Introduction
Ischemic stroke is one of the leading causes of
disability and mortality globally (1,2).
Interventions require the recovery of blood flow, which can lead to
reperfusion injury. Cerebral ischemia-reperfusion (CIR) injury is a
pathological process in which nerve damage induced by ischemia and
hypoxia is further aggravated following the short-term recovery of
blood perfusion (3). CIR leads to
mitochondrial dysfunction, inflammation, massive release of
reactive oxygen species (ROS), excessive glutamate excitotoxicity
and cell death, ultimately resulting in irreversible brain damage
(4). Progress has been made in
reperfusion therapy (5); however,
the therapeutic outcomes remain unsatisfactory. Therefore, improved
understanding of the pathological process of CIR injury and the
identification of novel treatment strategies is required.
MicroRNAs (miRNAs/miRs) are a class of small
endogenous noncoding RNAs (~22 nucleotides in length) that can
regulate the expression of genes at the post-transcriptional level
by binding to the 3′-untranslated region (3′-UTR) of target genes
(6–8). miRNAs have been reported to serve
important roles in the regulation of a variety of biological
processes, including proliferation, differentiation and apoptosis,
via the regulation of various target genes (7,9–11).
Increasing evidence has indicated that miRNAs serve important
regulatory roles in normal physiology and disease (12), including in the pathogenesis of CIR
injury (13,14). Numerous studies have reported that
various miRNAs are dysregulated during CIR injury, and that
targeting these miRNAs can effectively relieve neuronal injury
in vitro and in vivo (15–17).
miRNA-217 has been studied in various types of
cancers, including lung adenocarcinoma, acute myeloid leukemia,
liver and gastric cancers, and colorectal carcinoma (18–22).
miR-217 has been reported to be downregulated in glioma tissues and
cells, and overexpression of miR-217 was observed to reduce the
malignancy of glioma cells in vitro and decrease tumor
growth in vivo (23);
however, to the best of our knowledge, there is no data regarding
the expression of miR-217 in other neurological diseases, and
whether miR-217 is involved in regulating CIR injury remains
unknown.
Research into damage following CIR has increased in
previous years, and a large number of studies have demonstrated
that the mechanisms underlying the effects of CIR on brain damage
are complex (24). At present,
there is no treatment strategy to effectively treat CIR injury.
Therefore, finding new and effective CIR injury treatment methods
has important clinical significance. Novel roles for miRNAs in the
pathogenesis of CIR injury have been identified (13–17);
however, to the best of our knowledge, the role of miR-217 in CIR
injury remains unclear. Therefore, the aims of the present study
were to investigate the role of miR-217 in neuronal injury induced
by CIR using an in vitro cellular model induced by
oxygen-glucose deprivation and reoxygenation (OGD/R).
Materials and methods
Primary neuron culture
Primary rat cerebral cortical neurons were extracted
from Sprague-Dawley rat embryos (n=5) at embryonic day 16–18 as
previously described (25,26). In brief, brain cortex tissues were
extracted and dissected in Hanks' balanced salt solution (HBSS),
cut into ~1 mm3 cubes, washed with HBSS, and then
digested with 0.25% trypsin (37°C for 15 min). Then, DMEM-F12
(Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) was used to stop trypsin digestion. The
dissociated cells were seeded in 24-well plates and cultured for 24
h at 37°C. Then, the cells were cultured in neurobasal medium
(Gibco; Thermo Fisher Scientific, Inc.) containing 2% B-27, 2 mM
glutamine and 50 ug/ml gentamycin, and cells were incubated at 37°C
with 5% CO2. Sprague-Dawley rats were obtained from
Beijing Vital River Laboratory Animal Technology Co., Ltd., and
housed at 25±5°C with 50% humidity under a 12:12-h dark/light
cycle. Rats accessed to food and water ad libitum. Animal
experiments were conducted according to the the guidelines of the
National Institutes of Health for the Care and Use of Laboratory
Animals (27). The present study
was approved by the Animal Ethics Committee of the First People's
Hospital of Wenling (Wenling, China).
OGD/R model establishment
The OGD/R model was established in the present study
as previously described (26). In
brief, cortical neurons were plated into 6-well plates at a density
of 5×105 cells/ml (2 ml) and cultured overnight. Then,
the neurons were incubated in glucose-free DMEM in a hypoxic
incubator chamber (1% O2, 5% CO2 and 94%
N2) at 37°C for 6 h. After three washes with DMEM, cells
were incubated in DMEM supplemented with 4.5 g/l glucose at 37°C
with 5% CO2 for 24 h.
The same treatment was performed in the control
group without OGD/R exposure. Briefly, neurons in the control group
were cultured in DMEM under standard conditions for 6 h. After
three washes with DMEM, cells were incubated in DMEM at 37°C with
5% CO2 for 24 h.
Cell transfection
Cortical neurons were seeded in a 6-well plate
(1×106 cells/well) and incubated at 37°C for 24 h. Then,
100 nM miR-217 inhibitor (5′-UACUGCAUCAGGAACUGAUUGGA-3′; Bioneer
Corp.), 100 nM inhibitor control (5′-GCCUCCGGCUUCGCACCUCU-3′;
Bioneer Corp.), 2 µl control-small interfering RNA (siRNA; cat. no.
sc-36869; Santa Cruz Biotechnology, Inc.), 2 µl sirtuin 1
(SIRT1)-siRNA (cat. no. sc-40986; Santa Cruz Biotechnology, Inc.)
or 100 nM miR-217 inhibitor + 2 µl SIRT1-siRNA was transfected into
the cells using Lipofectamine® 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. At 24 h after transfection, transfection efficiency was
measured using reverse transcription-quantitative PCR (RT-qPCR). At
24 h following transfection with miR-217 inhibitor, inhibitor
control, or miR-217 inhibitor+SIRT1-siRNA, cells were subjected to
OGD/R induction.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was determined at 24 h following
OGD/R induction as previously described (26). Cell viability was determined via a
CCK-8 assay. In brief, cells were plated into a 96-well plate
(5×103 cells/well) and incubated at 37°C overnight.
