Introduction
Osteoarthritis (OA) is a chronic degenerative joint
disease that causes stiffness, restrictions in joint motion, and
chronic persistent pain (1,2).
Pathologically, OA is characterized by progressive articular
cartilage degeneration, subchondral bone rebuilding, and synovial
inflammation (3). Worldwide, knee
OA is a growing public health issue and a major cause of disability
in the elderly (4,5). Chronic pain is a hallmark symptom of
OA, which often necessitates the need for medical care and
contributes to a reduced quality of life, and increased healthcare
costs. However, current analgesic treatments for OA pain are often
insufficient or have potentially severe adverse effects. Therefore,
there is an essential need to elucidate the mechanisms of OA
pain.
Recent studies have shown that synovial inflammation
and chondrocyte cell death play prominent roles in OA progression
(6–8). Synovial macrophages can polarize
towards the proinflammatory M1 phenotype and secrete
proinflammatory cytokines, such as IL-1β, TNF-α, and IL-6, further
accelerating cartilage degeneration (9). Inhibiting the polarization of
synovial inflammation to the proinflammatory M1 phenotype may be a
vital strategy to alleviate synovial inflammation and OA pain.
Monoacylglycerol lipase (MAGL) is the enzyme responsible for
breaking down 2-arachidonoylglycerol (2-AG), and the inhibition of
MAGL enhances endocannabinoid signaling, reduces the levels of
proinflammatory metabolites, and produces anti-inflammatory and
anti-nociceptive effects (10–13).
Studies have found that the inhibition of MAGL can reduce acute
inflammatory pain and alleviate joint inflammation and pain
(14,15). However, the influence and mechanism
of MAGL on the polarization of synovial macrophages in OA remain to
be further studied.
Mitophagy, or mitochondrial autophagy, is a
selective process that mitigates inflammation and maintains
homeostasis by delivering damaged mitochondria to autophagosomes
for destruction (16,17). Studies have shown that mitophagy is
related to pain relief, including neuropathic pain and low back
pain (18–20). In addition, it was found that
enhanced mitophagy reversed LPS/IFN-γ-mediated M1 activation of
macrophages (21). However,
whether MAGL influences the levels of mitophagy of synovial
macrophages in OA remains unclear. Thus, here it was hypothesized
that MAGL may regulate the polarization of synovial macrophages by
targeting mitophagy.
In the present study, the primary aim was to explore
the potential role of MAGL in OA pain. MAGL accumulation in
synovial tissue and M1 polarization of synovial macrophages was
observed in OA patients and monoiodoacetate (MIA) mice model of OA.
Next, the effects of pharmacological inhibition and MAGL knockdown
on the polarization of macrophages were xassessed. Lastly, it was
determined that MAGL regulated the polarization of macrophages by
targeting mitophagy. These findings may provide a novel perspective
on the mechanism of MAGL for OA pain.
Materials and methods
Human OA patients
Written informed consent was obtained from each
patient for inclusion in the study and the use of their synovial
tissues for research. Synovial tissues were collected from patients
who underwent total knee replacement due to knee OA (OA group, n=5;
one woman, four men; age range, 64–74 years; median age, 69 years)
and those who underwent knee arthroscopic surgery due to anterior
cruciate ligament injury (Control group, n=5; one woman, four men;
age range, 63–71 years; median age, 66 years). This study was
performed in accordance with the Ethical Standards of the
Declaration of Helsinki and was approved by the Ethics Committee of
Suzhou Municipal Hospital (approval no. K-2021-066-K01).
Animal model
Male C57BL/6 (C57) mice weighing 25–35 g were
obtained from the Sino-British SIPPR/BK Lab (Shanghai, China). All
mice were provided ad libitum access to food and water,
housed at a temperature of 23–25°C and a humidity of 45–55%, with a
12/12-h light/dark cycle for 1 week prior to experimentation. The
mice were randomly divided into three groups (n=5 per group):
Control group, an MIA-OA group (MIA), and an MJN110 group (MIA +
MJN110).
The MIA model of OA pain in the mouse was
established as previously described (22). Briefly, the mice in the MIA-OA and
MJN110 groups were anesthetized with 50 mg/kg pentobarbital sodium
(intraperitoneal injection). The ipsilateral knee joint was trimmed
and wiped with alcohol, the knee was kept in a bent position to
identify the precise site for injection, and the 26 G needle was
inserted to inject MIA (0.1 mg/20 µl) (MilliporeSigma) through the
patellar tendon to the gap beneath the patella. A total of 3 weeks
after the MIA injection, the mice in the MJN110 group were
intraperitoneally injected with 1 mg/kg MJN110 (MilliporeSigma)
once a day for a week. On day 28, the mice were sacrificed
(CO2 euthanasia, the container was gradually filled with
carbon dioxide at a rate of 49.5% vol/min) for synovial tissue
collection.
All animal experiments were approved by the
Institutional Animal Care and Use Committee of Nanjing Medical
University (approval no. Y20210267).
Polarization of macrophages
In the logarithmic growth phase, macrophages
(RAW264.7, Beyotime Institute of Biotechnology, cat. no. C7505)
were selected and seeded into 6-well plates. After cell adhesion,
the cell culture medium (DMEM/F12, 10% FBS, 1%
Penicillin-Streptomycin) (Gibco; Thermo Fisher Scientific, Inc.)
was discarded and washed once with PBS. Then, the M1 polarization
induction medium containing 40 ng/ml LPS (MilliporeSigma) or M2
polarization induction medium containing 40 ng/ml IL-4
(MedChemExpress) was added as appropriate (23–25).
