Introduction
Renal fibrosis is a pathological injury process
involved in the progression of all chronic kidney diseases (CKDs)
to end-stage renal disease. Renal fibrosis and the development of
sclerotic lesions result in nephritic failure and a progressive
decline in renal function (1). The
high incidence of CKD has become a medical and public health
problem worldwide (2). Therefore,
understanding the pathophysiology of CKD is crucial for the
development of novel drugs and therapies aimed to limit CKD
progression (3,4). Unilateral ureteral obstruction (UUO)
in animals has been used as an in vivo model to study the
mechanisms of renal diseases (5).
UOO can lead to acute changes in renal function, inducing chronic
renal structural damage and promoting the rapid development of
renal interstitial fibrosis (6).
Compared with other models, UOO has the advantage of limiting the
lesions to one kidney, avoiding systemic toxic effects (7).
Obstructive renal injury leads to the formation of
tubular glomeruli, proximal tubular cell loss, immune cell
infiltration, collecting duct remodeling and interstitial fibrosis
(8,9). Neutrophils and macrophages infiltrate
the kidneys in the early phase of obstructive renal injury and
maintain their innate immune functions. These cells are involved in
kidney injury by synthesizing reactive oxygen species,
pro-inflammatory mediators and proteases (10). In contrast with the neutrophils
that are rapidly removed from the injury site, macrophages persist
during the recovery phase, and were suggested to contribute to
fibrosis (11,12).
Following recruitment to the damaged kidney,
macrophages can differentiate into two different subtypes depending
on the microenvironment: Classically activated (M1) and
alternatively activated (M2) macrophages (13). Pro-inflammatory M1 macrophages
differentiation is mediated by exposure to interferon-γ or
lipopolysaccharide, whereas anti-inflammatory M2 macrophages are
stimulated by T helper type 2 cytokines, such as interleukin (IL)-4
and IL-10 (14,15). M2 macrophages express arginase-1
(ARG1), whereas M1 macrophages express inducible nitric oxide
synthase (16). A previous study
demonstrated that IL-33, a member of the IL-1 family, cooperates
with other cytokines in promoting the transition from M0
macrophages to M2 (17).
Transforming growth factor (TGF)-β1 stimulates
fibroblasts to synthesize stress fibers and undergo further
differentiation to become myofibroblasts (18). IL-13 induces fibrosis by
stimulating the synthesis and activation of TGF-β1, or by directly
activating the synthetic and proliferative properties of
fibroblasts, epithelial cells and smooth muscle cells (19). Therefore, TGF-β1 and IL-13 are
essential for developing kidney fibrosis by stimulating the
synthesis of extracellular matrix proteins, particularly collagen.
In vitro studies found that IL-33 can promote IL-13-mediated
macrophage polarization to M2 phenotype (20).
IL-33 is an immunomodulatory cytokine (21). IL-33 is expressed in various
organs, and it was found to have the characteristics of an alarmin,
being able to promote pro-inflammatory responses (20). The interleukin 1 receptor like 1
(IL1RL1) receptor is a member of the Toll/IL-1 receptor
superfamily, and IL-33 is its agonist (22). A previous study demonstrated that
upregulation of the IL-33/IL1RL1 signaling pathway in the
obstructed kidney may promote tubular cell injury and interstitial
fibrosis (23). Additionally, a
previous study suggested that M2 macrophages, rather than M1
macrophages, are involved in renal fibrosis (24). Li et al reported that mature
(m)IL-33, via IL1RL1, enhanced bleomycin-induced pulmonary fibrosis
(25). However, the role of IL-33
in kidney disease remains unclear. Therefore, in the present study,
the relationship between IL-33 and macrophages was investigated,
and their roles in the development of renal fibrosis were examined
using a mouse model of UUO.
Materials and methods
Animals
Specific-pathogen free C57BL/6 male mice (n=63; age,
6–8 weeks; weight, 20–25 g) were purchased from the Institute of
Laboratory Animal Sciences (Beijing, China). All procedures were
approved by The Animal Experimental Ethics Committee of Jilin
University. Animals were housed in SPF level barrier environment
and kept at 22°C room temperature, 45±10% humidity, with a 12-h
light/dark cycle and free access to food and water.
Surgical procedure
A previous study demonstrated that isoflurane may
affect the function of immune cells (26). By contrast, Zhang et al
identified that the effect of isoflurane on immune function was
limited compared with intraperitoneal injections of chloral hydrate
and sodium pentobarbital (27).