Then, cells were transfected with or without miR-217 inhibitor,
inhibitor control or miR-217 inhibitor + SIRT1-siRNA for 24 h (in
equal quantities as aforementioned), followed by OGD/R treatment.
Subsequently, 10 µl CCK-8 solution (cat. no. C0038; Beyotime
Biotechnology) was added to each well and incubated for another 2 h
at 37°C. At the end of the experiment, cell viability was
calculated by measuring the absorbance at 450 nm using a
FLUOstar® Omega microplate reader (BMG Labtech
GmbH).
ELISA
The levels of tumor necrosis factor-α (TNF-α; cat.
no. PT516), and interleukin (IL)-6 (cat. no. PI326) and IL-1β (cat.
no. PI301; all Beyotime Institute of Biotechnology) in the
supernatant of cells were measured via sandwich ELISAs according to
the manufacturer's protocol.
Lactate dehydrogenase (LDH) assay
An LDH Cytotoxicity Assay kit (cat. no. C0016;
Beyotime Institute of Biotechnology) was used to determine cell
injury according to the manufacturer's protocols. In brief, cells
were lysed and then incubated with NADH and pyruvate at 37°C for 15
min. The absorbance value at a wavelength of 530 nm was determined
using a FLUOstar Omega microplate reader.
Cell apoptosis assay
To investigate the effects of miR-217 on the
apoptosis of cortical neurons, an Annexin V-FITC/propidium iodide
(PI) Apoptosis Detection kit (cat no. 70-AP101-100; MultiSciences
Biotech Co., Ltd.) was using according to the manufacturer's
protocols. Following the aforementioned treatments, cortical
neurons were stained with 5 µl Annexin V-FITC and 5 µl PI for 30
min at room temperature in the dark. Subsequently, a flow cytometer
(BD Biosciences) was used to analyze cell apoptosis. The apoptosis
rate (early + late) was calculated (percentage of cells in the
right quadrants) using WinMDI soft-ware (version 2.5; Purdue
University Cytometry Laboratories).
Evaluation of ROS, superoxide
dismutase (SOD) and malondialdehyde (MDA) levels
To analyze the levels of ROS in cells, a Reactive
Oxygen Species Assay Kit (cat. no. S0033; Beyotime Institute of
Biotechnology) was used according to the manufacturer's protocols.
A Total Superoxide Dismutase Assay kit with WST-8 (cat. no. S0101;
Beyotime Institute of Biotechnology) was used to determine the
level of SOD in cortical neurons according to the manufacturer's
protocols. A Lipid Peroxidation MDA Assay kit (cat no. S0131;
Beyotime Institute of Biotechnology) was used to determine the
levels of MDA.
Western blot assay
Proteins were extracted from cells using RIPA lysis
buffer (cat no. P0013E; Beyotime Institute of Biotechnology)
according to the manufacturer's protocols. A bicinchoninic acid
assay kit (Pierce; Thermo Fisher Scientific, Inc.) was used to
quantify the protein samples according to the manufacturer's
protocols. Equal amounts of protein samples (25 µg/lane) were
separated via SDS-PAGE on 12% gels, transferred onto polyvinylidene
difluoride membranes (EMD Millipore), and blocked in 5% skim milk
at room temperature for 1.5 h. Then, the membranes were incubated
with primary antibodies against SIRT1 (1:1,000; cat. no. 9475; Cell
Signaling Technology, Inc.), phosphorylated (p)-AMP-activated
protein kinase-α (AMPK-α; 1:1,000; cat. no. 50081; Cell Signaling
Technology, Inc.), AMPK-α (1:1,000; cat. no. 5831; Cell Signaling
Technology, Inc.), NF-κB p65 (1:1,000; cat. no. 8242; Cell
Signaling Technology, Inc.), p-p65 (1:1,000; cat. no. 3033; Cell
Signaling Technology, Inc.), and β-actin (1:1,000; cat. no. 4970;
Cell Signaling Technology, Inc.) overnight at 4°C, followed by
incubation with the horseradish peroxidase-conjugated anti-rabbit
IgG secondary antibody (1:2,000; cat no. 7074; Cell Signaling
Technology, Inc.) at room temperature for 2 h. Finally, protein
blots were visualized using chemiluminescent ECL reagent (EMD
Millipore) and quantified by densitometry (QuantityOne 4.5.0
software; Bio-Rad Laboratories, Inc.).
RT-qPCR
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols. A TaqMan MicroRNA
Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used for cDNA generation. Reaction conditions
for RT were: 50°C for 5 min and 80°C for 2 min. An SYBR Premix Ex
Taq™ II (TliRNaseH Plus) kit (Takara Bio, Inc.) was using to
analyze the synthesized cDNAs. U6 and GAPDH were used as internal
controls for miRNA and mRNA expression, respectively. Primer
sequences for PCR were as follows: miR-217, forward,
5′-CGCAGATACTGCATCAGGAA-3′ and reverse,
5′-CTGAAGGCAATGCATTAGGAACT-3′; SIRT1, forward,
5′-AATCCAGTCATTAAACGGTCTACAA-3′ and reverse,
5′-TAGGACCATTACTGCCAGAGG-3′; U6, forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′CGCTTCACGAATTTGCGTGTCAT3′; and GAPDH, forward,
5′-CTTTGGTATCGTGGAAGGACTC-3′ and reverse,
5′-GTAGAGGCAGGGATGATGTTCT-3′. The thermocycling conditions were as
follows: 95°C for 5 min, followed by 38 cycles of denaturation at
95°C for 15 sec and annealing/elongation at 60°C for 30 sec. The
relative gene expression was calculated using the 2−ΔΔCq
method (28).
