Introduction
Human dental pulp stem cells (hDPSCs) possess the
ability to proliferate and to differentiate into odontoblasts,
which enables them to participate in the reconstruction and repair
of diseased pulp tissue (1–3). At
present, dental caries, and the subsequent inflammation caused by
dental pathogens, are common and nonnegligible clinical problems
(4,5). Human carious dental pulp stem cells
(hCDPSCs) have attracted increasing levels of attention (6). Compared with normal hDPSCs, hCDPSCs
have a number of advantages, such as a high proliferation rate and
easy availability (6–9). hCDPSCs are considered to be ideal
‘seed’ cells in tissue engineering, although their characteristics
have yet to be fully elucidated.
Growth factors (GFs) are types of polypeptide or
glycoprotein that possess the biological properties of promoting
cell proliferation, differentiation and locomotion (10–12).
Among them, vascular endothelial growth factor (VEGF) and
insulin-like growth factor-1 (IGF-1) are two important GFs with
unique functions (13–15). VEGF has the capacity to trigger
newly forming vessels in order to provide a blood supply for bone
regeneration (16). IGF-1, a
member of the insulin-like peptide family, is a ubiquitous and
important peptide hormone and anti-apoptotic factor that exerts
important roles in organ apoptosis and differentiation (17). It has been reported that IGF-1 may
promote the proliferation of dental pulp stem cells (DPSCs) and
periodontal ligament stem cells (PDLSCs), and induce their
osteogenic/odontogenic differentiation (18,19).
It has been shown, that when either VEGF (25 ng/ml) or IGF-1 (100
ng/ml) was used, mesenchymal stem cells (MSCs) exhibited the
strongest proliferative capacity (19–21).
Furthermore, it was reported that the combined use of VEGF and
IGF-1 enhanced osteogenic differentiation of periosteum-derived
progenitor cells (PDPCs) and skin-derived MSCs (S-MSCs) (22). However, to the best of our
knowledge, the effects of VEGF and IGF-1, either alone or in
combination, on hCDPSCs has yet to be elucidated.
The phosphoinositide 3-kinase (PI3K)/Akt signaling
pathway is an important signal transduction pathway, which tightly
controls cell proliferation, migration, differentiation and
self-renewal (23,24). It has been reported that activation
of the AKT signaling pathway led to an improvement in the
proliferative and differentiation abilities of MSCs, thereby
enhancing stemness (25).
In the present study, IGF-1 and VEGF, when applied
either alone or in combination, were observed to exert an effect on
the proliferation, migration and differentiation of hCDPSCs in
vitro, and these effects were found to be associated with the
AKT signaling pathway. These findings add to our knowledge of the
cellular biological foundation for clinical application of hCDPSCs
in the treatment of dental pulp disease.
Materials and methods
Isolation and culture of hCDPSCs
hCDPSCs were obtained from the carious pulp tissues,
which were collected from teeth diagnosed with deep caries. The
diagnosis of deep caries was determined by endodontic specialists
on the basis of clinical assessment (26). A total of 20 patients (28–30 years
of age; 10 male and 10 female patients) were informed about the
nature of this research project, agreed to participate, and signed
informed consent forms for scientific experiments involving tooth
extraction in the Department of Stomatology of Nanfang Hospital,
Southern Medical University (Guangzhou, China). The study protocol
was performed according to a standard protocol approved by the
Ethics Committee of the Southern Medical University. The pulp was
carefully collected (1/3 of the apical pulp was discarded), cut
into small pieces, and digested with collagenase (3 mg/ml)/dispase
enzyme (4 mg/ml; Sigma-Aldrich; Merck KGaA) for 30 min. After 7–10
days, the primary cultured hCDPSCs were observed to be able to
climb out along the edge of the tissue blocks. When the confluence
of hCDPSCs reached ~80%, the cells were subcultured in new flasks.
Cells were cultured with HyClone™ α-Minimal Essential Medium
(α-MEM; Thermo Fisher Scientific, Inc.) containing 10% Gibco fetal
bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin (all
from Gibco; Thermo Fisher Scientific, Inc.). For all incubations in
the methods section, unless specified otherwise, cells were
cultured at 37°C in an atmosphere of 5% CO2.
Phenotypic identification of
hCDPSCs
Passage 3 hCDPSCs in the exponential growth phase
were collected by 0.25% Trypsin and centrifuged at 1,500 × g for 1
min at 37°C, and suspended in phosphate-buffered saline (PBS) to
prepare a single cell suspension with a density of
1×106/ml. The hCDPSCs were incubated with antihuman
CD29PE (1:300, cat. no. 557332;), CD44PE (1:300, cat. no. 562818),
CD45-PC5 (1:300, cat. no. 555484), CD90-PC5 (1:300, cat. no.
561972), CD105-PE (1:300, cat. no. 560839) and CD133-APC (1:300,
cat. no. 566596; all from BD Biosciences, Franklin Lakes, NJ, USA)
antibodies in different tubes at room temperature for 30 min,
before washing three times with PBS and resuspending the cells in
300 µl PBS. Fc blocking reagent was used to block the non-specific
detection of the Fc component of all antibodies. Flow cytometry (BD
Accuri C6, Becton Dickinson Biosciences) was used to detect the
positive rate of stem cell surface markers. All data were analyzed
by FlowJo Software (v10.0; FlowJo LLC).
Cell monoclonal assay and osteogenic
induction
hCDPSCs (2,000 cells) were inoculated into a 10 cm
culture dish at 37°C in an atmosphere of 5% CO2. After
14 days, hCDPSCs were fixed by 4% paraformaldehyde for 30 mins and
stained with Giemsa for 10 mins in 37°C Clones with >50 cells
were counted as one unit.
Passage 3 hCDPSCs were cultured with osteogenic
medium (10% FBS, 10 mmol/l sodium β-glycerophosphate, 50 mg/l
ascorbic acid and 0.1 µmol/l dexamethasone in α-MEM). After 7 days
of induction, hCDPSCs were fixed for 15 min with 4%
paraformaldehyde, and subsequently incubated with ALP stain for 10
min at room temperature. After 21 days of induction, hCDPSCs were
stained with Alizarin red for 10 min at room temperature following
fixation with 4% paraformaldehyde. After a thorough rinse, the
cells were observed and counted under a microscope a light
microscope with ×100 magnification.