Finally, the macrophages were incubated with 5% CO2 at
37°C for 36 h.
siRNA-mediated knockdown of MAGL in
macrophages
MAGL-siRNA was designed and synthesized by Gima Gene
Company and divided into a Control group (untransfected), a siRNA
Negative Control group (NC siRNA, sense 5′-UUCUCCGAACGUGUCACGUTT-3′
and antisense, 5′-ACGUGACACGUUCGGAGAATT-3′), and three target gene
transfection groups (MAGL siRNA 1 sense,
5′-GCUGGACAUGCUGGUAUUUTT-3′ and antisense,
5′-AAAUACCAGCAUGUCCAGCTT-3′; MAGL siRNA 2, sense
5′-CCAUGACCAUGUUGGCCAUTT-3′ and antisense,
5′-AUGGCCAACAUGGUCAUGGTT-3′; and MAGL siRNA 3 sense,
5′-GCCUACCUGCUCAUGGAAUTT-3′ antisense,
5′-AUUCCAUGAGCAGGUAGGCTT-3′). Macrophages in each group were seeded
in 6-well plates. The following experiments were performed in
accordance with the instructions of the Gima gene product. The
siRNA was diluted with buffer solution and gently mixed to prepare
the siRNA transfection diluent. Opti-MEM (200 µl) was diluted with
Lipofectamine® 3000 (5 µl) (Invitrogen; Thermo Fisher Scientific,
Inc.) and mixed and incubated for 5 min at room temperature. The
diluted Lipofectamine® with 100 pmol siRNA was gently mixed and
incubated for 20 min. The mixed solution of the complex was added
to the cell culture plate, and the cells were incubated for 48
h.
Flow cytometry
The cells were incubated in blocking buffer (0.5%
BSA in 1× PBS) for 30 min at room temperature and stained with
FITC-conjugated anti-inducible nitric oxide synthase (iNOS; 1:20,
BD Biosciences, cat. no. 610330) and PE-conjugated anti-arginase 1
(Arg1; 1:20, R&D Systems, cat. no. IC5868P) at 4°C in the dark
for 30 min. The cells were analyzed using a BD FACSAria™
II flow cytometer (BD Biosciences) and the BD FACSDiva™
software version 8.0 (BD Biosciences).
Immunohistochemical staining
Immunohistochemistry was performed as previously
described (26). Briefly, the
synovial tissues were cut into 7 µm thick sections. After blocking
in 5% goat serum in PBS for 1 h at room temperature, the tissue
sections were incubated with primary antibodies against anti-MAGL
(1:100, Abcam, cat. no. ab246902), anti-iNOS (1:200, ProteinTech
Group, Inc., cat. no. 22226-1), anti-Arg1 (1:200, ProteinTech
Group, Inc., cat. no. 16001-1), anti-CD80 (1:300, ProteinTech
Group, Inc., cat. no. 14292-1), or anti-CD206 (1:300, ProteinTech
Group, Inc., cat. no. 18704-1) overnight at 4°C followed by the
secondary antibody (1:1,000, Abcam, cat. no. ab6721) for 2 h at
room temperature. An Olympus CH30 (Olympus Corporation) microscope
was used to capture images (×200 or ×400).
Hematoxylin and eosin (H&E)
staining
H&E staining was performed and evaluated as
previously described (27). The
synovial tissues of the OA patients or OA mice were fixed with 4%
paraformaldehyde for 24 h at 4°C and decalcified in 10% EDTA for 2
weeks. The synovial tissues were embedded in paraffin before
cutting into 7 µm thick sections and then stained with H&E.
Evaluations were performed using an Olympus CH30 microscope (×200
or ×400). The inflammation score was evaluated by scoring the
severity of the infiltration of inflammatory cells as follows: 0,
no; 1, mild; 2, moderate; 3, severe.
Western blotting
Western blotting was performed as previously
described (28). The protein
concentrations of the synovial tissues or cells were determined
using a BCA Protein Assay kit (Thermo Fisher Scientific, Inc.).
Proteins (30 µg) were separated by SDS-PAGE on a 10% gel and
transferred to PVDF membranes (MilliporeSigma). The PVDF membranes
were blocked with 5% non-fat dry milk for 2 h, followed by
incubation with one of the following primary antibodies at 4°C
overnight: Anti-MAGL (1:1,000, Abcam, cat. no. ab246902), anti-iNOS
(1:1,000, ProteinTech Group, Inc., cat. no. 22226-1), anti-Arg1
(1:5,000, ProteinTech Group, Inc., cat. no. 16001-1), anti-TNF-α
(1:1,000, ABclonal, cat. no. A20851), anti-IL-1β (1:1,000,
ABclonal, cat. no. A16288), anti-IL-6 (1:1,000, ABclonal, cat. no.
A11115), anti-PTEN-induced kinase 1 (PINK1) (1:1,000, ProteinTech
Group, Inc., cat. no. 23274-1), anti-Parkin (1:1,000, ProteinTech
Group, Inc., cat. no. 14060-1), or anti-β-actin (1:5,000,
ProteinTech Group, Inc., cat. no. 81115-1). The following day, the
membranes were washed and incubated with the secondary antibody
(1:5,000, Abcam, cat. no. ab205718) for 2 h at room temperature.
The proteins were detected using Pierce ECL Western Blotting
Substrate (Thermo Fisher).