Dual-luciferase reporter assay
TargetScan bioinformatics software (release 7.1;
www.targetscan.org/vert_71) was used to
predict the targets of miR-217; a putative binding site between the
3′-UTR of SIRT1 and miR-217 was identified. Then, to determine
whether miR-217 directly binds to SIRT1, the target sequence
(WT-SIRT1; ATGCAGT) and a mutated sequence (MUT-SIRT1;
TACGTCA) were synthesized. To point-mutate the miR-217 binding
domain on the 3′-UTR of SIRT1, a QuikChange Site-Directed
Mutagenesis kit (Stratagene; Agilent Technologies, Inc.) was used
according to the manufacturer's protocols. Following digestion with
XhoI and NotI restriction enzymes, a pmiR RB Report™
reporter gene plasmid vector (Guangzhou RiboBio Co., Ltd.) were
combined with synthesized WT-SIRT1 or MUT-SIRT1 to construct
recombinant plasmids, SIRT1-WT or SIRT1-MUT. Neurons
(5×104 cells/well) were co-transfected with 50 ng
SIRT1-WT or 50 ng SIRT1-MUT, 25 ng pRL-TK (expressing
Renilla luciferase as the internal control; Promega
Corporation), and 50 nM miR-217 mimic
(5′-UACUGCAUCAGGAACUGAUUGGA-3′) or 50 nM mimic control
(5′-UUUGUACUACACAAAAGUACUG-3′) using Lipofectamine 2000 according
to the manufacturer's protocols. At 24 h following transfection,
luciferase activity was determined using a dual-luciferase assay
system (Promega Corporation) and normalized to the Renilla
luciferase activity.
Statistical analysis
Experiments in the present study were performed
three times. Experimental analysis was conducted using SPSS 17.0
software (SPSS, Inc.). Data were presented as the mean ± standard
deviation. Differences between groups were determined using
Student's t-test or one-way ANOVA with a Bonferroni post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
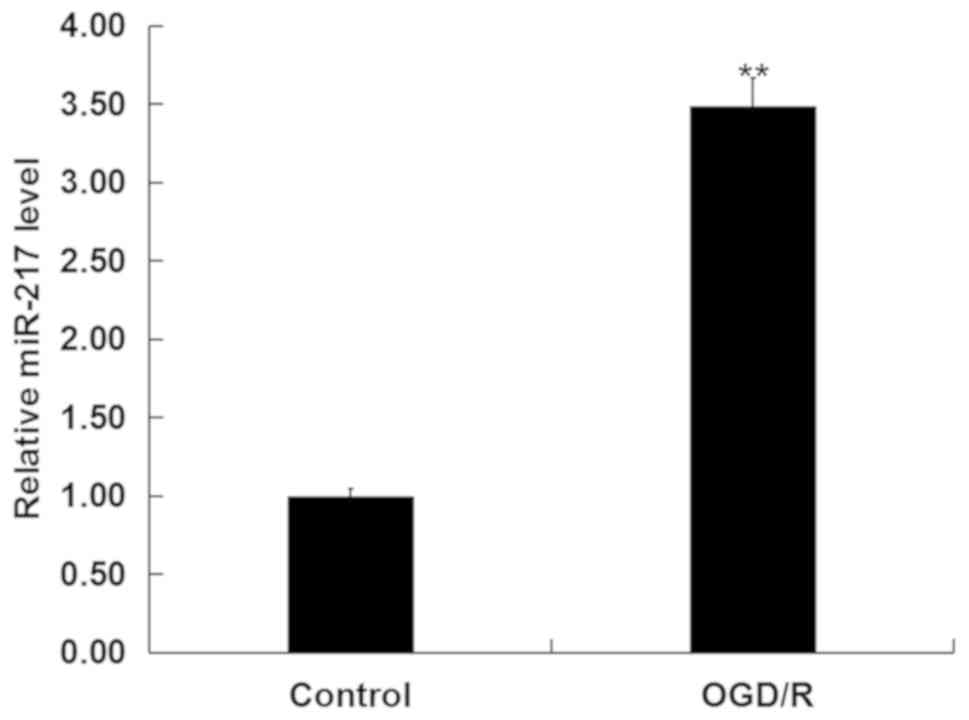
miR-217 is upregulated in
OGD/R-treated neurons
To investigate whether miR-217 was involved in CIR
injury in vitro, the levels of miR-217 in neurons following
OGD/R or control treatment was determined via RT-qPCR analysis. As
presented in Fig. 1, compared with
the control, OGD/R treatment significantly upregulated the
expression of miR-217 in neurons.