Effect of VEGF and IGF-1 on
proliferation and migration of hCDPSCs
Passage 3 hCDPSCs in the exponential growth phase
were collected by 0.25% Trypsin and centrifuged at 1,500 × g for 1
min at 37°C, and inoculated (1,500 cells) into 96-well plates, and
induced by the addition of no agent (blank control), IGF-1 (100
ng/ml), VEGF (25 ng/ml), or VEGF (25 ng/ml) + IGF-1 (100 ng/ml),
respectively. Cell proliferation was analyzed using a Cell Counting
Kit-8 (CCK-8) assay (Beyotime Institute of Biotechnology).
According to the manufacturer's protocol, on days 1, 3, 5, 7 and 9,
the absorbance at 490 nm wavelength was measured following
incubation with CCK8 reagent for 3 h. Similarly, after the cells
were cultured in the different experimental group set-ups,
migration assay was performed in a Transwell chamber. Serum-free
medium was added in the upper chamber, and 5×104 cells
were inoculated in each well. 10% FBS medium was added into the
lower chamber. The cells were incubated at 37°C in an atmosphere of
5% CO2 for 24 h. Subsequently, the supernatant was
discarded, the cells were fixed in 4% paraformaldehyde for 30 min
and stained by 0.1% crystal violet in 37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells in the different
groups using TRIzol®, and total RNA was
reverse-transcribed into cDNA according to the instructions of the
RT kit employed (Qiagen GmbH). The content of RNA was quantified
(Nanodrop; Thermo Fisher Scientific, Inc.). SYBR Green was
purchased from Qiagen GmbH and the sequences of the RT-PCR primers
(Thermo Fisher Scientific, Inc.) are given in Table I. GAPDH was used as reference gene.
The following thermocycling conditions were used: 95°C for 20 sec,
annealing at 60°C for 30 sec, and extension at 72°C for 30 sec (40
cycles). Each assay was performed in triplicate, and quantification
was performed using the 2−ΔΔCq method (27).
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| Gene | Sequence |
|---|
| RUNX2 | F:
5′TGGTTACTGTCATGGCGGGTA3′ |
|
| R:
5′TCTCAGATCGTTGAACCTTGCTA3′ |
| BSP | F:
5′CACTGGAGCCAATGCAGAAGA3′ |
|
| R:
5′TGGTGGGGTTGTAGGTTCAAA3′ |
| ALP | F:
5′GAGATGTTGTCCTGACACTTGTG3′ |
|
| R:
5′AGGCTTCCTCCTTGTTGGGT3′ |
| VEGF | F:
5′AGGGCAGAATCATCACGAAGT3′ |
|
| R:
5′AGGGTCTCGATTGGATGGCA3′ |
| PDGF | F:
5′TGGCAGTACCCCATGTCTGAA3′ |
|
| R:
5′CCAAGACCGTCACAAAAAGGC3′ |
| GAPDH | F:
5′ACAACTTTGGTATCGTGGAAGG3′ |
|
| R:
5′GCCATCACGCCACAGTTTC3′ |
Western blot analysis
Passage 3 hCDPSCs from the different groups were
collected by 0.25% Trypsin and centrifuged at 1,500 × g for 1 min
at 37°C and washed twice with ice-cold PBS, and subsequently the
total protein was extracted using RIPA buffer (Beyotime Institute
of Biotechnology) (28). Aliquots
of 40 µg protein from each sample were subjected to SDSPAGE (10%
gels), and the proteins were subsequently transferred on to PVDF
membranes (Life Sciences, Ann Arbor, MI, USA). The membranes were
subsequently blocked with TBST containing 5% defatted milk powder
at room temperature for 1 h, and then incubated overnight with
primary antibodies against RUNX2 (1:500, cat. no. 12556), BSP
(1:500, cat. no. 5468S), ALP (1:500, cat. no. 8681), VEGF (1:500,
cat. no. 2463), PDGF (1:500, cat. no. 3169), AKT (1:500, cat. no.
4685), phosphorylated (p)-AKT (1:500, cat. no. 9614) and cyclin D1
(1:500, cat. no. 2978; all antibodies from Cell Signaling
Technology, Danvers, MA, USA) at 4°C overnight. The membranes were
subsequently washed with PBS three times. Peroxidase-conjugated
goat anti-rabbit or goat anti-mouse was used as the secondary
antibody (cat. no. 4414 or 4410S, respectively; 1:1,000 dilution;
Cell Signaling Technology), and the membranes were incubated with
secondary antibody for 1 h at room temperature, prior to subsequent
exposure to an Odyssey 2-colour infrared laser imaging system
(LI-COR Biosciences, Lincoln, NE, USA). The semi-quantitative
results for each western blot were measured using the Image-Pro
Plus 6.0 program (Media Cybernetics, Inc., Rockville, MD, USA).
In vitro Matrigel™ tube formation
assay
Passage 3 hCDPSCs in the exponential growth phase
were collected by 0.25% Trypsin and centrifuged at 1,500 × g for 1
min at 37°C, and cell suspensions (1×106 cells) were
prepared. Matrigel™ was coated onto 12-well plates according to the
manufacturer's protocol (29,30).
The cells were gently mixed, inoculated on to the aggregated
Matrigel™, and subsequently were incubated in 37°C in an atmosphere
of 5% CO2 for 7 h. The condition of the vessels was
observed under a light microscope, and the density of vessels was
determined using Image-Pro Plus 6.0 program (Media Cybernetics,
Inc.).
Statistical analysis
SPSS 17.0 was used for statistical analysis. Data
are expressed as the mean ± standard deviation. Comparisons among
groups was performed using one-way ANOVA, and post-hoc comparisons
were performed by the least significant difference (LSD) t test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Identification of hCDPSCs
Passage 3 hCDPSCs were collected and cultured for
stem cell identification. The cells exhibited the property of
monoclonal formation, as well as osteogenic differentiation and
mineralization (Fig. 1A-C). The
flow cytometry experiments revealed positive expression of CD29,
CD44, CD90 and CD105, and negative expression of CD45 and CD133
(Fig. 1D). According to the
criteria for defining multipotent MSCs, the isolated human carious
dental pulp cells were identified as MSCs.