Since UUO and sham surgery are rapid procedures, the induction of
anesthesia was performed using the recommended concentration of 4%
isoflurane and continuous inhalation of 2% isoflurane was selected
as maintain anesthesia after induction, as previously reported
(14,28). For surgical procedures, mice were
placed on a temperature-controlled operating table. A small
incision was performed on the left flank of the mouse, and the
intestines were gently displaced from the abdomen and covered with
sterile saline-soaked gauze. The left ureter was exposed and
ligated with a 6-0 silk suture (Johnson and Johnson). The
intestines were placed back in the abdomen, and the muscle, fascia
and skin were sutured with sterile surgical 4-0 silk (Johnson and
Johnson). Prophylactic povidone-iodine (10%) was applied to the
abdominal wound. For the sham surgery group, surgery was performed
as aforementioned; however, the ureter was not ligated. Renal
tissue and blood were harvested after 1, 3, 5, 7, 9, 11 and 14 days
(n=6 in the day 7 and 14 groups; n=3 in the other groups).
mIL-33 treatment
Mice were treated with IL-33 to investigate its
effect on UUO-induced fibrosis (n=6). In the present study, 1 mg
recombinant murine mIL-33 (PeproTech, Inc.) was injected
intraperitoneally on the same day of UOO surgery, and 1 and 2 days
after UUO surgery. Mice treated with mIL-33 were sacrificed on day
7.
Macrophage depletion
Macrophage depletion was performed to test whether
macrophages were involved in IL-33-mediated pro-fibrotic effects
(n=6). Clodronate liposomes enter the macrophage membranes via
endocytosis, and intracellular clodronate induces macrophage
apoptosis (29). Kidney macrophage
depletion was achieved by intraperitoneal injection of 200 µl (~1
mg) clodronate (ClodLip BV) or control liposomes on the same day of
UUO surgery (11,30), and 3 days after UUO surgery. Mice
were sacrificed on day 7.
Cytokine measurements
Serum levels of IL-33 and additional inflammatory
cytokines, including IL-13 and TGF-β1, were determined using
commercial ELISA kits (Cusabio Biotech Co., Ltd.; IL-33 cat. no.
CSB-E14393; IL-13 cat. no. CSB-E04602m; and TGF-β1 cat. no.
CSB-E04726m) according to the manufacturer's protocol. The
experiments were performed three times.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total renal RNA was extracted from a quarter of each
animal's kidney using the Eastep Super Total RNA extraction kit
(Promega Corporation), according to the manufacturer's protocol.
RNA concentration was determined spectrophotometrically at 260 and
280 nm. A total of 2 µg RNA was used to perform RT using the
All-in-One First-Strand cDNA Synthesis kit (GeneCopoeia, Inc.):
37°C 60 min for reverse transcription reaction, 85°C for 5 min for
termination of reaction. The FastStart Universal SYBR Green master
mix (Roche Diagnostics) was used for qPCR, performed on a Light
Cycler 480 (Roche Diagnostics). Thermal cycling conditions were
95°C for 10 min followed by 40 cycles of 95°C for 15 sec, 60°C for
30 sec. Double distilled water was used as the negative control for
the target and housekeeping gene β-actin used as an internal
control. The sequences of the primers used were as follows
(25): IL13 forward,
5′-GAATCCAGGGCTACACAGAAC-3′ and reverse,
5′-AACATCACACAAGACCAGACTC-3′; IL33 forward,
5′-ACTATGAGTCTCCCTGTCCTG-3′ and reverse, 5′-ACGTCACCCCTTTGAAGC-3′;
IL1RL1 forward, 5′-TCTGTGGAGTACTTTGTTCACC-3′ and reverse,
5′-TCTGCTATTCTGGATACTGCTTTC-3′; TGF-β1 forward,
5′-CCATGAGGAGCAGGAAGG-3′ and reverse,
5′-ACAGCAAAGATAACAAACTCCAC-3′; collagen 3 forward,
5′-TCTCTAGACTCATAGGACTGACC-3′ and reverse,
5′-TTCTTCTCACCCTTCTTCATCC-3′; ARG1 forward,
5′-AGTGTTGATGTCAGTGTGAGC-3′ and reverse,
5′-GAATGGAAGAGTCAGTGTGGT-3′, as previously reported.
Renal histology and
immunohistochemical staining
The kidneys were dissected and cut into two parts.