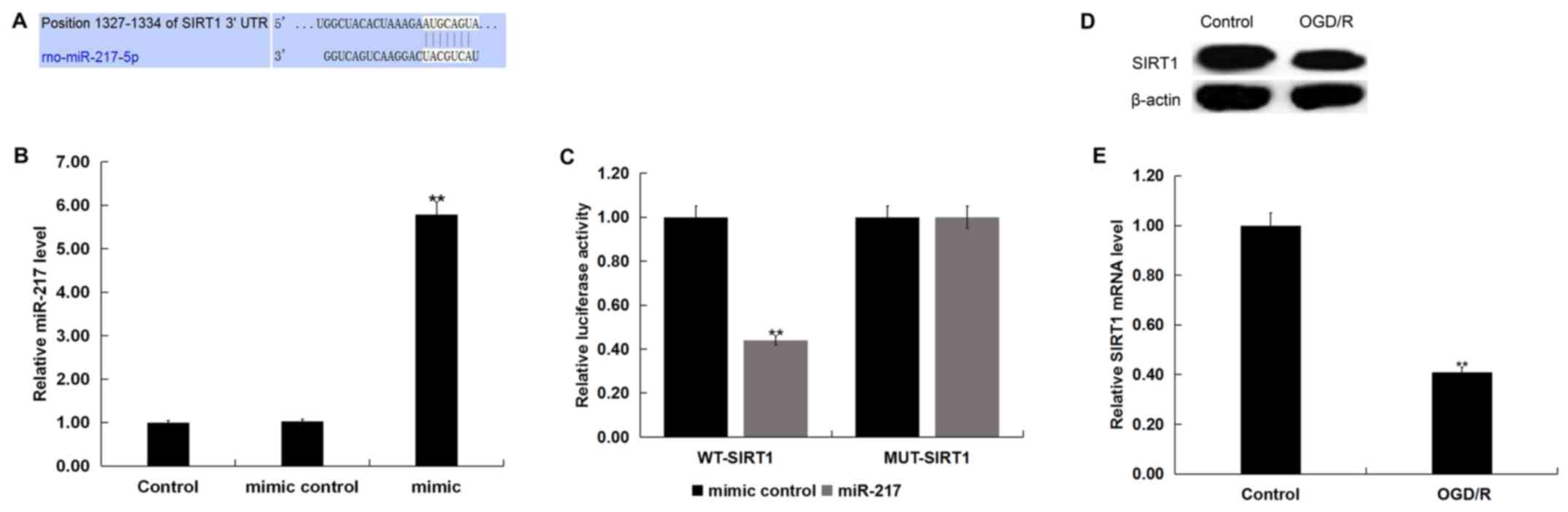
SIRT1 is a target gene of miR-217
TargetScan was used to predict the potential targets
of miR-217, and a putative binding site between SIRT1 and miR-217
was identified (Fig. 2A). To
demonstrate the association between miR-217 and SIRT1, a luciferase
reporter assay was conducted. miR-217 mimic was used to
significantly upregulated miR-217 expression in neurons (Fig. 2B). As presented in Fig. 2C, compared with cells
co-transfected with WT-SIRT1 and mimic control, luciferase activity
was significantly decreased in neurons co-transfected with WT-SIRT1
and miR-217 mimic. The results indicated that miR-217 directly
targeted SIRT1.
Subsequently, the protein and mRNA levels of SIRT1
in neurons following control or OGD/R treatment were determined via
western blot and RT-qPCR analyses, respectively. As presented in
Fig. 2D, OGD/R treatment markedly
reduced SIRT1 protein expression in neurons. Additionally, compared
with the control group, the mRNA levels of SIRT1 in neurons were
significantly downregulated following OGD/R treatment (Fig. 2E).
Downregulation of miR-217 alleviates
OGD/R-induced neuronal injury
To investigate the role of miR-217 in OGD/R-induced
neuronal injury, neurons were transfected with miR-217 inhibitor,
inhibitor control, control-siRNA, SIRT1-siRNA or miR-217 inhibitor
+ SIRT1-siRNA for 24 h, then subjected to OGD/R induction. The
transfection efficiency was measured by RT-qPCR and/or western
blotting. As presented in Fig. 3A,
compared with the transfection control group, miR-217 inhibitor
significantly downregulated the expression of miR-217 in neurons,
whereas the mRNA levels of SIRT1 in neurons were decreased
following SIRT1-siRNA transfection (Fig. 3B). The protein levels of SIRT1 in
neurons were also reduced following SIRT1-siRNA transfection
(Fig. 3D). Furthermore, it was
demonstrated that miR-217 inhibitor significantly increased the
mRNA expression of SIRT1 in neurons, and that this upregulation was
attenuated by SIRT1-siRNA (Fig.