Immunofluorescence staining
The synovial tissues were permeabilized in 0.1%
Triton X-100 for 20 min and then blocked with 5% bovine serum
albumin for 1 h at room temperature. Then the synovial tissues were
incubated with anti-MAGL (1:200, Abcam, cat. no. ab246902) and
anti-iNOS (1:200; Invitrogen; Thermo Fisher Scientific, Inc.; cat.
no. MA5-17139) antibodies overnight at 4°C, followed by incubation
with the secondary antibody (1:1,000, Abcam, cat. nos. ab150084 and
ab150113) for 2 h at room temperature in the dark. An Olympus
Fluoview FV3000 (Olympus Corporation) confocal microscope was used
to capture images.
Transmission electron microscopy
The synovial tissues were cut into 1 mm3
segments, preserved in 3% glutaraldehyde for 2 h at 4°C, treated
with 1% osmic acid for 2 h at 4°C, and dried with various acetone
concentrations before encasing in resin. The samples were sliced
into extremely thin sections (90 nm) using an ultramicrotome. A
Hitachi H-7560 (Hitachi) transmission electron microscope was used
to capture images.
Mechanical threshold test
The mechanical pain threshold of mice was measured
using an electronic von Frey (Ugo Basile S.R.L, cat. no. 38450)
(29). Briefly, the mice were
individually placed in a cage with a grid floor for 1 h to adapt to
the new environment. An increasing force (g) was applied on the
plantar surface of the hind using rigid 0.5 mm diameter
polypropylene tips. The mechanical threshold was based on the
pressure at which paw withdrawal occurred. This test was done
before, and on days 3, 7, 10, 14, 17, 21, and 28 after the
injection of MIA. The mechanical threshold was tested thrice, and
the mean value was used each time.
Thermal threshold test
The thermal pain threshold of mice was measured
using a hot plate (Ugo Basile S.R.L., cat. no. 35250) as described
previously (30). The mice were
placed on a metal surface of the hot plate equipment maintained at
55±0.1°C. The hot plate was surrounded by a transparent plastic
barrier. The latency to jumping off the plate or licking a hind paw
was recorded; 30 seconds was used as a cut-off time to protect the
paws against injury.
Statistical analysis
All experiments were performed at least three times.
Data are presented as the mean ± SD. Statistical analysis was
performed using GraphPad Prism version 8.0.1 (GraphPad Software,
Inc.). Normality and homogeneity were evaluated using Shapiro-Wilk
and Levene tests, respectively. Differences between groups were
compared using an unpaired Student's t-test (two groups), a
Mann-Whitney U test (two groups), a Kruskal-Wallis followed by
Dunn's test, or a one-way or two-way ANOVA followed by a Tukey's
post hoc test for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
MAGL accumulates in patients with knee
OA with the polarization of macrophages towards an M1
phenotype
To investigate the potential role of MAGL in OA, we
first collected synovial tissues from patients who underwent total
knee replacement due to knee OA (OA group, n=5) and from patients
who underwent arthroscopic knee surgery due to anterior cruciate
ligament injury (Control group, n=5) to perform H&E staining.
As shown in Fig. 1A and B, the
synovial tissues of the OA group were thickened, and there was a
greater degree of infiltration of inflammatory cells compared with
the Control group. Additionally, the synovial tissue score in the
OA group was significantly increased compared with that of the
Control group (P<0.01). Immunohistochemical staining and western
blotting were performed to identify the MAGL levels in the synovial
tissue. As shown in Fig. 1C and D,
compared with the Control group, the average optical density of
MAGL in the OA group was significantly increased (P<0.01). The
protein expression levels of MAGL in the synovial tissues of the
Control group were low, while that of the OA group was
significantly higher (P<0.01) (Fig.
1E and F).
 | Figure 1.MAGL accumulates in OA patients and
polarizes macrophages towards an M1 phenotype. (A) Representative
images of the synovial tissues stained with H&E (upper panel,
×200, scale bar 50 µm; lower panel, ×400, scale bar 20 µm). (B)
Inflammation scores of the synovial tissues. (C and D)
Immunohistochemical staining for MAGL in synovial tissues (upper
panel, ×200, scale bar 50 µm; lower panel, ×400, scale bar 20 µm).
(E) Western blots of MAGL expression in synovial tissues. (F)
Western blotting analysis of MAGL in synovial tissues of each
group. (G-J) Immunohistochemical staining of iNOS, CD80, Arg1, and
CD206 in synovial tissues (left ×200, scale bar 50 µm; right ×400,
scale bar 20 µm). (K and L) Immunofluorescence staining of MAGL
(red) with iNOS (green) in the synovial tissues (scale bar 100 µm).
n=5 per group. *P<0.05, **P<0.01 vs. control group. ns, not
significant. MAGL, monoacylglycerol lipase; OA, osteoarthritis. |
To further determine the polarization phenotype of
synovial macrophages from knee OA patients, the expression levels
of the M1 polarization markers, iNOS and CD80, and the M2
polarization markers, Arg1 and CD206, were determined by
immunohistochemical staining. As shown in Fig. 1G and H, compared with the Control
group, the mean optical density of iNOS and CD80 in the OA group
was significantly higher (P<0.01). The expression levels of Arg1
and CD206 were low in the synovial tissues of both the Control
group and the OA group, and there was no significant difference in
the average optical density between the Control group and OA group
(P>0.05) (Fig. 1I and J).
Furthermore, it was found that the colocalization of MAGL with the
M1 macrophage marker iNOS in the synovial tissues of the OA group
increased when compared with that of the Control group (P<0.05)
(Fig. 1K and L). Hence, these
results indicated that MAGL was upregulated in patients with knee
OA, with synovial macrophages exhibiting polarization towards an M1
phenotype.