Each kidney was cut lengthwise and half of each kidney (n=6) was
fixed using 10% formalin for at least 24 h (room temperature;
20–24°C). After fixation, the tissue was dehydrated with gradient
ethanol, transparent with n-butanol and embedded with paraffin, and
5-µm sections. Following xylene dewaxing and rehydration in
descending ethanol series, the sections were stained with
hematoxylin and eosin, and Masson's trichrome staining solution, to
assess fibrosis. For immunohistochemistry, Xylene dewaxing and
transparent; rehydration in descending ethanol series, then an
additional antigen retrieval step was performed by heating samples
in an EDTA-based buffer (pH 9.0) at 95–100°C for 15 min. blocked
with 5% BSA (AR0004, Wuhan Boster Biological Technology, Ltd.) for
30 min at 37°C. The primary antibodies used were anti-IL-33 (1:200;
cat. no. AF3626; R&D Systems, Inc.) and anti-adhesion G
protein-coupled receptor E1 (ADGRE1; 1:200; cat. no. MAB5580;
R&D Systems, Inc.). Samples were incubated with primary
antibody for 10 h at 4°C. The secondary antibodies used were
Horseradish Peroxidase-conjugated Affinipure Rabbit Anti-Goat IgG
(1:200; cat. no. SA00001-4; ProteinTech Group, Inc.) and
Horseradish Peroxidase-conjugated Affinipure Goat Anti-Rat IgG
(1:200; cat. no. SA00001-15; ProteinTech Group, Inc.). The
secondary antibodies incubation was 37°C for 60 min. Images were
obtained using an Olympus BX51 microscope and DP2-BSW imaging
system (Olympus Corporation). Masson's trichrome stained samples
were analyzed using ImageJ software (National Institutes of Health,
version 1.4.3.) and regions stained in blue were quantified. In
total, 5 randomly selected fields of view were analyzed for each
sample. IL-33-positive cells and macrophages (ADGRE1-positive
cells) were counted in each microscopic field, and the mean number
of positive cells per high-power field (magnification, ×400) was
calculated.
Western blot assay
Kidney tissue proteins were extracted using RIPA
assay lysis buffer (cat. no. DE101; Beijing Transgen Biotech Co.,
Ltd.), and protein concentrations were determined using a
bicinchoninic protein assay kit (cat. no. E162-01; Gene-star
Biosolutions Co., Ltd.). Protein separation was performed by 10%
SDS-PAGE. The separated proteins were transferred to a PVDF
membrane (EMD Millipore) which was incubated with 5% non-fat milk
in Tris buffer for 1 h at room temperature to block non-specific
binding. The membranes were then incubated overnight at 4°C with a
primary antibody against ARG1 (1:1,000; cat. no. 93668; Cell
Signaling Technology, MA). The PVDF membrane was washed with TBST
(% 0.05 Tween20; cat. no. E175-01; Gene-star Co., Ltd.) three
times, followed by incubation with a secondary horseradish
peroxidase-labeled anti-rabbit immunoglobulin G (1:2,000; cat. no.
SA00001-2; ProteinTech Group, Inc.) for 1 h at room temperature.
Then the PVDF membrane was washed with TBST three times, 10 min
each time. β-Tubulin (cat. no. HC101; Beijing Transgen Biotech Co.,
Ltd.) was used as a protein loading control, the membranes were
incubated with primary antibody β-Tubulin overnight at 4°C and then
processed for detection by ECL (cat. no. abs920; Absin Bioscience,
Inc.), using an integrated chemiluminescence imaging system
(ChemiScope 6000; Clinx Science Instruments Co., Ltd.) and
ChemiCapturePAD V4.0.12.812 (Clinx Science Instruments Co., Ltd.)
for detection and imaging.
Statistical analyses
All data are presented as the mean ± SD from three
independent experiments (n=6 in each experiment, except for the UUO
and sham groups at days 1, 3, 5, 9 and 11, where n=3). Student's
t-test was used to compare two groups. One-way ANOVA followed by
Student-Newman-Keuls post hoc test was used to compare multiple
groups. P<0.05 was considered to indicate a statistically
significant difference. Analyses were performed using GraphPad
Prism software (version 5.0; GraphPad Software, Inc.).
Results
Protein expression levels of IL-33 and
IL1RL1 are increased in a mouse model of UUO
Hematoxylin and eosin, and Masson's trichrome
staining showed increased inflammatory cell infiltration and
collagen deposition in the kidney after UUO induction (Fig. 1A, B and D). The present data
suggested that UUO mice exhibited renal inflammation and
interstitial fibrosis.
Immunohistochemical analysis of renal tissue
sections from sham and UUO mice suggested that renal tubular
epithelial cells expressed high levels of IL-33 (Fig. 1C and E). Moreover, increased
expression levels of IL-33 were detected in the UUO group compared
with the sham surgery group. The mRNA expression level of collagen
3 was also assessed. The expression level of collagen 3 increased
in UUO mice compared with the sham group (Fig. 1F). The serum level of IL-33
increased significantly in the UUO and sham operation groups 1 day
after surgery (Fig. 1G). This
effect may be due to the stimulation induced by the surgical
trauma, which promoted the infiltration of local inflammatory cells
and consequent secretion of IL-33. On the following days, the serum
levels of IL-33 in the sham group were similar to day 0, whereas
the serum levels of IL-33 in the UUO group were significantly
increased compared with the sham group. In addition, the expression
levels of IL-33 and IL1RL1 in renal tissue were increased compared
with the sham group at days 3, 5, 7, 9, 11 and 14 after surgery
(Fig. 1H and I).