3C). miR-217 inhibitor also enhanced the protein expression of
SIRT1 in neurons, in a manner that was attenuated by SIRT1-siRNA
(Fig. 3E).
Then, the effects of miR-217 inhibitor on
OGD/R-induced injury were determined via CCK-8 and LDH assays.
Consistent with a previous study (25), OGD/R treatment significantly
reduced cell viability and increased LDH release. The effects of
OGD/R treatment were inhibited by miR-217 downregulation, and this
inhibition was attenuated by SIRT1-siRNA (Fig. 4A and B). Additionally, miR-217
inhibition suppressed the OGD/R treatment-induced apoptosis of
neurons (Fig. 4C and D).
Collectively, the data indicated that miR-217 downregulation
attenuated OGD/R-induced neuronal injury.
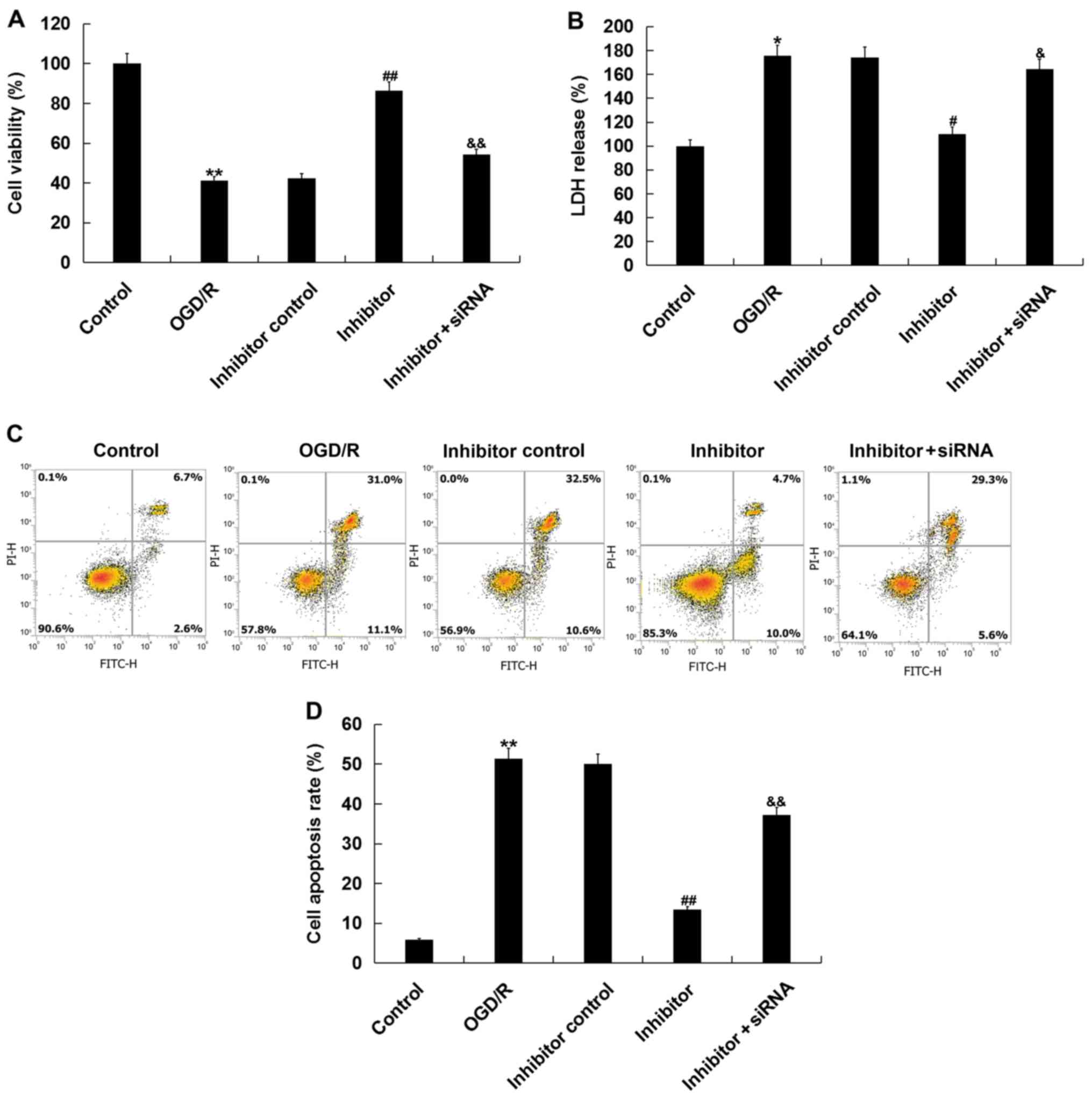 | Figure 4.Effects of miR-217 inhibitor on
OGD/R-induced neuronal injury. Neurons were transfected with
miR-217 inhibitor, inhibitor control or miR-217 inhibitor + sirtuin
1-siRNA for 24 h, followed by OGD/R treatment. (A) Cell viability
was detected using a Cell Counting Kit-8 assay. (B) LDH release was
measured by an LDH assay. (C and D) Cell apoptosis was determined
using flow cytometry. Data are presented as the mean ± standard
deviation. *P<0.05, **P<0.01 vs. control;
#P<0.05, ##P<0.01 vs. OGD/R;
&P<0.05, &&P<0.01 vs.
inhibitor. miR-217, microRNA-217; OGD/R, oxygen-glucose deprivation
and reoxygenation; LDH, lactate dehydrogenase; siRNA, small
interfering RNA; PI, propidium iodide. |
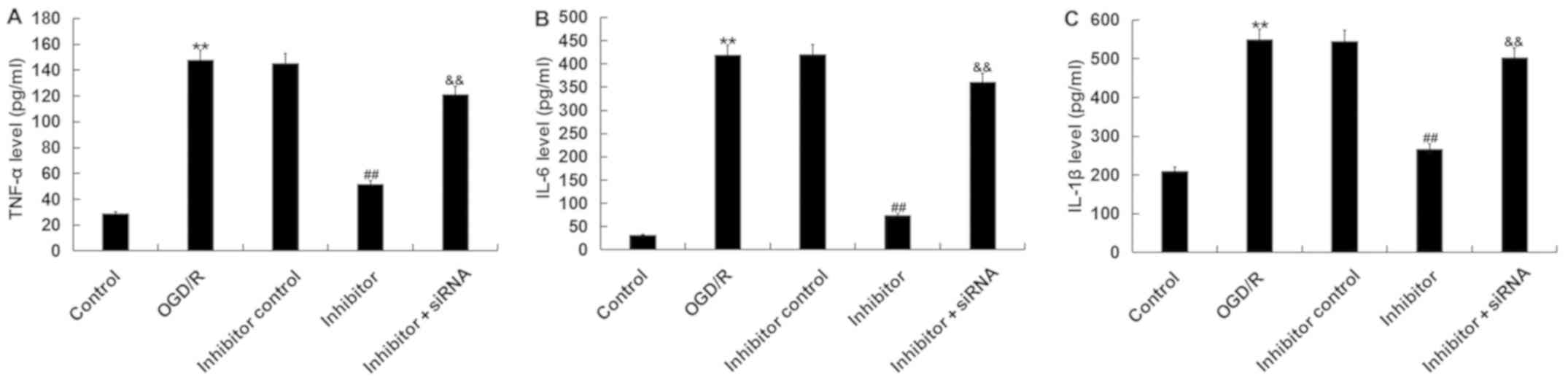
Downregulation of miR-217 alleviates
OGD/R-induced inflammatory response
To further investigate the biological function of
miR-217 in the regulation of OGD/R injury, the effects of miR-217
inhibitor on OGD/R-induced inflammation were determined. The levels
of TNF-α, IL-6 and IL-1β were detected by ELISA. As presented in
Fig. 5, the increased levels of
TNF-α, IL-6 and IL-1β following OGD/R were significantly reduced by
miR-217 inhibitor; however, these reductions were prevented by
SIRT1-siRNA.