Effects of VEGF and IGF-1 on
proliferation and migration of hCDPSCs
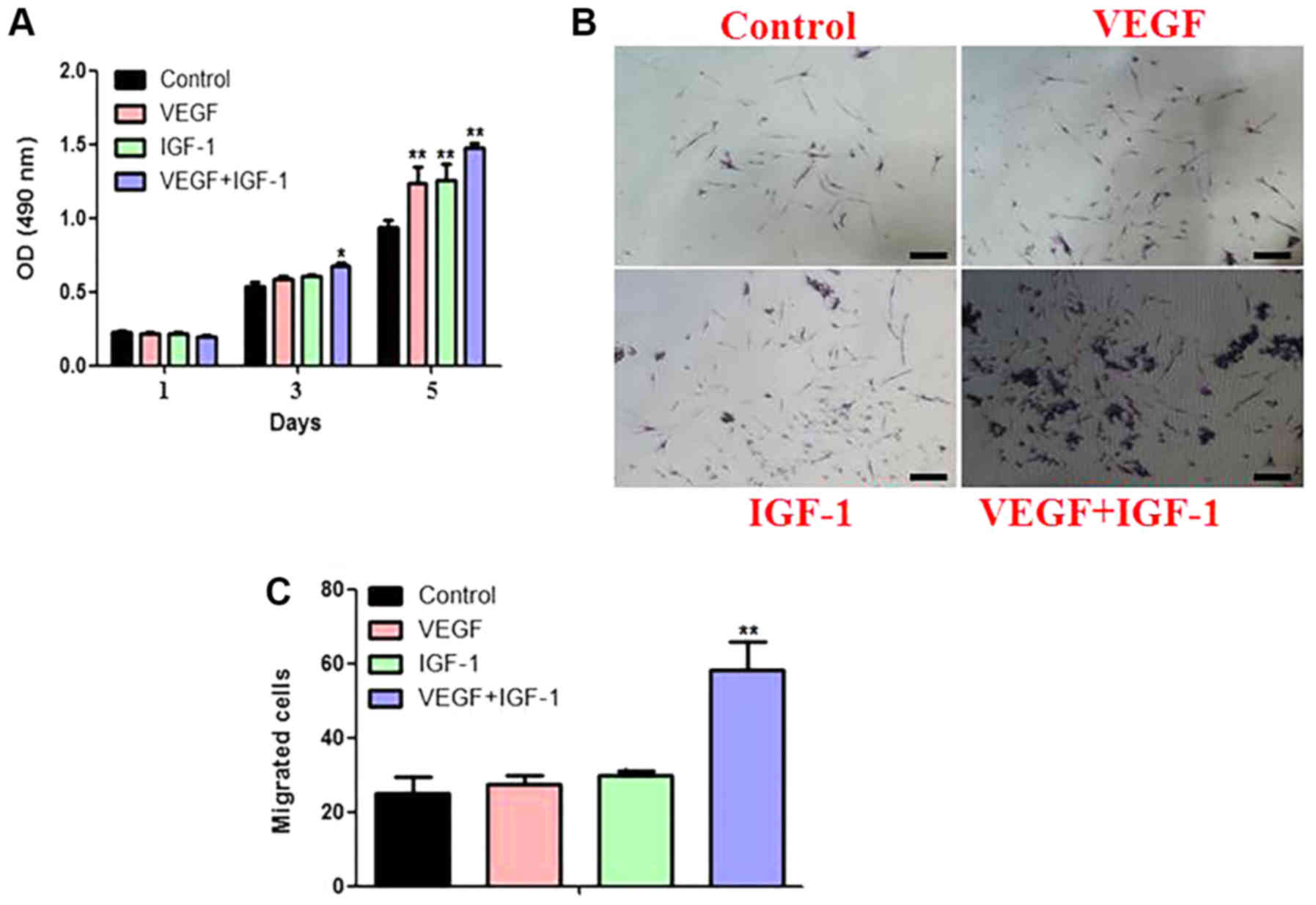
CCK8 assay revealed that the proliferation of
hCDPSCs could be stimulated by adding either VEGF or IGF-1 alone,
whereas the addition of VEGF and IGF-1 in combination led to a
further enhancement in the proliferation rate of the hCDPSCs
(Fig. 2A).
Transwell assay was subsequently used to detect the
migratory ability of hCDPSCs upon treatment with VEGF and IGF-1,
either separately or in combination. The results demonstrated that
there was a synergistic effect of VEGF/IGF-1 treatment on cell
migration, and the effect of combined treatment was greater than
that of either of these two GFs when added in isolation (Fig. 2B).
Effects of VEGF and IGF-1 on
osteogenic differentiation of hCDPSCs in vitro
For these experiments, five experimental groups were
established: Blank control (Con), ordinary osteogenic medium (OM),
osteogenic medium plus VEGF (VEGF + OM), osteogenic medium plus
IGF-1 (IGF-1 + OM), and osteogenic medium plus VEGF and IGF-1 (VEGF
+ IGF-1 + OM). Alizarin red staining and ALP staining revealed
that, compared with the OM group, the osteogenic ability of the
VEGF + IGF-1 + OM group revealed a significant enhancement in the
staining intensity (Fig. 3A-C).
The corresponding RT-qPCR and western blotting results revealed
that the mRNA and protein expression levels of the
osteogenesisassociated genes (i.e., Runx2, BSP and ALP) increased
significantly in the VEGF + OM and IGF-1 + OM groups, whereas the
largest increase was observed for the VEGF + IGF-1 + OM group
(Fig. 4A-C).
 | Figure 3.Osteogenic differentiation of hCDPSCs
upon treatment with VEGF and IGF-1. (A) The osteogenic
differentiation capability of hCDPSCs was examined using Alizarin
red staining, with (B) subsequent quantification of the results.
