periodontal ligament stem cells in vitro.
Mol Med Rep. 9:235–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He L, Lin Y, Hu X, Zhang Y and Wu H: A
comparative study of platelet-rich fibrin (PRF) and platelet-rich
plasma (PRP) on the effect of proliferation and differentiation of
rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 108:707–713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhattarai N, Gunn J and Zhang M:
Chitosan-based hydrogels for controlled, localized drug delivery.
Adv Drug Deliv Rev. 62:83–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Riva R, Ragelle H, des Rieux A, Duhem N,
Jérôme C and Préat V: Chitosan and chitosan derivatives in drug
delivery and tissue engineering. Adv Polym Sci. 244:19–44. 2011.
View Article : Google Scholar
|
|
13
|
Sonia TA and Sharma CP: Chitosan and its
derivatives for drug delivery perspective. Adv Polym Sci.
243:23–53. 2011. View Article : Google Scholar
|
|
14
|
Mengatto LN, Helbling IM and Luna JA:
Recent advances in chitosan films for controlled release of drugs.
Recent Pat Drug Deliv Formul. 6:156–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar AS and Ramaswamy NM: Chitosan
microspheres as potential vaccine delivery systems. Int J Drug
Deliv. 3:43–50. 2011. View Article : Google Scholar
|
|
16
|
Taşkın AK, Yaşar M, Ozaydın I, Kaya B, Bat
O, Ankaralı S, Yıldırım U and Aydın M: The hemostatic effect of
calcium alginate in experimental splenic injury model. Ulusal
Travma Acil Cerrahi Derg. 19:195–199. 2013. View Article : Google Scholar
|
|
17
|
Pinkas O and Zilberman M: Effect of
hemostatic agents on properties of gelatin-alginate soft tissue
adhesives. J Biomater Sci Polym Ed. 25:555–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He Q, Gong K, Ao Q, Ma T, Yan Y, Gong Y
and Zhang X: Positive charge of chitosan retards blood coagulation
on chitosan films. J Biomater Appl. 27:1032–1045. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lam PL, Lee KK, Wong RS, Cheng GY, Cheng
SY, Yuen MC, Lam KH, Gambari R, Kok SH and Chui CH: Development of
hydrocortisone succinic acid/and 5-fluorouracil/chitosan
microcapsules for oral and topical drug deliveries. Bioorg Med Chem
Lett. 22:3213–3218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Prins HJ, Braat AK, Gawlitta D, Dhert WJ,
Egan DA, Tijssen-Slump E, Yuan H, Coffer PJ, Rozemuller H and
Martens AC: In vitro induction of alkaline phosphatase levels
predicts in vivo bone forming capacity of human bone marrow stromal
cells. Stem Cell Res. 12:428–440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Casati MZ, de Vasconcelos Gurgel BC,
Gonçalves PF, Pimentel SP, da Rocha Nogueira Filho G, Nociti FH Jr
and Sallum EA: Platelet-rich plasma does not improve bone
regeneration around peri-implant bone defects - a pilot study in
dogs. Int J Oral Maxillofac Surg. 36:132–136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schaaf H, Streckbein P, Lendeckel S,
Heidinger KS, Rehmann P, Boedeker RH and Howaldt HP: Sinus lift
augmentation using autogenous bone grafts and platelet-rich plasma:
Radiographic results. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 106:673–678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dohan Ehrenfest DM, de Peppo GM, Doglioli
P and Sammartino G: Slow release of growth factors and
thrombospondin-1 in Choukroun's platelet-rich fibrin (PRF): A gold
standard to achieve for all surgical platelet concentrates
technologies. Growth Factors. 27:63–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marx RE: Platelet-rich plasma (PRP): What
is PRP and what is not PRP? Implant Dent. 10:225–228. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park HC, Kim SG, Oh JS, You JS, Kim JS,
Lim SC, Jeong MA, Kim JS, Jung C, Kwon YS and Ji H: Early bone
formation at a femur defect using CGF and PRF grafts in adult dogs:
A comparative study. Implant Dent. 25:387–393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu Z and Kipnis J: Thrombospondin 1-a key
astrocyte-derived neurogenic factor. FASEB J. 24:1925–1934. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao C, Isenberg JS and Popel AS: Human
expression patterns: Qualitative and quantitative analysis of
thrombospondin-1 under physiological and pathological conditions. J
Cell Mol Med. 22:2086–2097. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murphy-Ullrich JE and Suto MJ:
Thrombospondin-1 regulation of latent TGF-β activation: A
therapeutic target for fibrotic disease. Matrix Biol. 68-69:28–43.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghadami M, Makita Y, Yoshida K, Nishimura
G, Fukushima Y, Wakui K, Ikegawa S, Yamada K, Kondo S, Niikawa N
and Tomita Ha: Genetic mapping of the Camurati-Engelmann disease
locus to chromosome 19q13.1-q13.3. Am J Hum Genet. 66:143–147.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Le Bousse-Kerdilès MC and Martyré MC: Dual
implication of fibrogenic cytokines in the pathogenesis of fibrosis
and myeloproliferation in myeloid metaplasia with myelofibrosis.
Ann Hematol. 78:437–444. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang CY, Li XD, Hao ZH and Xu D:
Insulin-like growth factor-1 improves diabetic cardiomyopathy
through antioxidative and anti-inflammatory processes along with
modulation of Akt/GSK-3β signaling in rats. Korean J Physiol
Pharmacol. 20:613–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pierce GF, Tarpley JE, Yanagihara D,
Mustoe TA, Fox GM and Thomason A: Platelet-derived growth factor
(BB homodimer), transforming growth factor-beta 1, and basic
fibroblast growth factor in dermal wound healing. Neovessel and
matrix formation and cessation of repair. Am J Pathol.
140:1375–1388. 1992.PubMed/NCBI
|
|
33
|
Hu K and Olsen BR: The roles of vascular
endothelial growth factor in bone repair and regeneration. Bone.
91:30–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu Y, Chen L, Zhou C, Yang J, Hou Y and
Wang W: Development and evaluation of alginate-chitosan gastric
floating beads loading with oxymatrine solid dispersion. Drug Dev
Ind Pharm. 42:456–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He M, Zhang X, Yao W, Wang C, Shi L and
Zhou P: Construction of alternate layered chitosan/alginate
composite hydrogels and their properties. Mater Lett. 200:43–46.
2017. View Article : Google Scholar
|
|
36
|
Coppi G and Iannuccelli V:
Alginate/chitosan microparticles for tamoxifen delivery to the
lymphatic system. Int J Pharm. 367:127–132. 2009. View Article : Google Scholar : PubMed/NCBI
|