Downregulation of miR-217 alleviates
OGD/R-induced oxidative stress
It was then investigated as to whether transfection
with miR-217 inhibitor induced an effect on OGD/R-induced oxidative
stress; it was observed that compared with the control, OGD/R
treatment significantly enhanced the levels of ROS and MDA, and
reduced those of SOD in neurons. Downregulation of miR-217
significantly decreased ROS production (Fig. 6A), reduced the levels of MDA
(Fig. 6B) and increased those of
SOD (Fig. 6C) in OGD/R-treated
neurons, whereas these alterations were attenuated by SIRT1
silencing. The results indicated that miR-217 downregulation
attenuated OGD/R-induced oxidative stress.
 | Figure 6.Effects of miR-217 inhibitor on
oxidative stress in OGD/R-treated neurons. Neurons were transfected
with miR-217 inhibitor, inhibitor control or miR-217 inhibitor +
sirtuin 1-siRNA for 24 h, followed by OGD/R treatment. Then, (A)
ROS, (B) MDA and (C) SOD levels were determined. Data are presented
as the mean ± standard deviation. *P<0.05, **P<0.01 vs.
control; #P<0.05, ##P<0.01 vs. OGD/R;
&P<0.05, &&P<0.01 vs.
inhibitor. miR-217, microRNA-217; ROS, reactive oxygen species;
OGD/R, oxygen-glucose deprivation and reoxygenation; siRNA, small
interfering RNA; MDA, malondialdehyde; SOD, superoxide
dismutase. |
Effects of miR-217 inhibitor on the
SIRT1/AMPK-α/NF-κB pathway in OGD/R-treated neurons
Finally, the SIRT1/AMPK-α/NF-κB pathway in
OGD/R-treated neurons was analyzed to investigate the molecular
mechanisms underlying the effects of miR-217 on OGD/R-induced
neuronal injury. As presented in Fig.
7, compared with the control, OGD/R treatment significantly
reduced SIRT1 mRNA and protein levels (Fig. 7A and B), decreased AMPK-α protein
phosphorylation levels (Fig. 7A and
C) and promoted p65 phosphorylation (Fig. 7A and D) in neurons. Compared with
the OGD/R treatment group, miR-217 downregulation significantly
upregulated SIRT1 and p-AMPK-α expression, and decreased p-p65
levels; these effects were eliminated by SIRT1 downregulation.
 | Figure 7.Effects of miR-217 inhibitor on
SIRT1/AMPK-α/NF-κB pathway in OGD/R-treated neurons. Neurons were
transfected with miR-217 inhibitor, inhibitor control or miR-217
inhibitor + SIRT1-siRNA for 24 h, followed by OGD/R treatment.
Then, the (A) protein and (B) mRNA expression levels of SIRT1 were
determined by western blot and reverse transcription-quantitative
PCR analyses, respectively. Additionally, the protein levels of
p-AMPK-α, AMPK-α, p65, and p-p65 were measured by western blotting.
The ratios of (C) p-AMPK-α/AMPK-α and (D) p-p65/p65 normalized to
β-actin were calculated and presented. Data are presented as the
mean ± standard deviation. **P<0.01 vs. control;
##P<0.01 vs. OGD/R; &P<0.05,
&&P<0.01 vs. inhibitor. miR-217,
microRNA-217; OGD/R, oxygen-glucose deprivation and reoxygenation;
siRNA, small interfering RNA; SIRT1, sirtuin 1; p, phosphorylated;
AMPK-α, AMP-activated protein kinase-α. |
Discussion
Recent studies have reported that miR-217 serves an
important role in the development of tumors (18–22).
miR-217 is involved in the apoptosis of human podocyte cells by
targeting TNF ligand superfamily member 11 in membranous
nephropathy (29). Another study
demonstrated that miR-217 promotes fibroblast senescence (30). Additionally, miR-217 was identified
as a key regulator in ethanol-induced hepatic inflammation
(31); however, the role of
miR-217 in neuronal injury induced by CIR injury remains unclear.
In the present study, the results indicated that miR-217 was
upregulated in OGD/R-treated neurons, and its downregulation
relieved OGD/R treatment-induced neuronal injury, as revealed by
increased cell viability, reduced LDH release and decreased cell
apoptosis. Additionally, it was observed that miR-217
downregulation notably inhibited OGD/R-induced inflammatory
responses and oxidative stress. Furthermore, the findings of the
present study revealed that SIRT1 was a direct target of miR-217,
and that the effects of miR-217 inhibitor on OGD/R-treated neurons
were eliminated by SIRT1 silencing.
A number of experimental studies have revealed that
the mechanisms underlying the effects of CIR on brain damage are
complex (24,25). At present, there is no strategy to
effectively treat CIR injury. Therefore, finding novel and
effective methods has important clinical relevance. The emerging
roles of miRNAs in the pathogenesis of CIR injury have been
identified in numerous studies (12–16).
In the present study, the role of miR-217 in neuron injury induced
by OGD/R was investigated.
An in vitro cellular model of CIR injury was
established by treating neurons with OGD/R. The OGD/R model is a
cell injury model widely used to study CIR injury in vitro
(24,26,32).
Then, the levels of miR-217 in neurons subjected to control or
OGD/R treatment were determined, and the results revealed that
compared with the control group, miR-217 was significantly
upregulated in OGD/R-treated neurons. Subsequently, it was revealed
that SIRT1 was a direct target of miR-217 that was downregulated in
neurons treated with OGD/R. SIRT1 is a NAD+-dependent
deacetylase important for apoptosis, cell cycle, mitochondrial
function and metabolism (33), and
serves an important role in the regulation of inflammatory
responses and oxidative stress (33–37).
To explore the potential function of miR-217 in the regulation of
OGD/R-induced neuronal injury, loss-of-function experiments were
performed by transfection with miR-217 inhibitor. The results
suggested that miR-217 downregulation significantly mitigated
OGD/R-induced neuronal injury, as determined by increased cell
viability, reduced LDH release and decreased cell apoptosis.
Additionally, miR-217 downregulation notably suppressed
inflammatory responses and oxidative stress enhanced by OGD/R
treatment in neurons.
The activation of the AMPK-α-SIRT1 pathway can
inhibit NF-κB inflammation (38).