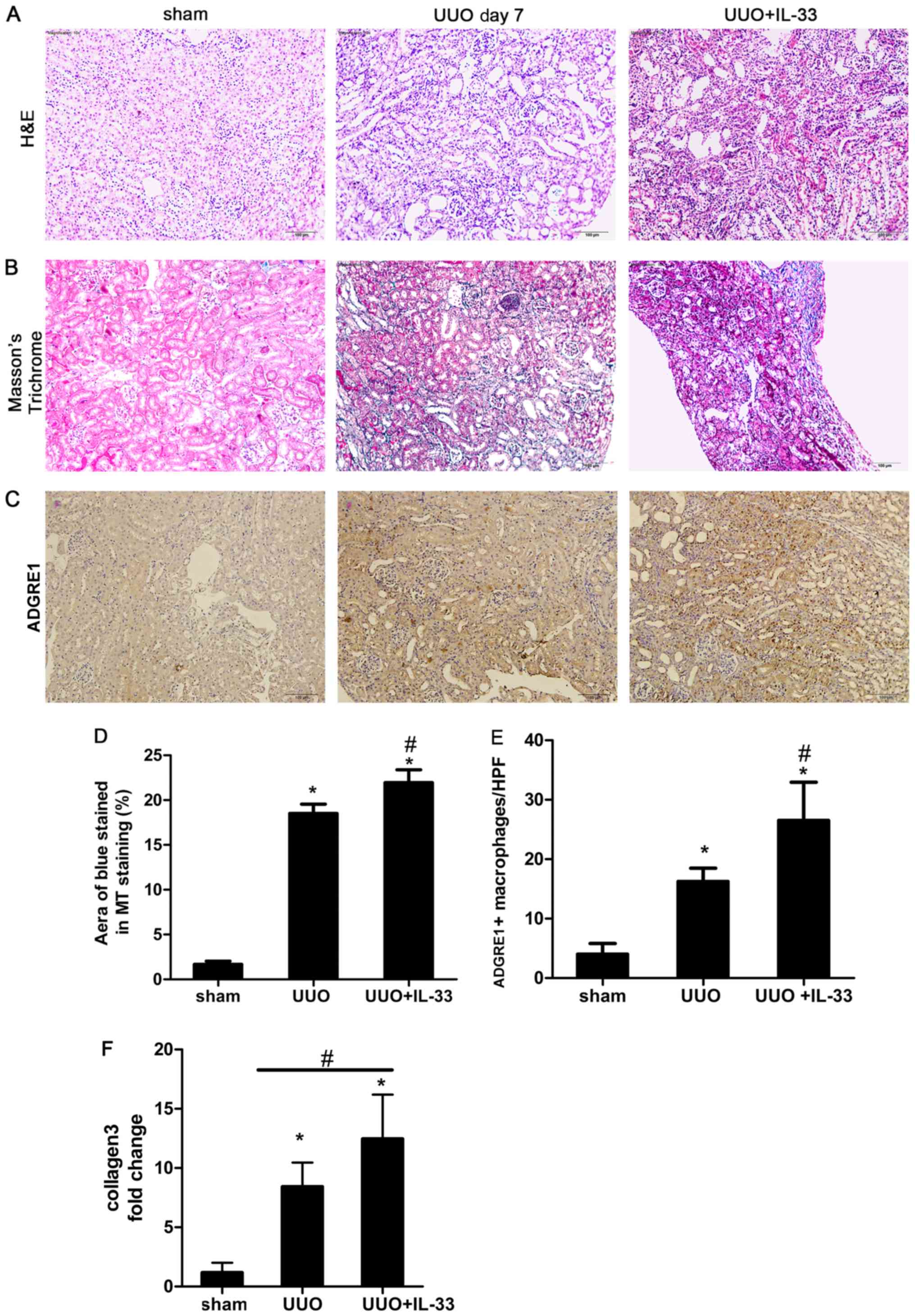
Recombinant mIL-33 exacerbates
UUO-induced renal fibrosis and macrophage infiltration in mice
kidneys
Mice were injected intraperitoneally with mIL-33 on
the same day of UUO surgery. Sham-operated and UUO mice were used
as control groups. Kidney tissues were harvested on day 7.
Exogenous mIL-33 significantly increased UUO-induced renal
inflammation (Fig. 2A) and
collagen deposition (Fig. 2B and
D) compared with the sham surgery group. Treatment with IL-33
significantly increased the expression level of collagen compared
with the UOO group (P<0.05; Fig.
2F).