**P<0.01 vs. control. (C) The results from ALP staining are
shown. For the description of the experimental groups (Control, OM,
VEGF+OM, IGF-1+OM and VEGF+IGF-1+OM), see the Results section.
Scale bar, 200 µm. hCDPSCs, human carious dental pulp stem cells;
IGF-1, insulinlike growth factor 1; VEGF, vascular endothelial
growth factor; ALP, alkaline phosphatase. |
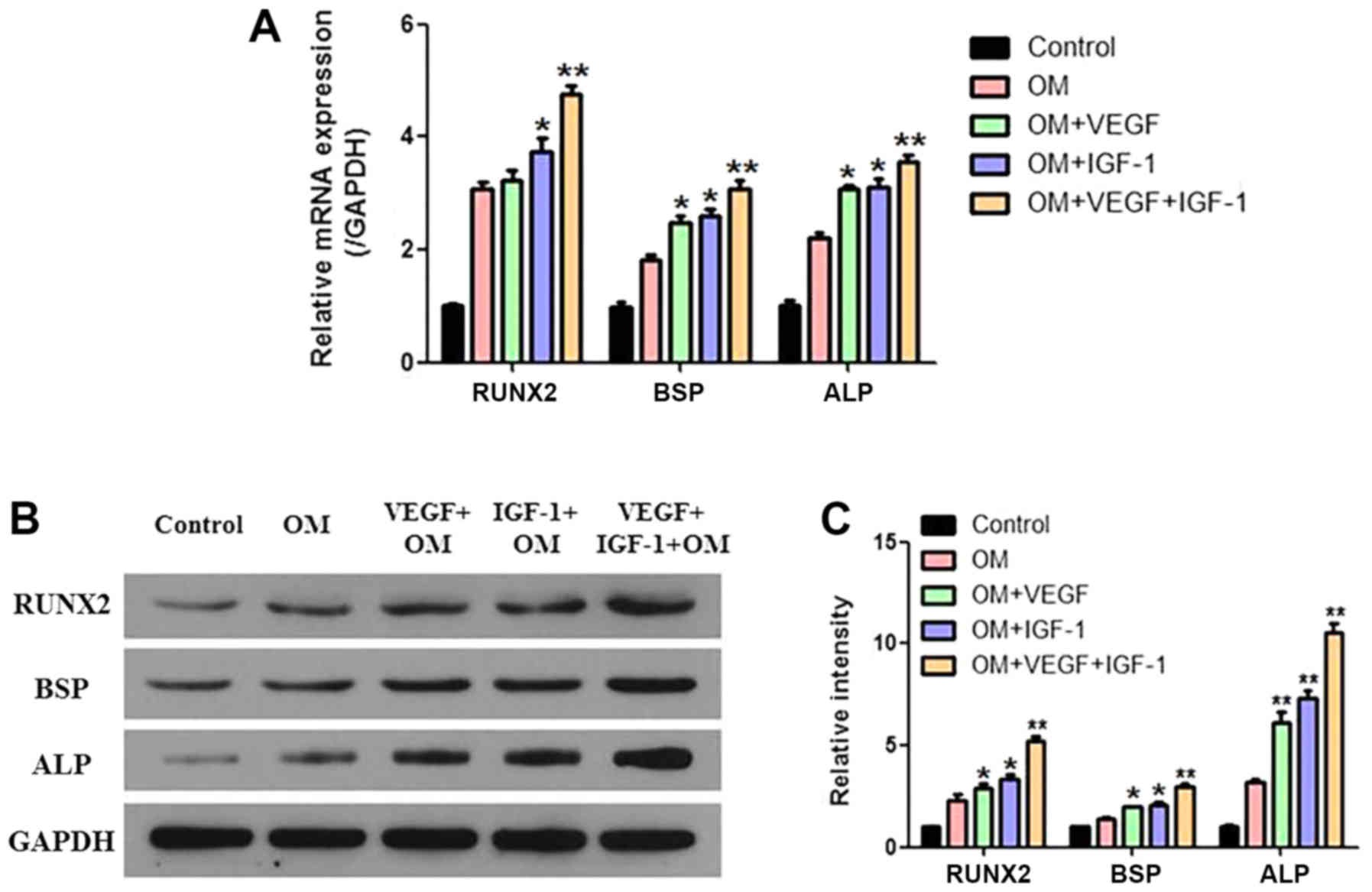 | Figure 4.Analysis of the
osteogenesis-associated genes and proteins of hCDPSCs upon
treatment with VEGF and IGF-1. (A) The mRNA expression levels of
the RUNX2, BSP and ALP genes were examined by RT-qPCR. (B) The
protein expression levels of RUNX2, BSP and ALP were examined by
western blotting, and (C) the relative protein expression levels
were normalized against GAPDH. *P<0.05, **P<0.01 vs. control.
For the description of the experimental groups (Control, OM,
VEGF+OM, IGF-1+OM and VEGF+IGF-1+OM), see the Results section.
hCDPSCs, human carious dental pulp stem cells; IGF-1, insulin-like
growth factor 1; VEGF, vascular endothelial growth factor; RUNX2,
Runt-related transcription factor 2; BSP, bone sialoprotein; ALP,
alkaline phosphatase; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase. |
Effects of VEGF and IGF-1 on
angiogenic differentiation of hCDPSCs in vitro
Tube formation assays revealed that the number of
tubules could be increased by adding either VEGF or IGF-1 alone,
whereas the addition of VEGF and IGF in combination led to a
further increase in the angiogenic ability of hCDPSCs (Fig. 5B and C). The RT-qPCR and western
blotting results exhibited a similar trend to that of the
osteogenesis-induction assay: The expression of vessel
formation-associated genes and proteins (i.e., VEGF and PDGF)
increased significantly in the OM + VEGF and OM +I GF-1 groups,
whereas the VEGF + IGF-1 + OM group exhibited the greatest
enhancement (Fig. 5A, D and
E).
 | Figure 5.Angiogenic differentiation of hCDPSCs
upon treatment with VEGF and IGF-1. (A) mRNA expression levels of
VEGF and PDGF were examined by RT-qPCR. (B and C) Tube-formation
ability was examined in the different groups. Magnification, ×200.