Activation of AMPK/SIRT1 signaling attenuated TNF-α-induced MMP-3
expression in human nucleus pulposus cells (39). Additionally, activation of the
AMPK/SIRT1 pathway protected PC12 cells against cisplatin-induced
neurotoxicity (40). The pathway
has also been reported to relieve oxidized low-density
lipoprotein-induced endothelial cell injury (41). These results indicated that
AMPK/SIRT1 signaling serves important roles in cell injury and
inflammation regulation. Furthermore, a recent study revealed that
AMPK/SIRT1 pathway activation was involved in the protective
effects of lncRNA SNHG12 on CIR injury (42). Therefore, to investigate the
molecular mechanisms underlying the effects of miR-217 inhibitor on
OGD/R-induced neuronal injury, the activity of the
SIRT1/AMPK-α/NF-κB pathway was analyzed in the present study.
Results revealed that OGD/R treatment significantly reduced SIRT1
mRNA and protein levels, decreased AMPK-α phosphorylation, and
increased p65 phosphorylation level in neurons; these effects were
reversed by miR-217 inhibitor. Of note, all the observed effects of
miR-217 inhibitor on OGD/R-treated neurons were attenuated by SIRT1
silencing.
In conclusion, the results of the present study
demonstrated that miR-217 was upregulated in OGD/R-treated neurons,
and that its inhibition attenuated OGD/R-induced neuronal injury
via the upregulation of SIRT1 and the SIRT1/AMPK-α/NF-κB pathway.
Of note, this is a preliminary study of the role of miR-217 in CIR
injury, and further research is required to more comprehensively
and convincingly determine the precise roles and mechanisms of
miR-217 in CIR injury. For example, the effects of miR-217 on CIR
injury in vivo should be investigated, in addition to the
effects of SIRT1 manipulation alone on CIR injury. Furthermore, in
the present study, experiments were performed at only one time
point (24 h following OGD/R treatment); thus, additional time
points should be investigated in the future. Finally, an
investigation into the association between miR-217 expression and
the clinical characteristics and prognosis of patients with CIR
injury is required to confirm the clinical relevance of miR-217 in
CIR injury. In-depth research into these issues will be conducted
in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medical and
Health Science and Technology Planning Project of Zhejiang Province
(grant no. 2018KY918).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GR and WZ contributed to study design, data
collection, statistical analysis, data interpretation and
manuscript preparation. SS contributed to data collection and
interpretation. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of The First People's Hospital of Wenling.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Feigin VL: Stroke epidemiology in the
developing world. Lancet. 365:2160–2161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldstein LB, Adams R, Becker K, Furberg
CD, Gorelick PB, Hademenos G, Hill M, Howard G, Howard VJ, Jacobs
B, et al: Primary prevention of ischemic stroke A statement for
healthcare professionals from the stroke council of the American
heart association. Circulation. 103:163–182. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jean WC, Spellman SR, Nussbaum ES and Low
WC: Reperfusion injury after focal cerebral ischemia: The role of
inflammation and the therapeutic horizon. Neurosurgery.
43:1382–1397. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schrepfer E and Scorrano L: Mitofusins,
from mitochondria to metabolism. Mol Cell. 61:683–694. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhaskar S, Stanwell P, Cordato D, Attia J
and Levi C: Reperfusion therapy in acute ischemic stroke: Dawn of a
new era? BMC Neurol. 18:82018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghildiyal M and Zamore PD: Small silencing
RNAs: An expanding universe. Nat Rev Genet. 10:94–108. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soifer HS, Rossi JJ and Saetrom P:
MicroRNAs in disease and potential therapeutic applications. Mol
Ther. 15:2070–2079. 2017. View Article : Google Scholar
|
|
10
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Connell RM, Rao DS, Chaudhuri AA and
Baltimore D: Physiological and pathological roles for microRNAs in
the immune system. Nat Rev Immunol. 10:111–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mendell JT and Olson EN: MicroRNAs in
stress signaling and human disease. Cell. 148:1172–1187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Y, Lei Y, Yu F, Changfeng F, Song W and
Xuming M: MicroRNAs expression and function in cerebral ischemia
reperfusion injury. J Mol Neurosci. 53:242–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu Y, Deng H, Xu S and Zhang J: MicroRNAs
regulate mitochondrial function in cerebral ischemia-reperfusion
injury. Int J Mol Sci. 16:24895–24917. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang P, Liang X, Lu Y, Zhao X and Liang J:
MicroRNA-93 downregulation ameliorates cerebral ischemic injury
through the Nrf2/HO-1 defense pathway. Neurochem Res. 41:2627–2635.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang N, Zhang L, Lu Y, Zhang M, Zhang Z,
Wang K and Lv J: Down-regulation of microRNA-142-5p attenuates
oxygen-glucose deprivation and reoxygenation-induced neuron injury
through up-regulating Nrf2/ARE signaling pathway. Biomed
Pharmacother. 89:1187–1195. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi F, Dong Z, Li H, Liu X, Liu H and Dong
R: MicroRNA-137 protects neurons against ischemia/reperfusion
injury through regulation of the notch signaling pathway. Exp Cell
Res. 352:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu AN, Qu HJ, Yu CY and Sun P: Knockdown
of LINC01614 inhibits lung adenocarcinoma cell progression by
up-regulating miR-217 and down-regulating FOXP1. J Cell Mol Med.
22:4034–4044. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang LP, Wang JP and Wang XP: HOTAIR
contributes to the growth of liver cancer via targeting miR-217.
Oncol Lett. 15:7963–7972. 2018.PubMed/NCBI
|
|
20
|
Yan J, Wu G, Chen J, Xiong L, Chen G and
Li P: Downregulated miR-217 expression predicts a poor outcome in
acute myeloid leukemia. Cancer Biomark. 22:73–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Safaralizadeh R, Ajami N, Nemati M,
Hosseinpourfeizi M, Azimzadeh Isfanjani A and Moaddab SY:
Disregulation of miR-216a and miR-217 in gastric cancer and their
clinical significance. J Gastrointest Cancer. 50:78–83. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu B, Du Q, Li H, Liu HY, Ye X, Zhu B,
Zhai Q and Li XX: Diagnostic potential of serum exosomal colorectal
neoplasia differentially expressed long non-coding RNA (CRNDE-p)
and microRNA-217 expression in colorectal carcinoma. Oncotarget.