IL-33 was previously identified to influence the
phenotype and function of macrophages (31). In addition, ADGRE1 is a well-known
macrophage marker (5). In the
present study, to investigate the relationship between macrophages
and IL-33 in obstructive renal injury, immunostaining of ADGRE1 was
performed on renal tissue. The infiltration of ADGRE1 positive
macrophages increased in UUO mice treated with recombinant mIL-33
compared with the sham and UUO groups (Fig. 2C and E). The number of macrophages
was higher in the renal tubule area compared with other regions
(Fig. 2C).
UUO induces IL-33 production, which
promotes renal fibrosis through macrophages, increasing the
secretion of IL-13 and TGF-β1
A previous study identified that IL-13 and TGF-β1
are key cytokines required for the development of fibrosis
(19). As mentioned above, IL-33
exacerbated UUO-induced renal fibrosis and the expression levels of
these cytokines. However, macrophage depletion (Fig. 3A and D) decreased renal
inflammation and interstitial fibrosis (Fig. 3B, C, E and F), and abrogated the
expression levels of IL-13 and TGF-β1 (Fig. 3I-L). Additionally, macrophage
ablation decreased the expression levels of IL-33 and IL1RL1
(Fig. 3G and H). The present
findings suggested that macrophages served an important role in the
mechanism underlying IL-33/IL1RL1 role in promoting renal fibrosis
through the production of fibrogenic factors, including TGF-β1 and
IL-13.
 | Figure 3.UUO induces interleukin IL-33, which
promotes renal fibrosis via macrophages. Mice were subjected to
sham surgery, UUO or UUO + IL-33, and treated with clodronate or
control liposomes. Mouse kidneys were harvested 7 days after
surgery. (A) Immunohistochemical staining of ADGRE1 suggested that
macrophages were ablated by clodronate treatment. (B) H&E
staining suggested that macrophage depletion reduced UUO-induced
inflammatory cell infiltration. (C) MT staining suggested that
macrophage depletion reduced UUO-induced renal fibrosis. (D)
Quantification of ADGRE1 positive cells. (E) Quantification of MT
staining results. (F) Collagen 3 relative mRNA expression levels.
(G) IL-33 relative mRNA expression levels. (H) IL1RL1 relative mRNA
expression levels. (I) IL-13 serum levels. (J) IL-13 relative mRNA
expression levels. (K) TGF-β1 serum levels. (L) TGF-β1 relative
mRNA expression levels. Magnification, ×200. Data are expressed as
the mean ± SD from three independent experiments. *P<0.05 vs.
sham; #P<0.05 vs. respective Lip PBS group. H&E,
hematoxylin and eosin; ADGRE1, adhesion G protein-coupled receptor
E1; UUO, unilateral ureteral obstruction; MT, Masson's trichrome;
Lip, liposomes; Clod, clodronate; IL, interleukin; IL1RL1,
interleukin 1 receptor like 1; TGF, transforming growth factor. |
IL-33 increases UUO-induced renal
fibrosis by promoting the polarization of macrophages to M2
phenotype
Previous in vitro studies demonstrated that
IL-33 increased the IL-13-mediated macrophage polarization to M2
phenotype (25). In the present
study, the mRNA expression level of ARG1, an M2 marker (32), was investigated. Treatment with
IL-33 increased the mRNA expression of ARG1 in UUO mice (Fig. 4A). During UUO-induced renal
fibrosis, the protein expression level of ARG1 increased compared
with the sham-operated group, and exogenous IL-33 increased this
effect (Fig. 4B).
Discussion
Although the IL-33/IL1RL1 pathway has been studied
in skin, heart, liver and lung fibrosis (33), only a limited number of studies
have investigated this pathway in kidney fibrosis. Upregulation of
the IL-33/IL1RL1 signaling pathway in human serum may promote renal
interstitial fibrosis; however, whether this process is related to
macrophages remains unclear (23).
Previous studies investigated the polarization of macrophages to M2
in the process of renal fibrosis (24,34),
but the mechanisms underlying macrophage polarization have yet to
be fully elucidated. In the present study, IL-33 was identified to
increase UUO-induced renal fibrosis in mice. The present results
suggested that this effect was mediated by the production of IL-13
and TGF-β1 by M2 macrophages. These cytokines are powerful
activators of fibroblasts, and are able to stimulate fibroblast
proliferation and function, increasing collagen synthesis and
inducing renal fibrosis (35,36).
The present data suggested that the
IL-33/IL1RL1/macrophage pathway is involved in the UUO-mediated
renal fibrotic process. Compared with the control group, the
expression levels of IL-33 and IL1RL1 in UUO-induced renal fibrotic
tissues were significantly increased between days 3 and 14
following surgery. Moreover, the immunohistochemical and
histopathological results of the kidney tissue analyses suggested
that IL-33 was primarily synthesized in the renal tubule area.