Pharmacological inhibition of MAGL
promotes polarization of macrophages towards an M2 phenotype and
alleviates pain behaviors in OA mice
To further investigate the role of MAGL in OA,
MJN110, a potent pharmacological inhibitor of MAGL, was
intraperitoneally injected in MIA-induced OA mice once a day for a
week. First, the expression levels of MAGL in the synovial tissue
were detected. As shown in Fig. 2A and
B, compared with the Control group, the average optical density
of MAGL in the MIA-OA group was significantly increased
(P<0.01), and the average optical density of MAGL in the MJN110
group was statistically significantly lower than that in MIA-OA
group (P<0.01). Next, the effect of MAGL inhibition on the
polarization of synovial macrophages in MIA-induced OA mice was
investigated by detecting the expression levels of iNOS and Arg1 in
the synovial tissues. As shown in Fig.
2C and D, the mean optical density of iNOS in the MIA-OA group
was significantly increased compared with the Control and MJN110
groups (P<0.01). There was no significant difference in the
expression levels of Arg1 in the Control group and the MIA-OA group
(P>0.05). Compared with the MIA-OA group, the average optical
density of Arg1 in the MJN110 group was significantly increased
(P<0.01) (Fig. 2E and F).
 | Figure 2.Inhibition of MAGL promotes the
polarization of macrophages towards an M2 phenotype and alleviates
pain behaviors in OA mice. (A-F) Immunohistochemical staining of
MAGL, iNOS, and Arg1 in synovial tissues (upper panel, ×200, scale
bar 50 µm; lower panel, ×400, scale bar 20 µm). (G) Representative
images of the synovial tissues stained with H&E upper panel,
×200, scale bar 50 µm; lower panel, ×400, scale bar 20 µm). (H)
Inflammation scores of the synovial tissues. (I) The mechanical
pain threshold of mice was measured by electronic von Frey. (J) The
thermal pain threshold of mice was measured using a hot plate (n=5
per group). *P<0.05, **P<0.01 vs. MIA-OA group;
#P<0.05 vs. control group. ns, not significant; MAGL,
monoacylglycerol lipase; OA, osteoarthritis; H&E, hematoxylin
and eosin; MIA, monoiodoacetate. |
To further identify the role of MAGL inhibition in
OA pain treatment, H&E staining, and mechanical and thermal
threshold tests were performed. Based on the staining results
(Fig. 2G and H), it was found that
the synovial tissue score of the MJN110 group was significantly
lower when compared with the MIA-OA group (P<0.01). The results
of the mechanical pain and thermal pain tests are shown in Fig. 2I and J. Compared with the Control
group, the thresholds of mechanical and thermal pain in the MIA-OA
group were significantly reduced after 7 days of MIA injection,
which lasted until day 28 (P<0.05). After 7 days of continuous
administration of MJN110, the thresholds of mechanical and thermal
pain were significantly higher than those of the MIA-OA group
(P<0.05). These results suggest that the inhibition of MAGL
promotes the polarization of synovial macrophages towards an M2
phenotype and alleviates pain behaviors in OA mice.
MAGL accumulates in M1-polarized mice
macrophages in vitro
Next, the function of MAGL in vitro was
explored. Mice macrophages were divided into a Control group, the
M1 group (LPS polarization inducing), and the M2 (IL-4 polarization
inducing) group. The representative pictures of the cellular
morphologies of the three groups are shown in Fig. 3A. Initially, flow cytometry
experiments were used to verify the effectiveness of the induction
medium. As shown in Fig. 3B,
compared with the M2 group, the proportion of iNOS-positive cells
in the M1 group was significantly higher. In comparison, the
proportion of Arg1-positive cells in the M1 group was significantly
lower than that in the M2 group (P<0.01). Meanwhile, western
blotting was used to determine the trends of protein levels of iNOS
and Arg1 in macrophages in the three groups (Fig. 3C-E).
Subsequently, western blotting was used to examine
the expression of MAGL in the macrophages in the three groups. As
shown in Fig. 3F-G, compared with
the Control group, the protein expression levels of MAGL in the
macrophages of the M1 group significantly increased. In contrast,
the protein expression levels of MAGL of the M2 group significantly
decreased compared with that of the M1 group (P<0.01).