(D) The protein expression levels of VEGF and PDGF were detected by
western blotting, and (E) the relative protein expression level was
normalized against GAPDH. *P<0.05, **P<0.01 vs. control. For
the description of the experimental groups (Control, VEGF, IGF-1
and VEGF+IGF-1), see the Results section. hCDPSCs, human carious
dental pulp stem cells; IGF-1, insulin-like growth factor 1; VEGF,
vascular endothelial growth factor; PDGF, platelet-derived growth
factor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. |
Effect of VEGF and IGF-1 on the AKT
signaling pathway in hCDPSCs in vitro
Compared with the control group, the expression
levels of p-AKT and cyclin D1 were increased upon addition of
either VEGF or IGF-1 alone, whereas the expression of these two
proteins was further increased by adding VEGF and IGF-1 in
combination to activate the AKT signaling pathway of the hCDPSCs
(Fig. 6). These results
demonstrated that the combination of VEGF and IGF-1 elicited a
synergistic effect (Fig. 6).
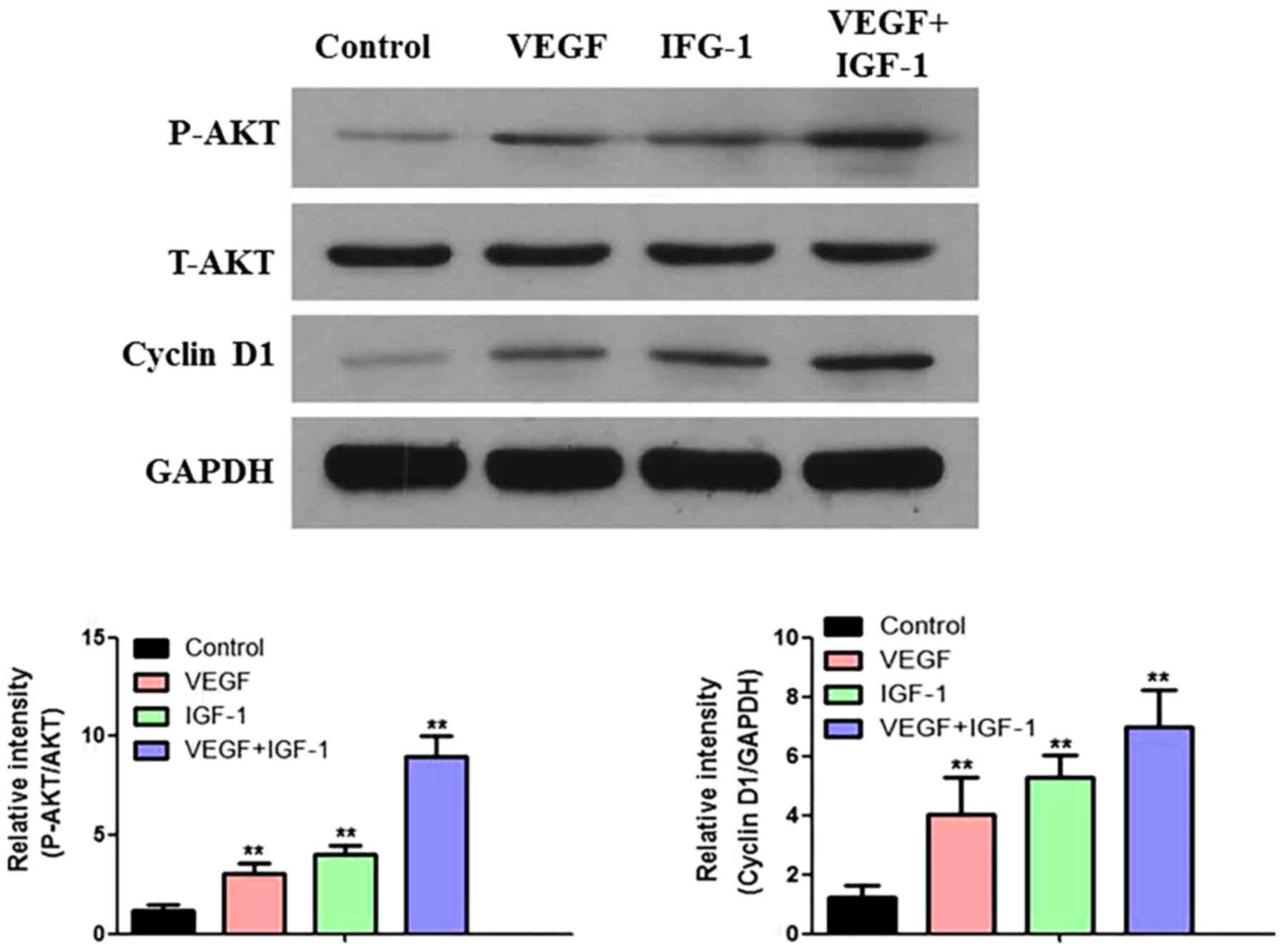 | Figure 6.Effect of AKT signaling pathway in
hCDPSCs upon treatment with VEGF and IGF-1. The protein expression
levels of P-AKT, T-AKT and cyclin D1 were detected by western
blotting, and the relative protein expression levels were
calculated. **P<0.01 vs. control. For the description of the
experimental groups (Control, VEGF, IGF-1 and VEGF+IGF-1), see the
Results section. hCDPSCs, human carious dental pulp stem cells;
IGF-1, insulin-like growth factor 1; VEGF, vascular endothelial
growth factor; P-AKT, phosphorylated AKT; T-AKT, total AKT. |
Discussion
Currently, hDPSCs are commonly used as ‘seed’ cells
in bone tissue engineering due to their high self-renewal capacity
and stemness (3,31). hCDPSCs are a unique type of dental
stem cell, since they are derived from the pulp in deep carious
teeth (7,8). This special environmental stimulus
enables the stronger proliferation and osteogenic differentiation
capability of hCDPSCs, and therefore they are now recognized as a
potential source for regenerative medicine (7,8).
GFs are critical in tissue regeneration, as they
have important roles in regulating cell functions (32,33).