Treatment with mIL-33 increased renal fibrosis and macrophage
infiltration induced by UUO. Additionally, mIL-33 increased the
expression levels of IL-13 and TGF-β1, suggesting that IL-33 may be
a fibrogenic factor. Following macrophage ablation in the UOO
group, the expression levels of IL-33 and its receptor, IL1RL1,
were decreased to similar levels as the sham group. Additionally,
renal fibrosis was decreased, and the serum levels of IL-13 and
TGF-β1 were reduced. Furthermore, following macrophage ablation,
treatment with mIL-33 was not sufficient to reverse these effects
in UUO mice. Collectively, the present results suggested that the
role of mIL-33/IL1RL1 in promoting fibrosis in UUO mice was
primarily due to the action of macrophages and the resulting
increase in fibrogenic cytokines.
Wang et al (37) found that the majority of
macrophages expressed M2 markers, and only a small number expressed
M1 markers during renal fibrosis. However, this previous study did
not investigate the relationship between macrophages and IL-33
during renal fibrosis. Pan et al (24) demonstrated that macrophages
recruited to the fibrotic region were polarized to the M2 subtype
after renal injury in UUO mice. In acute fibrotic lesions, M1
inflammatory macrophages can transdifferentiate to M2 macrophages
(5,38). However, the mechanism of macrophage
polarization into the M2 subtype during renal fibrosis is not
clear. In the present study, IL-33 was identified to serve an
important role in this process. During UUO-induced renal fibrosis,
the expression level of ARG1, a surface marker of M2 macrophages,
was significantly higher compared with the sham group, and this
effect was further increased by treatment with IL-33. A previous
in vitro study found that IL-33 increased the IL-13-mediated
polarization of nonpolarized M0 macrophages into M2 macrophages
(25). In addition, it was
previously identified that in vitro-differentiated M2
macrophages express TGF-β1, an important fibrogenic factor
(39). The present study
identified that the expression levels of IL-13, IL-33, ARG1 and
TGF-β1 increased during UUO-induced renal fibrosis, and that, in
obstructive renal injury, IL-13 and IL-33 acted on recruited
macrophages, polarizing them to the M2 phenotype. Additionally, M2
macrophages were previously found to synthesize TGF-β1, which,
together with IL-13, may promote the development of renal fibrosis
(31).
The immune system serves an important role in the
progression of CKD. Specifically, the stimulation of the immune
system in the kidney can result in the recruitment and in the
activation of immune cells. Dysregulated tissue repair processes
are an important cause of fibrosis (40). During acute kidney injury (AKI)
caused by sterile tissue damage, resident macrophages and dendritic
cells are activated. Resident macrophages recruit more white blood
cells and initiate the immune response to clear cell debris and
dead tissue (10,41). These repair mechanisms are
important, but immune cells may prolong the inflammatory process in
response to certain chemokines, exacerbating kidney damage
(42). The present results
suggested that, during UUO-induced renal fibrosis, the inflammatory
cytokine IL-33 activated and polarized recruited macrophages,
leading to the secretion of IL-13 and TGF-β1, which were able to
increase renal injury and fibrosis.
Current treatment methods for AKI and CKD involve
supportive and alternative therapies. To the best of the authors'
knowledge, targeted therapy to treat AKI and CKD has not yet been
used in a clinical setting. A number of previous studies on
experimental ischemic AKI demonstrated that cell therapy with
dendritic and regulatory T cells was able to promote tissue repair
(43,44). Additionally, a previous study
demonstrated the efficacy of treatments aimed to targets factors
that are involved in the recruitment, activation, or are produced
by macrophages (45). The present
study investigating the mechanism underlying IL-33-mediated
macrophages regulation may provide new insights into the
development of novel therapeutic strategies to treat renal
fibrosis.
Acknowledgements
The authors would like to thank Dr Dong Li
(Department of Immunology, College of Basic Medicine, Jilin
University) for assistance in animal breeding, drug treatment and
design of the primers used in the qPCR experiments, and Dr Jinyan
Yu (Department of Respiratory and Critical Care Medicine, The
Second Hospital of Jilin University) for assistance in the
immunohistochemical experiments.
Funding
The present study was supported by The Natural
Science Foundation from the Department of Science and Technology of
Jilin Province (grant no. 20160101129JC), the Special Medical
Foundation from The Department of Finance of Jilin Province (grant
no. 2017 0125), and Jilin Province Science and Technology Plan
Outstanding Youth Talent Fund Project (grant no.
20180520137JH).
Availability of data and materials
All data generated or analyzed for the present study
are included in this published paper.