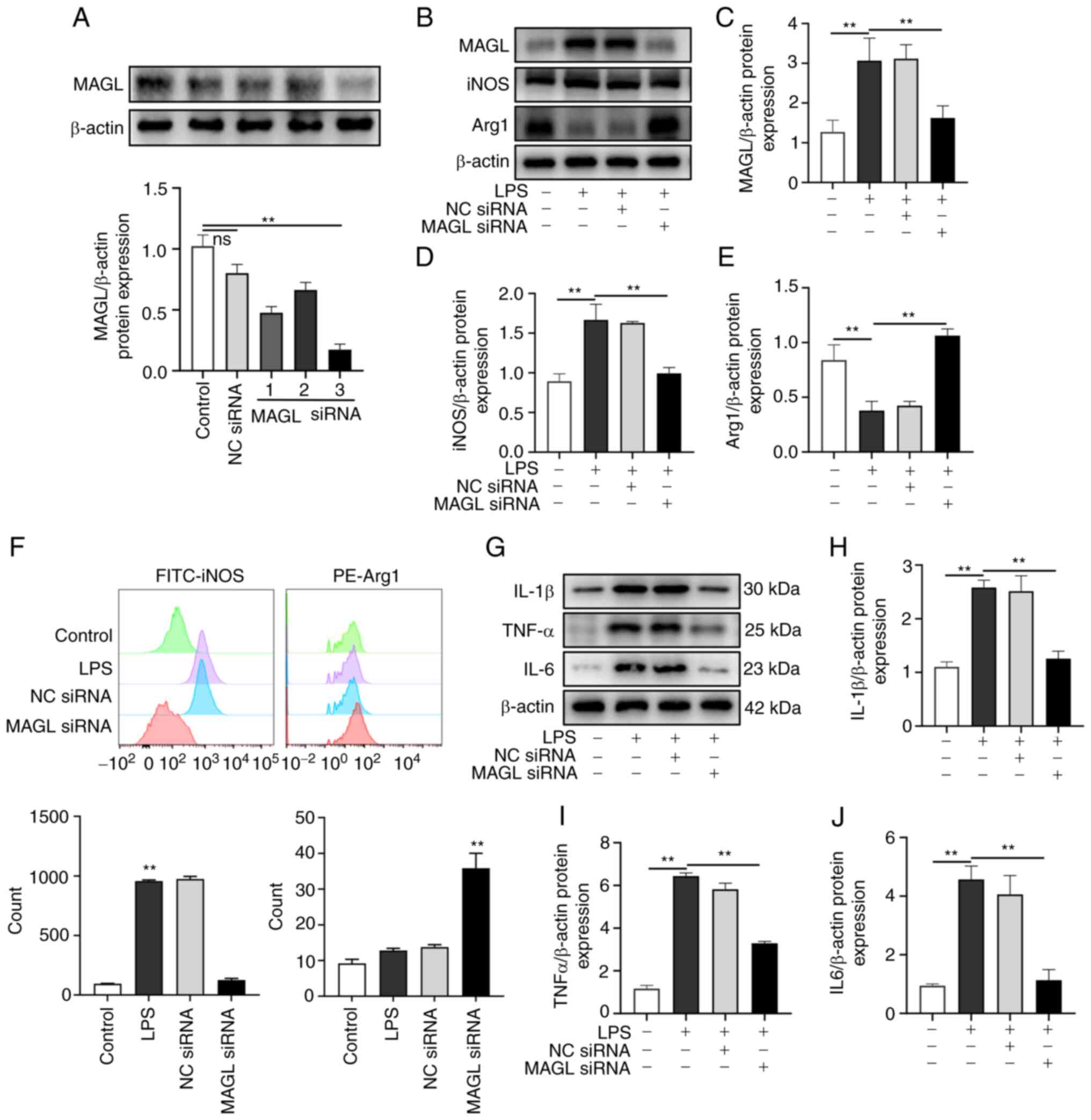
MAGL knockdown suppresses the
polarization of M1 macrophages and promotes polarization towards an
M2 phenotype in vitro
Mice macrophages (LPS polarization inducing) were
transfected with MAGL siRNA or NC siRNA (Fig. 4A). Western blotting and flow
cytometry experiments confirmed the effect on the polarization of
macrophages. As shown in Fig.
4B-E, the protein expression levels of MAGL and iNOS in the LPS
group significantly increased compared with the Control group
(P<0.01), and the protein expression levels of iNOS
significantly decreased following MAGL knockdown when compared with
the LPS group. In contrast, Arg1 expression significantly increased
(P<0.01). As shown in Fig. 4F,
compared with the Control group, the proportion of iNOS-positive
cells in the LPS group was significantly higher. In contrast, the
proportion of Arg1 positive cells was significantly higher than
that in the LPS group after MAGL knockdown (P<0.01). The
expressions of inflammatory factors IL-1β, TNF-α, and IL-6 were
also examined. As shown in Fig.
4G-J, compared with the control group, the expressions of
IL-1β, TNF-α, and IL-6 significantly increased after M1
polarization (P<0.01), while MAGL knockdown significantly
reduced the expression of IL-1β, TNF-α, and IL-6 compared with the
LPS group (P<0.01). Based on the above results, MAGL knockdown
suppressed polarization towards an M1 phenotype and promoted
polarization towards the M2 phenotype.
MAGL knockdown enhances mitophagy of
M1 macrophages in vitro
As shown in the electron microscopy images in
Fig. 5A, mitochondria with
abnormal morphology with mitophagy were observed in the synovial
tissues of patients in the Control and OA groups. Fluorescence
imaging and mitophagy protein markers were evaluated further to
verify the possible role of MAGL on mitophagy. The mitophagosome is
a red fluorescent probe, which is bound to mitochondria in the cell
by chemical bonds, while the lysosome is a green fluorescent probe;
thus, yellow fluorescence is observed when they are colocalized.
The amount of yellow fluorescence represented the levels of
mitophagy. As shown in Fig. 5B,
there was almost no yellow fluorescence in the Control group, only
a little yellow fluorescence in the LPS group, and a considerable
amount of yellow fluorescence in the macrophages in the MAGL siRNA
group. The PINK1 and Parkin expression levels in macrophages were
examined by western blotting. As shown in Fig. 5C-E, compared with the LPS group,
the protein expression levels of PINK1 and Parkin in the MAGL siRNA
group were significantly increased (P<0.01). The above results
indicated that MAGL knockdown by siRNA increased mitophagy levels
in M1 macrophages.
Discussion
Knee OA is a common degenerative disease
characterized by joint pain and cartilage destruction, which is
more common in the elderly. The incidence of OA has increased with
the advent of an aging society but is also becoming increasingly
diagnosed in the younger population as well (31). Chronic persistent knee joint pain
is the primary clinical manifestation of patients with OA of the
knee, and it can seriously affect a patient's quality of life. The
specific mechanism by which OA pain manifests is unclear. However,
it is generally accepted that synovial inflammation caused by the
release of inflammatory factors leads to the destruction of the
synovium and cartilage is an essential factor of OA pain (32). Therefore, regulating a synovial
inflammatory response and reducing the levels of proinflammatory
cytokines form the basis for alleviating OA pain.