8:83745–83753. 2017.PubMed/NCBI
|
|
23
|
Zheng J, Liu X, Xue Y, Gong W, Ma J, Xi Z,
Que Z and Liu Y: TTBK2 circular RNA promotes glioma malignancy by
regulating miR-217/HNF1β/Derlin-1 pathway. J Hematol Oncol.
10:522017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sahu S, Nag DS, Swain A and Samaddar DP:
Biochemical changes in the injured brain. World J Biol Chem.
8:21–31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Zhao F, Zhao Y, Wang J, Pei L,
Sun N and Shi J: Hypoxia induces an increase in intracellular
magnesium via transient receptor potential melastatin 7 (TRPM7)
channels in rat hippocampal neurons in vitro. J Biol Chem.
286:20194–20207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu X, Li M, Hou M, Huang W and Song J:
MicroRNA-135a alleviates oxygen-glucose deprivation and
reoxygenation-induced injury in neurons through regulation of
GSK-3β/Nrf2 signaling. J Biochem Mol Toxicol. 2:e221592018.
View Article : Google Scholar
|
|
27
|
Bayne K: Revised guide for the care and
use of laboratory animals available. American physiological
society. Physiologist. 39:199208–199211. 1996.
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li J, Liu B, Xue H, Zhou QQ and Peng L:
miR-217 Is a useful diagnostic biomarker and regulates human
podocyte cells apoptosis via targeting TNFSF11 in membranous
nephropathy. Biomed Res Int. 2017:21687672017.PubMed/NCBI
|
|
30
|
Wang B, Du R, Xiao X, Deng ZL, Jian D, Xie
HF and Li J: Microrna-217 modulates human skin fibroblast
senescence by directly targeting DNA methyltransferase 1.
Oncotarget. 8:33475–33486. 2017.PubMed/NCBI
|
|
31
|
Yin H, Liang X, Jogasuria A, Davidson NO
and You M: miR-217 regulates ethanol-induced hepatic inflammation
by disrupting sirtuin 1-lipin-1 signaling. Am J Pathol.
185:1286–1296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xin L, Junhua W, Long L, Jun Y and Yang X:
Exogenous hydrogen sulfide protects SH-SY5Y cells from OGD/rinduced
injury. Curr Mol Med. 17:563–567. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nogueiras R, Habegger KM, Chaudhary N,
Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT
and Tschöp MH: Sirtuin 1 and sirtuin 3: Physiological modulators of
metabolism. Physiol Rev. 92:1479–1514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
da Cunha MSB and Arruda SF:
Tucum-do-cerrado (Bactris setosa Mart.) may promote anti-aging
effect by Upregulating SIRT1-Nrf2 pathway and attenuating oxidative
stress and inflammation. Nutrients. 9:E12432017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rada P, Pardo V, Mobasher MA,
García-Martínez I, Ruiz L, González-Rodríguez Á, Sanchez-Ramos C,
Muntané J, Alemany S, James LP, et al: SIRT1 controls acetaminophen
hepatotoxicity by modulating inflammation and oxidative stress.
Antioxid Redox Signal. 28:1187–1208. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chan SH, Hung CH, Shih JY, Chu PM, Cheng
YH, Lin HC and Tsai KL: SIRT1 inhibition causes oxidative stress
and inflammation in patients with coronary artery disease. Redox
Biol. 13:301–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng YY, Kao CL, Ma HI, Hung CH, Wang CT,
Liu DH, Chen PY and Tsai KL: SIRT1-related inhibition of
pro-inflammatory responses and oxidative stress are involved in the
mechanism of nonspecific low back pain relief after exercise
through modulation of Toll-like receptor 4. J Biochem. 158:299–308.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tian Y, Ma J, Wang W, Zhang L, Xu J, Wang
K and Li D: Resveratrol supplement inhibited the NF-κB inflammation
pathway through activating AMPKα-SIRT1 pathway in mice with fatty
liver. Mol Cell Biochem. 422:75–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang XH, Zhu L, Hong X, Wang YT, Wang F,
Bao JP, Xie XH, Liu L and Wu XT: Resveratrol attenuated
TNF-α-induced MMP-3 expression in humannucleus pulposus cells by
activating autophagy via AMPK/SIRT1 signaling pathway. Exp Biol Med
(Maywood). 241:848–853. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ferreira RS, Dos Santos NAG, Bernardes CP,
Sisti FM, Amaral L, Fontana ACK and Dos Santos AC: Caffeic acid
phenethyl ester (CAPE) protects PC12 cells against
cisplatin-induced neurotoxicity by activating the AMPK/SIRT1,
MAPK/Erk, and PI3k/Akt signaling pathways. Neurotox Res. Apr
23–2019.(Epub ahead of print). doi: 10.1007/s12640-019-00042-w.
View Article : Google Scholar
|
|
41
|
Zhao D, Sun X, Lv S, Sun M, Guo H, Zhai Y,
Wang Z, Dai P, Zheng L, Ye M and Wang X: Salidroside attenuates
oxidized low-density lipoprotein-induced endothelial cell injury
via promotion of the AMPK/SIRT1 pathway. Int J Mol Med.
43:2279–2290. 2019.PubMed/NCBI
|
|
42
|
Yin WL, Yin WG, Huang BS and Wu LX: LncRNA
SNHG12 inhibits miR-199a to upregulate SIRT1 to attenuate cerebral
ischemia/reperfusion injury through activating AMPK signaling
pathway. Neurosci Lett. 690:188–195. 2019. View Article : Google Scholar : PubMed/NCBI
|