Authors' contributions
HL conceived and designed the experiments. YL
performed the experiments and wrote the manuscript. JL provided
reagents and materials, and analyzed the data. TY prepared the
figures and performed the western blot analysis. BY interpreted the
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures were approved by The Animal
Experimental Ethics Committee of Jilin University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barnes JL and Glass WFII: Renal
interstitial fibrosis: A critical evaluation of the origin of
myofibroblasts. Contrib Nephrol. 169:73–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hill NR, Fatoba ST, Oke JL, Hirst JA,
O'Callaghan CA, Lasserson DS and Hobbs FD: Global prevalence of
chronic kidney disease-A systematic review and meta-analysis. PLoS
One. 11:e01587652016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao J, Wang L, Cao A, Jiang M, Chen X and
Peng W: Renal tubulointerstitial fibrosis: A review in animal
models. J Integr Nephrol Androl. 2:75–80. 2015. View Article : Google Scholar
|
|
4
|
Imig JD and Ryan MJ: Immune and
inflammatory role in renal disease. Compr Physiol. 3:957–976.
2013.PubMed/NCBI
|
|
5
|
Xiao X, Gaffar I, Guo P, Wiersch J,
Fischbach S, Peirish L, Song Z, El-Gohary Y, Prasadan K, Shiota C
and Gittes GK: M2 macrophages promote beta-cell proliferation by
up-regulation of SMAD7. Proc Natl Acad Sci USA. 111:E1211–E1220.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chevalier RL, Forbes MS and Thornhill BA:
Ureteral obstruction as a model of renal interstitial fibrosis and
obstructive nephropathy. Kidney Int. 75:1145–1152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forbes MS, Thornhill BA and Chevalier RL:
Proximal tubular injury and rapid formation of atubular glomeruli
in mice with unilateral ureteral obstruction: A new look at an old
model. Am J Physiol Renal Physiol. 301:F110–F117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Forbes MS, Thornhill BA, Minor JJ, Gordon
KA, Galarreta CI and Chevalier RL: Fight-or-flight: Murine
unilateral ureteral obstruction causes extensive proximal tubular
degeneration, collecting duct dilatation, and minimal fibrosis. Am
J Physiol Renal Physiol. 303:F120–F129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferrario F, Castiglione A, Colasanti G,
Barbiano di Belgioioso G, Bertoli S and D'Amico G: The detection of
monocytes in human glomerulonephritis. Kidney Int. 28:513–519.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
Inflammatory processes in renal fibrosis. Nat Rev Nephrol.
10:493–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim MG, Kim SC, Ko YS, Lee HY, Jo SK and
Cho W: The role of M2 macrophages in the progression of chronic
kidney disease following acute kidney injury. PLoS One.
10:e01439612015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guiteras R, Flaquer M and Cruzado JM:
Macrophage in chronic kidney disease. Clin Kidney J. 9:765–771.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guiteras R, Sola A, Flaquer M, Hotter G,
Torras J, Grinyó JM and Cruzado JM: Macrophage overexpressing NGAL
ameliorated kidney fibrosis in the UUO mice model. Cell Physiol
Biochem. 42:1945–1960. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Colin S, Chinetti-Gbaguidi G and Staels B:
Macrophage phenotypes in atherosclerosis. Immunol Rev. 262:153–166.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flavell RA, Sanjabi S, Wrzesinski SH and
Licona-Limón P: The polarization of immune cells in the tumour
environment by TGFbeta. Nat Rev Immunol. 10:554–567. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liew FY, Girard JP and Turnquist HR:
Interleukin-33 in health and disease. Nat Rev Immunol. 16:676–689.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ricardo SD, van Goor H and Eddy AA:
Macrophage diversity in renal injury and repair. J Clin Invest.
118:3522–3530. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wynn TA and Ramalingam TR: Mechanisms of
fibrosis: Therapeutic translation for fibrotic disease. Nat Med.
18:1028–1040. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lott JM, Sumpter TL and Turnquist HR: New
dog and new tricks: Evolving roles for IL-33 in type 2 immunity. J
Leukoc Biol. 97:1037–1048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kurowska-Stolarska M, Hueber A, Stolarski
B and McInnes IB: Interleukin-33: A novel mediator with a role in
distinct disease pathologies. J Intern Med. 269:29–35. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Staurengo-Ferrari L, Trevelin SC, Fattori
V, Nascimento DC, de Lima KA, Pelayo JS, Figueiredo F, Casagrande
R, Fukada SY, Teixeira MM, et al: Interleukin-33 receptor (ST2)
deficiency improves the outcome of staphylococcus aureus-induced
septic arthritis. Front Immunol. 9:9622018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen WY, Chang YJ, Su CH, Tsai TH, Chen
SD, Hsing CH and Yang JL: Upregulation of Interleukin-33 in
obstructive renal injury. Biochem Biophys Res Commun.