MAGL regulates the endocannabinoid system by
hydrolyzing 2-AG and plays a vital role in inflammatory responses,
analgesia, and neuroprotection (11,33,34).
In the present study, the role and mechanism of MAGL in OA pain
were assessed. It was found that the synovial tissue inflammation
score and the expression levels of MAGL increased significantly in
OA patients and MIA-induced OA mice. MJN110, a potent
pharmacological inhibitor of MAGL, was intraperitoneally injected
for 7 consecutive days in OA mice, and this reduced the synovial
tissue inflammation score significantly, and the thresholds of
mechanical and thermal pain were significantly increased following
MAGL inhibition, indicating that MAGL could affect the regulation
of OA pain.
Synovial inflammation plays a vital role in the
pathological process of OA and is an essential factor in OA pain.
The infiltration of inflammatory cells in synovial tissues further
aggregates a variety of inflammatory and immune cells, especially
macrophages, which are widely involved in the inflammatory cascade
(35,36). Macrophages are highly plastic and
can polarize towards M1 or M2 phenotypes following specific cues
from the environment, and each phenotype exerts contrasting
functions. M1 macrophages secrete proinflammatory cytokines such as
IL-1β, IL-6, and TNFα, which aggravate the inflammatory response
and stimulate nociceptive receptors of peripheral sensory nerves.
In contrast, M2 macrophages secrete IL-10 and other
anti-inflammatory cytokines, which can reduce the inflammatory
response (37,38). However, whether MAGL modulates OA
pain by affecting macrophage polarization in synovial tissue
requires further study. The present study showed that the synovial
macrophages of OA patients and OA mice were primarily polarized
towards an M1 phenotype. The pharmacological inhibition of MAGL by
injecting MJN110 showed that MAGL inhibition promoted synovium
macrophage polarization from an M1 phenotype towards an M2
phenotype in OA mice. Additionally, double immunofluorescence
staining showed that MAGL co-localization with an M1 polarization
marker increased in OA patients. However, the mechanism involved in
regulating macrophage polarization by MAGL requires further
study.
Mitophagy degrades damaged mitochondria in cells and
regulates cell metabolism through autophagy, which is critical for
cell function and mitochondrial network function, and it plays a
vital role in the maintenance of homeostasis and protects nerve
cells by removing damaged mitochondria (39–41).
In a model of neuropathic pain, it was found that the secretion of
proinflammatory cytokines was decreased by enhanced mitophagy
levels, which significantly alleviated the response to pain
(42). PINK1 and Parkin are
important molecules regulating mitophagy and play an essential role
in maintaining mitochondrial function. Typically, the expression
levels of PINK1 are low, and PINK1 is blocked from entering the
inner mitochondrial membrane and thus accumulates on the outer
mitochondrial membrane when mitochondria are damaged, and it
recruits Parkin to the damaged mitochondria at the same time
(43–46). It was found that after treating
cells with Carbonyl Cyanide m-Chlorophenylhydrazine for 3 h to
induce mitophagy, increased protein expression of PINK1 was
detected (47). In the present
study, it was found that mitophagosome and lysosome colocalization
significantly increased under confocal microscopy using a mitophagy
detection kit in the MAGL siRNA group, and the protein expression
levels of PINK1 and Parkin, indicating that mitophagy levels in M1
macrophages increased after MAGL knockdown.
The present study has several limitations. First,
the study did not include knockout mice. The gene knockout mice may
help further elucidate the role of MAGL in regulating mitophagy and
polarization of synovial macrophages. Second, male C57 mice were
used to establish the MIA-OA model in these experiments. The role
of MAGL in OA pain in aged female mice should thus be also
assessed. Third, the detailed molecular mechanism of MAGL
inhibiting mitophagy requires elucidation.
In conclusion, the present study demonstrated that
MAGL accumulated in the synovial tissues of patients and mice with
OA, and inhibition of MAGL promoted the polarization of synovial
macrophages from an M1 towards an M2 phenotype and alleviated pain
in OA mice. Based on these results, it is proposed that MAGL
regulates synovial macrophage polarization by inhibiting mitophagy
in OA.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding from by the Science
and Technology Development Plan of Suzhou Science and Technology
Bureau (grant no. SKJY2021115) and the Science and Technology
Development Fund of Nanjing Medical University (grant no.
NMUB2020257).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW and DG conceived, designed the study and revised
the manuscript. CG and MC performed the experiments, analyzed the
data and drafted the manuscript. XL analyzed the data. CG, MC and
CW confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was performed in accordance with the
Ethical Standards of the Declaration of Helsinki and was approved
by the Ethics Committee of Suzhou Municipal Hospital (approval no.
K-2021-066-K01). All animal experiments were approved by the
Institutional Animal Care and Use Committee of Nanjing Medical
University (approval no. Y20210267).
Patient consent for publication
All patients provided informed consent for
publication of their data.
Competing interests
The authors declare no competing interests.
Glossary
Abbreviations
Abbreviations:
|
OA
|
osteoarthritis
|
|
MAGL
|
monoacylglycerol lipase
|
|
2-AG
|
2-arachidonoylglycerol
|
|
MIA
|
monoiodoacetate
|
|
iNOS
|
inducible nitric oxide synthase
|
|
Arg1
|
arginase 1
|
|
H&E
|
hematoxylin and eosin
|
|
PINK1
|
PTEN-induced kinase 1
|
References
|
1
|
Hodgkinson T, Kelly DC, Curtin CM and
O'Brien FJ: Mechanosignalling in cartilage: An emerging target for
the treatment of osteoarthritis. Nat Rev Rheumatol. 18:67–84. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quicke JG, Conaghan PG, Corp N and Peat G:
Osteoarthritis year in review 2021: Epidemiology & therapy.