Previous studies have reported that the combination of IGF-1 and
other GFs (such as PDGF-B or BMP-2) may affect cell proliferation,
promote osteogenesis, and help the reconstruction of
toothsupporting tissues (34–36).
IGF-1 has been identified to stimulate osteogenic differentiation
via the mitogen-activated protein kinase signaling pathway,
suggesting that IGF-1 has a role in regeneration of the periodontal
tissue (37). VEGF has the
potential of triggering neovascularization, which is able to
regulate both normal and pathological conditions, and provides
blood supply for tissue regeneration (38,39).
Angiogenesis is essential for bone reconstruction, as new bone
formation relies on a suitable blood supply to provide cells and
nutrients (40). Therefore, the
selection of suitable GFs has become an important research focus in
tissueengineering therapy.
In the present study, the CCK8 assay results
demonstrated that the combination of VEGF and IGF-1 exerted a
synergistic effect on proliferation. Transwell assay revealed that
the migratory ability of hCDPSCs upon combined treatment of VEGF
and IGF-1 was markedly stronger compared with that of treating with
VEGF or IGF-1 alone. This finding indicated that there was a
synergistic effect resulting from treating the cells with VEGF and
IGF-1 in combination. It has been previously shown that, when
either VEGF (25 ng/ml) or IGF-I (100 ng/ml) were used, MSCs
exhibited the strongest proliferative capacity (19–21).
Furthermore, it was reported that the combined use of VEGF and
IGF-1 enhanced osteogenic PDPCs and S-MSCs (22). Therefore, based on the findings of
these previous studies, a combination of IGF-1 (100 ng/ml) and VEGF
(25 ng/ml) was used in the present study to investigate the effects
of IGF-1 and VEGF on proliferation, migration, osteogenesis and
vascularization of hCDPSCs.
RUNX2 is a highly conserved transcription factor,
known as the most important regulator in osteoblast and odontoblast
differentiation (41). Runx2
activates boneassociated genes and promotes mineralization in the
early stage of osteoblast differentiation (41,42).
ALP is the enzyme that is predominantly involved in both bone and
tooth mineralization, and elevated levels of ALP may be considered
as an early marker of odontoblast differentiation and dentin
formation (43). BSP is an
important product during osteogenic differentiation, which may
clearly indicate the level of ALP (44). The results of RT-PCR and western
blotting in the present study demonstrated that VEGF and IGF-1
exerted a synergistic effect on bone formation, which was more
effective than using either of the drugs alone. It has been
reported that the combined use of VEGF and IGF-1 could induce the
differentiation of S-MSCs into osteoblasts, and that the IGF-1 and
insulin signaling pathways may function as important mediators in
terms of Runx2 activity (45).
The results from the alizarin red and ALP staining
experiments in the present study revealed that the combined use of
VEGF and IGF-1 could promote the osteogenesis of hCDPSCs. VEGF has
been widely studied as a major regulator in angiogenesisassociated
processes, in which the molecule exerts both direct and indirect
functions (46). VEGF stimulates
cell proliferation and migration, whereas on the other hand, it
increases the permeability of blood vessels and allows plasma
proteins to leak out of blood vessels, a process that serves an
important role in remodeling the extracellular matrix to adapt to
angiogenesis (46,47). The addition of VEGF during the
early stage of bone repair may lead to an improvement in the blood
supply of the microenvironment and the formation of new bone
(47). The results of the present
study showed that the single application of either VEGF or IGF-1
was sufficient to promote vascular differentiation of hCDPSCs,
whereas the combined use of VEGF and IGF-1 elicited a synergistic
effect.
The PI3K/Akt pathway is a tyrosine kinase
receptor-mediated signaling system, which exists widely in various
types of cell (23). It is an
important signal-transduction pathway involved in the regulation of
cell growth, proliferation and differentiation (24). Activated Akt can induce changes in
a series of downstream factors [including mammalian target of
rapamycin, (mTOR), glycogen synthase kinase 3 (GSK3), Bax, NF-κB,
caspases, etc.] and participate in the regulation of cell growth,
differentiation, division and migration (24). The phosphorylation level of Akt may
also reflect the activity of whole signaling pathway (48). It has been shown that IGF-1
promotes the vascular differentiation of adipose-derived stem cells
and endothelial cells via the PI3K/AKT signaling pathway (48). The activation of autophagic
activity is able to enhance the osteogenic differentiation of
hBMSCs, thereby providing a novel therapeutic target for
osteoporosis treatment (49).
Therefore, it is possible to surmise that activation of the
PI3K-AKT pathway may give rise to the observed effects associated
with the mTOR pathway and autophagy activity during the osteogenic
differentiation of hCDPSCs.
In conclusion, the present study has identified that
the AKT signaling pathway may be activated by the use of VEGF or
IGF-1 alone, whereas the AKT signaling pathway can be further
activated by the combined use of VEGF and IGF-1. This suggests that
the combined use of VEGF and IGF-1 may have the additive effects
through AKT signaling pathway.
Acknowledgements
Not applicable.
Funding
This study was supported by grant from the National
Natural Science Foundation of China (grant no. 8187041227).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BW and WL designed the study. WL, WX and JL guided
the experiments. WL, YC and YP performed the experiments. WL and WX
collected and processed the clinical data. JL, YC and YP analyzed
and interpreted the patient data. BW and WL wrote the paper, and BW
reviewed and edited the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study protocol was performed according to a
standard protocol approved by the Ethics Committee of the Southern
Medical University. All patients signed written informed consent
forms.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xuan K, Li B, Guo H, Sun W, Kou X, He X,
Zhang Y, Sun J, Liu A, Liao L, et al: Deciduous autologous tooth
stem cells regenerate dental pulp after implantation into injured
teeth. Sci Transl Med. 10:eaaf32272018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xia Y, Chen H, Zhang F, Bao C, Weir MD,
Reynolds MA, Ma J, Gu N and Xu HHK: Gold nanoparticles in
injectable calcium phosphate cement enhance osteogenic
differentiation of human dental pulp stem cells. Nanomedicine.