473:1026–1032. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan B, Liu G, Jiang Z and Zheng D:
Regulation of renal fibrosis by macrophage polarization. Cell
Physiol Biochem. 35:1062–1069. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li D, Guabiraba R, Besnard AG, Komai-Koma
M, Jabir MS, Zhang L, Graham GJ, Kurowska-Stolarska M, Liew FY,
McSharry C and Xu D: IL-33 promotes ST2-dependent lung fibrosis by
the induction of alternatively activated macrophages and innate
lymphoid cells in mice. J Allergy Clin Immunol. 134:1422–1432.e11.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Inada T, Yamanouchi Y, Jomura S, Sakamoto
S, Takahashi M, Kambara T and Shingu K: Effect of propofol and
isoflurane anaesthesia on the immune response to surgery.
Anaesthesia. 59:954–959. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang WL, Liu MY, Zhang ZC and Duan CY:
Effect of different anesthesia methods on erythrocyte immune
function in mice. Asian Pac J Trop Med. 6:995–998. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hohlbaum K, Bert B, Dietze S, Palme R,
Fink H and Thöne-Reineke C: Severity classification of repeated
isoflurane anesthesia in C57BL/6JRj mice-Assessing the degree of
distress. PLoS One. 12:e01795882017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van Rooijen N and Hendrikx E: Liposomes
for specific depletion of macrophages from organs and tissues.
Methods Mol Biol. 605:189–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ferenbach DA, Sheldrake TA, Dhaliwal K,
Kipari TM, Marson LP, Kluth DC and Hughes J: Macrophage/monocyte
depletion by clodronate, but not diphtheria toxin, improves renal
ischemia/reperfusion injury in mice. Kidney Int. 82:928–933. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aimo A, Migliorini P, Vergaro G, Franzini
M, Passino C, Maisel A and Emdin M: The IL-33/ST2 pathway,
inflammation and atherosclerosis: Trigger and target? Int J
Cardiol. 267:188–192. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sanson M, Distel E and Fisher EA: HDL
induces the expression of the M2 macrophage markers arginase 1 and
Fizz-1 in a STAT6-dependent process. PLoS One. 8:e746762013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Molofsky AB, Savage AK and Locksley RM:
Interleukin-33 in tissue homeostasis, injury, and inflammation.
Immunity. 42:1005–1019. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu CC, Chien CT and Chang TC: M2
macrophage polarization modulates epithelial-mesenchymal transition
in cisplatin-induced tubulointerstitial fibrosis. Biomedicine
(Taipei). 6:52016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Biernacka A, Dobaczewski M and
Frangogiannis NG: TGF-β signaling in fibrosis. Growth Factors.
29:196–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ramalingam TR, Gieseck RL, Acciani TH, M
Hart K, Cheever AW, Mentink-Kane MM, Vannella KM and Wynn TA:
Enhanced protection from fibrosis and inflammation in the combined
absence of IL-13 and IFN-γ. J Pathol. 239:344–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang S, Meng XM, Ng YY, Ma FY, Zhou S,
Zhang Y, Yang C, Huang XR, Xiao J, Wang YY, et al: TGF-β/Smad3
signalling regulates the transition of bone marrow-derived
macrophages into myofibroblasts during tissue fibrosis. Oncotarget.
7:8809–8822. 2016.PubMed/NCBI
|
|
38
|
Ikezumi Y, Suzuki T, Yamada T, Hasegawa H,
Kaneko U, Hara M, Yanagihara T, Nikolic-Paterson DJ and Saitoh A:
Alternatively activated macrophages in the pathogenesis of chronic
kidney allograft injury. Pediatr Nephrol. 30:1007–1017. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu J, Cao Q, Zheng D, Sun Y, Wang C, Yu X,
Wang Y, Lee VW, Zheng G, Tan TK, et al: Discrete functions of M2a
and M2c macrophage subsets determine their relative efficacy in
treating chronic kidney disease. Kidney Int. 84:745–755. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tecklenborg J, Clayton D, Siebert S and
Coley SM: The role of the immune system in kidney disease. Clin Exp
Immunol. 192:142–150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yatim KM and Lakkis FG: A brief journey
through the immune system. Clin J Am Soc Nephrol. 10:1274–1281.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kurts C, Panzer U, Anders HJ and Rees AJ:
The immune system and kidney disease: Basic concepts and clinical
implications. Nat Rev Immunol. 13:738–753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li L, Huang L, Ye H, Song SP, Bajwa A, Lee
SJ, Moser EK, Jaworska K, Kinsey GR, Day YJ, et al: Dendritic cells
tolerized with adenosine A2AR agonist attenuate acute kidney
injury. J Clin Invest. 122:3931–3942. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lai LW, Yong KC and Lien YH: Pharmacologic
recruitment of regulatory T cells as a therapy for ischemic acute
kidney injury. Kidney Int. 81:983–992. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sean Eardley K and Cockwell P: Macrophages
and progressive tubulointerstitial disease. Kidney Int. 68:437–455.
2005. View Article : Google Scholar : PubMed/NCBI
|