Osteoarthritis Cartilage. 30:196–206. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klein JC, Keith A, Rice SJ, Shepherd C,
Agarwal V, Loughlin J and Shendure J: Functional testing of
thousands of osteoarthritis-associated variants for regulatory
activity. Nat Commun. 10:24342019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Neill TW and Felson DT: Mechanisms of
osteoarthritis (OA) pain. Curr Osteoporos Rep. 16:611–616. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y, Ying L, Chen W, Cui ZX, Zhu Q, Liu
X, Zheng H, Liang D and Zhu Y: Accelerating the 3D T1ρ
mapping of cartilage using a signal-compensated robust tensor
principal component analysis model. Quant Imaging Med Surg.
11:3376–3391. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou J, Deng S, Fang H, Du X, Peng H and
Hu Q: Circular RNA circANKRD36 regulates Casz1 by targeting miR-599
to prevent osteoarthritis chondrocyte apoptosis and inflammation. J
Cell Mol Med. 25:120–131. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deligiannidou GE, Papadopoulos RE,
Kontogiorgis C, Detsi A, Bezirtzoglou E and Constantinides T:
Unraveling natural products' role in osteoarthritis management-an
overview. Antioxidants (Basel). 9:3482020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kraus VB, McDaniel G, Huebner JL, Stabler
TV, Pieper CF, Shipes SW, Petry NA, Low PS, Shen J, McNearney TA
and Mitchell P: Direct in vivo evidence of activated macrophages in
human osteoarthritis. Osteoarthritis Cartilage. 24:1613–1621. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou F, Mei J, Yang S, Han X, Li H, Yu Z,
Qiao H and Tang T: Modified ZIF-8 nanoparticles attenuate
osteoarthritis by reprogramming the metabolic pathway of synovial
macrophages. ACS Appl Mater Interfaces. 12:2009–2022. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Omran Z: New disulfiram derivatives as
MAGL-selective inhibitors. Molecules. 26:32962021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gil-Ordóñez A, Martín-Fontecha M,
Ortega-Gutiérrez S and López-Rodríguez ML: Monoacylglycerol lipase
(MAGL) as a promising therapeutic target. Biochem Pharmacol.
157:18–32. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jha V, Biagi M, Spinelli V, Di Stefano M,
Macchia M, Minutolo F, Granchi C, Poli G and Tuccinardi T:
Discovery of monoacylglycerol lipase (MAGL) inhibitors based on a
pharmacophore-guided virtual screening study. Molecules. 26:782020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun J, Zhou YQ, Chen SP, Wang XM, Xu BY,
Li DY, Tian YK and Ye DW: The endocannabinoid system: Novel targets
for treating cancer induced bone pain. Biomed Pharmacother.
120:1095042019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wiskerke J, Irimia C, Cravatt BF, De Vries
TJ, Schoffelmeer ANM, Pattij T and Parsons LH: Characterization of
the effects of reuptake and hydrolysis inhibition on interstitial
endocannabinoid levels in the brain: An in vivo microdialysis
study. ACS Chem Neurosci. 3:407–417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Philpott HT and McDougall JJ: Combatting
joint pain and inflammation by dual inhibition of monoacylglycerol
lipase and cyclooxygenase-2 in a rat model of osteoarthritis.
Arthritis Res Ther. 22:92020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Q, Lei JH, Bao J, Wang H, Hao W, Li
L, Peng C, Masuda T, Miao K, Xu J, et al: BRCA1 Deficiency: BRCA1
deficiency impairs mitophagy and promotes inflammasome activation
and mammary tumor metastasis (Adv. Sci. 6/2020). Adv Sci (Weinh).
7:20700332020. View Article : Google Scholar
|
|
17
|
Duan R, Xie H and Liu ZZ: The role of
autophagy in osteoarthritis. Front Cell Dev Biol. 8:6083882020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shao S, Xu CB, Chen CJ, Shi GN, Guo QL,
Zhou Y, Wei YZ, Wu L, Shi JG and Zhang TT: Divanillyl sulfone
suppresses NLRP3 inflammasome activation via inducing mitophagy to
ameliorate chronic neuropathic pain in mice. J Neuroinflammation.
18:1422021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yi MH, Shin J, Shin N, Yin Y, Lee SY, Kim
CS, Kim SR, Zhang E and Kim DW: PINK1 mediates spinal cord
mitophagy in neuropathic pain. J Pain Res. 12:1685–1699. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin J, Zhuge J, Zheng X, Wu Y, Zhang Z, Xu
T, Meftah Z, Xu H, Wu Y, Tian N, et al: Urolithin A-induced
mitophagy suppresses apoptosis and attenuates intervertebral disc
degeneration via the AMPK signaling pathway. Free Radic Biol Med.
150:109–119. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patoli D, Mignotte F, Deckert V, Dusuel A,
Dumont A, Rieu A, Jalil A, Van Dongen K, Bourgeois T, Gautier T, et
al: Inhibition of mitophagy drives macrophage activation and
antibacterial defense during sepsis. J Clin Invest. 130:5858–5874.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pitcher T, Sousa-Valente J and Malcangio
M: The monoiodoacetate model of osteoarthritis pain in the mouse. J
Vis Exp. 537462016.PubMed/NCBI
|
|
23
|
Zheng XF, Hong YX, Feng GJ, Zhang GF,
Rogers H, Lewis MA, Williams DW, Xia ZF, Song B and Wei XQ:
Lipopolysaccharide-induced M2 to M1 macrophage transformation for
IL-12p70 production is blocked by Candida albicans mediated
up-regulation of EBI3 expression. PLoS One. 8:e639672013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu L, Guo H, Song A, Huang J, Zhang Y,
Jin S, Li S, Zhang L, Yang C and Yang P: Progranulin inhibits
LPS-induced macrophage M1 polarization via NF-кB and MAPK pathways.