14:35–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martin-Del-Campo M, Rosales-Ibanez R,
Alvarado K, Sampedro JG, Garcia-Sepulveda CA, Deb S, San Román J
and Rojo L: Strontium folate loaded biohybrid scaffolds seeded with
dental pulp stem cells induce in vivo bone regeneration in critical
sized defects. Biomater Sci. 4:1596–1604. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Farges JC, Alliot-Licht B, Renard E,
Ducret M, Gaudin A, Smith AJ and Cooper PR: Dental pulp defence and
repair mechanisms in dental caries. Mediators Inflamm.
2015:2302512015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fernandez MR, Goettems ML, Demarco FF and
Correa MB: Is obesity associated to dental caries in Brazilian
schoolchildren? Braz Oral Res. 31:e832017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Louvrier A, Euvrard E, Nicod L, Rolin G,
Gindraux F, Pazart L, Houdayer C, Risold PY, Meyer F and Meyer C:
Odontoblastic differentiation of dental pulp stem cells from
healthy and carious teeth on an original PCL-based 3D scaffold. Int
Endod J. 51 (Suppl 4):e252–e263. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gnanasegaran N, Govindasamy V and Abu
Kasim NH: Differentiation of stem cells derived from carious teeth
into dopaminergic-like cells. Int Endod J. 49:937–949. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Werle SB, Lindemann D, Steffens D, Demarco
FF, de Araujo FB, Pranke P and Casagrande L: Carious deciduous
teeth are a potential source for dental pulp stem cells. Clin Oral
Investig. 20:75–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma D, Gao J, Yue J, Yan W, Fang F and Wu
B: Changes in proliferation and osteogenic differentiation of stem
cells from deep caries in vitro. J Endod. 38:796–802. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sinclair KL, Mafi P, Mafi R and Khan WS:
The use of growth factors and mesenchymal stem cells in
orthopaedics: In particular, their use in Fractures and Non-Unions:
A systematic review. Curr Stem Cell Res Ther. 12:312–325. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Augustyniak E, Trzeciak T, Richter M,
Kaczmarczyk J and Suchorska W: The role of growth factors in stem
cell-directed chondrogenesis: A real hope for damaged cartilage
regeneration. Int Orthop. 39:995–1003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hankenson KD, Gagne K and Shaughnessy M:
Extracellular signaling molecules to promote fracture healing and
bone regeneration. Adv Drug Deliv Rev. 94:3–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim Y and Liu JC: Protein-engineered
microenvironments can promote endothelial differentiation of human
mesenchymal stem cells in the absence of exogenous growth factors.
Biomater Sci. 4:1761–1772. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trosan P, Javorkova E, Zajicova A, Hajkova
M, Hermankova B, Kossl J, Krulova M and Holan V: The Supportive
Role of insulin-like growth factor-I in the differentiation of
murine mesenchymal stem cells into corneal-like cells. Stem Cells
Dev. 25:874–881. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen CY, Tseng KY, Lai YL, Chen YS, Lin FH
and Lin S: Overexpression of insulin-like growth factor 1 enhanced
the osteogenic capability of aging bone marrow mesenchymal stem
cells. Theranostics. 7:1598–1611. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi C, Zhao Y, Yang Y, Chen C, Hou X, Shao
J, Yao H, Li Q, Xia Y and Dai J: Collagen-binding VEGF targeting
the cardiac extracellular matrix promotes recovery in porcine
chronic myocardial infarction. Biomater Sci. 6:356–363. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rybalko VY, Pham CB, Hsieh PL, Hammers DW,
Merscham-Banda M, Suggs LJ and Farrar RP: Controlled delivery of
SDF-1α and IGF-1: CXCR4(+) cell recruitment and functional skeletal
muscle recovery. Biomater Sci. 3:1475–1486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng X, Huang D, Lu X, Feng G, Xing J, Lu
J, Xu K, Xia W, Meng Y, Tao T, et al: Insulin-like growth factor 1
can promote proliferation and osteogenic differentiation of human
dental pulp stem cells via mTOR pathway. Dev Growth Differ.
56:615–624. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu Y, Mu J, Fan Z, Lei G, Yan M, Wang S,
Tang C, Wang Z, Yu J and Zhang G: Insulin-like growth factor 1
enhances the proliferation and osteogenic differentiation of human
periodontal ligament stem cells via ERK and JNK MAPK pathways.
Histochem Cell Biol. 137:513–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bai Y, Li P, Yin G, Huang Z, Liao X, Chen
X and Yao Y: BMP-2, VEGF and bFGF synergistically promote the
osteogenic differentiation of rat bone marrow-derived mesenchymal
stem cells. Biotechnol Lett. 35:301–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JH, Um S, Jang JH and Seo BM: Effects
of VEGF and FGF-2 on proliferation and differentiation of human
periodontal ligament stem cells. Cell Tissue Res. 348:475–484.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dicarlo M, Bianchi N, Ferretti C, Orciani
M, Di Primio R and Mattioli-Belmonte M: Evidence supporting a
paracrine effect of IGF-1/VEGF on human mesenchymal stromal cell
commitment. Cells Tissues Organs. 201:333–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Liu X, Li H, Chen C, Hu B, Niu X,
Li Q, Zhao B, Xie Z and Wang Y: Exosomes/tricalcium phosphate
combination scaffolds can enhance bone regeneration by activating
the PI3K/Akt signaling pathway. Stem Cell Res Ther. 7:1362016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu X, Zheng S, Ye Y, Wu Y, Lin K and Su J:
Enhanced osteogenic differentiation and bone regeneration of
poly(lactic-co-glycolic acid) by graphene via activation of
PI3K/Akt/GSK-3β/β-catenin signal circuit. Biomater Sci.
6:1147–1158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iyer S, Viernes DR, Chisholm JD, Margulies
BS and Kerr WG: SHIP1 regulates MSC numbers and their osteolineage
commitment by limiting induction of the PI3K/Akt/βcatenin/Id2 axis.
Stem Cells Dev. 23:2336–2351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma D, Cui L, Gao J, Yan W, Liu Y, Xu S and
Wu B: Proteomic analysis of mesenchymal stem cells from normal and
deep carious dental pulp. PLoS One. 9:e970262014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan Y, Chen J, Yu Y, Dai K, Wang J and Liu
C: Enhancement of BMP-2-mediated angiogenesis and osteogenesis by
2-N,6-O-sulfated chitosan in bone regeneration. Biomater Sci.
6:431–439. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gangadaran P, Rajendran RL, Lee HW,
Kalimuthu S, Hong CM, Jeong SY, Lee SW, Lee J and Ahn BC:
Extracellular vesicles from mesenchymal stem cells activates VEGF
receptors and accelerates recovery of hindlimb ischemia. J Control
Release. 264:112–126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thekkeparambil Chandrabose S, Sriram S,
Subramanian S, Cheng S, Ong WK, Rozen S, Kasim NHA and Sugii S:
Amenable epigenetic traits of dental pulp stem cells underlie high
capability of xeno-free episomal reprogramming. Stem Cell Res Ther.
9:682018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schreier C, Rothmiller S, Scherer MA,
Rummel C, Steinritz D, Thiermann H and Schmidt A: Mobilization of
human mesenchymal stem cells through different cytokines and growth
factors after their immobilization by sulfur mustard. Toxicol Lett.
293:105–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alarcin E, Lee TY, Karuthedom S, Mohammadi
M, Brennan MA, Lee DH, Marrella A, Zhang J, Syla D, Zhang YS, et
al: Injectable shear-thinning hydrogels for delivering osteogenic
and angiogenic cells and growth factors. Biomater Sci. 6:1604–1615.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moeller M, Pink C, Endlich N, Endlich K,
Grabe HJ, Volzke H, Dörr M, Nauck M, Lerch MM, Köhling R, et al:
Mortality is associated with inflammation, anemia, specific
diseases and treatments, and molecular markers. PLoS One.
12:e1759092017. View Article : Google Scholar
|
|
34
|
Schminke B, Vom Orde F, Gruber R,
Schliephake H, Burgers R and Miosge N: The pathology of bone tissue
during peri-implantitis. J Dent Res. 94:354–361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chang PC, Cirelli JA, Jin Q, Seol YJ,
Sugai JV, D'Silva NJ, Danciu TE, Chandler LA, Sosnowski BA and
Giannobile WV: Adenovirus encoding human platelet-derived growth
factor-B delivered to alveolar bone defects exhibits safety and
biodistribution profiles favorable for clinical use. Hum Gene Ther.
20:486–496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang C, Hong FF, Wang CC, Li L, Chen JL,
Liu F, Quan RF and Wang JF: TRIB3 inhibits proliferation and
promotes osteogenesis in hBMSCs by regulating the ERK1/2 signaling
pathway. Sci Rep. 7:103422017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen FM, Chen R, Wang XJ, Sun HH and Wu
ZF: In vitro cellular responses to scaffolds containing two
microencapulated growth factors. Biomaterials. 30:5215–5224. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Koo T, Park SW, Jo DH, Kim D, Kim JH, Cho
HY, Kim J, Kim JH and Kim JS: CRISPR-LbCpf1 prevents choroidal
neovascularization in a mouse model of age-related macular
degeneration. Nat Commun. 9:18552018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Du J, Xie P, Lin S, Wu Y, Zeng D, Li Y and
Jiang X: Time-Phase sequential utilization of adipose-derived
mesenchymal stem cells on mesoporous bioactive glass for
restoration of critical size bone defects. ACS Appl Mater
Interfaces. 10:28340–28350. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deng L, Hu G, Jin L, Wang C and Niu H:
Involvement of microRNA-23b in TNF-α-reduced BMSC osteogenic
differentiation via targeting runx2. J Bone Miner Metab.
36:648–660. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim KM and Jang WG: Zaluzanin C (ZC)
induces osteoblast differentiation through regulating of osteogenic
genes expressions in early stage of differentiation. Bioorg Med
Chem Lett. 27:4789–4793. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Z, Tan J, Lei L, Sun W, Wu Y, Ding P
and Chen L: The positive effects of secreting cytokines IL-17 and
IFN-γ on the early-stage differentiation and negative effects on
the calcification of primary osteoblasts in vitro. Int
Immunopharmacol. 57:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Han Y, Jin Y, Lee SH, Khadka DB, Cho WJ
and Lee KY: Berberine bioisostere Q8 compound stimulates osteoblast
differentiation and function in vitro. Pharmacol Res. 119:463–475.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Qiao M, Shapiro P, Kumar R and Passaniti
A: Insulin-like growth factor-1 regulates endogenous RUNX2 activity
in endothelial cells through a phosphatidylinositol
3-kinase/ERK-dependent and Akt-independent signaling pathway. J
Biol Chem. 279:42709–42718. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Heldin J, O'Callaghan P, Hernández Vera R,
Fuchs PF, Gerwins P and Kreuger J: FGD5 sustains vascular
endothelial growth factor A (VEGFA) signaling through inhibition of
proteasome-mediated VEGF receptor 2 degradation. Cell Signal.
40:125–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gurkan A, Tekdal GP, Bostanci N and
Belibasakis GN: Cytokine, chemokine, and growth factor levels in
peri-implant sulcus during wound healing and osseointegration after
piezosurgical versus conventional implant site preparation:
Randomized, controlled, split-mouth trial. J Periodontol.
90:616–626. 2019.PubMed/NCBI
|
|
47
|
Lin S, Zhang Q, Shao X, Zhang T, Xue C,
Shi S, Zhao D and Lin Y: IGF-1 promotes angiogenesis in endothelial
cells/adipose-derived stem cells coculture system with activation
of PI3K/Akt signal pathway. Cell Prolif. 50:2017. View Article : Google Scholar :
|
|
48
|
Wan Y, Zhuo N, Li Y, Zhao W and Jiang D:
Autophagy promotes osteogenic differentiation of human bone marrow
mesenchymal stem cell derived from osteoporotic vertebrae. Biochem
Biophys Res Commun. 488:46–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pantovic A, Krstic A, Janjetovic K, Kocic
J, Harhaji-Trajkovic L, Bugarski D and Trajkovic V: Coordinated
time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy
controls osteogenic differentiation of human mesenchymal stem
cells. Bone. 52:524–531. 2013. View Article : Google Scholar : PubMed/NCBI
|