BMC Immunol. 21:322020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuda M, Aizawa S, Tsuboi I, Hirabayashi Y,
Harada T, Hino H and Hirai S: Imbalanced M1 and M2 macrophage
polarization in bone marrow provokes impairment of the
hematopoietic microenvironment in a mouse model of hemophagocytic
lymphohistiocytosis. Biol Pharm Bull. 45:1602–1608. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He X, Xiao J, Li Z, Ye M, Lin J, Liu Z,
Liang Y, Dai H, Jing R and Lin F: Inhibition of PD-1 alters the
SHP1/2-PI3K/Akt axis to decrease M1 polarization of alveolar
macrophages in lung ischemia-reperfusion injury. Inflammation.
46:639–654. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mussawy H, Zustin J, Luebke AM, Strahl A,
Krenn V, Rüther W and Rolvien T: The histopathological synovitis
score is influenced by biopsy location in patients with knee
osteoarthritis. Arch Orthop Trauma Surg. 142:2991–2997. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen M, Zhang Y, Wang H, Yang H, Yin W, Xu
S, Jiang T, Wang M, Wu F and Yu W: Inhibition of the norepinephrine
transporter rescues vascular hyporeactivity to catecholamine in
obstructive jaundice. Eur J Pharmacol. 900:1740552021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nunes MA, Toricelli M, Schöwe NM, Malerba
HN, Dong-Creste KE, Farah DMAT, De Angelis K, Irigoyen MC, Gobeil
F, Araujo Viel T and Buck HS: Kinin B2 receptor activation prevents
the evolution of Alzheimer's disease pathological characteristics
in a transgenic mouse model. Pharmaceuticals (Basel). 13:2882020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kikuchi M, Takase K, Hayakawa M, Hayakawa
H, Tominaga S and Ohmori T: Altered behavior in mice overexpressing
soluble ST2. Mol Brain. 13:742020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen H, Wu J, Wang Z, Wu Y, Wu T, Wu Y,
Wang M, Wang S, Wang X, Wang J, et al: Trends and patterns of knee
osteoarthritis in China: A longitudinal study of 17.7 million
adults from 2008 to 2017. Int J Environ Res Public Health.
18:88642021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Conaghan PG, Cook AD, Hamilton JA and Tak
PP: Therapeutic options for targeting inflammatory osteoarthritis
pain. Nat Rev Rheumatol. 15:355–363. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kasatkina LA, Rittchen S and Sturm EM:
Neuroprotective and immunomodulatory action of the endocannabinoid
system under neuroinflammation. Int J Mol Sci. 22:54312021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baggelaar MP, Maccarrone M and van der
Stelt M: 2-Arachidonoylglycerol: A signaling lipid with manifold
actions in the brain. Prog Lipid Res. 71:1–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thomson A and Hilkens CMU: Synovial
macrophages in osteoarthritis: The key to understanding
pathogenesis? Front Immunol. 12:6787572021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Woodell-May JE and Sommerfeld SD: Role of
inflammation and the immune system in the progression of
osteoarthritis. J Orthop Res. 38:253–257. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Griffin TM and Scanzello CR: Innate
inflammation and synovial macrophages in osteoarthritis
pathophysiology. Clin Exp Rheumatol. 37 (Suppl 120):S57–S63.
2019.
|
|
38
|
Chandrasekaran P, Izadjoo S, Stimely J,
Palaniyandi S, Zhu X, Tafuri W and Mosser DM: Regulatory
macrophages inhibit alternative macrophage activation and attenuate
pathology associated with fibrosis. J Immunol. 203:2130–2140. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoo SM and Jung YK: A molecular approach
to mitophagy and mitochondrial dynamics. Mol Cells. 41:18–26.
2018.PubMed/NCBI
|
|
40
|
Lou G, Palikaras K, Lautrup S,
Scheibye-Knudsen M, Tavernarakis N and Fang EF: Mitophagy and
neuroprotection. Trends Mol Med. 26:8–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pickles S, Vigié P and Youle RJ: Mitophagy
and quality control mechanisms in mitochondrial maintenance. Curr
Biol. 28:R170–R185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang S, Deng Z, Ma Y, Jin J, Qi F, Li S,
Liu C, Lyu FJ and Zheng Q: The role of autophagy and mitophagy in
bone metabolic disorders. Int J Biol Sci. 16:2675–2691. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu Y, Tang Y, Lu J and Zhang W, Zhu Y,
Zhang S, Ma G, Jiang P and Zhang W: PINK1-mediated mitophagy
protects against hepatic ischemia/reperfusion injury by restraining
NLRP3 inflammasome activation. Free Radic Biol Med. 160:871–886.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yi S, Zheng B, Zhu Y, Cai Y, Sun H and
Zhou J: Melatonin ameliorates excessive PINK1/Parkin-mediated
mitophagy by enhancing SIRT1 expression in granulosa cells of PCOS.
Am J Physiol Endocrinol Metab. 319:E91–E101. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li R and Chen J: Salidroside protects
dopaminergic neurons by enhancing PINK1/Parkin-mediated mitophagy.
Oxid Med Cell Longev. 2019:93410182019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Miller S and Muqit MMK: Therapeutic
approaches to enhance PINK1/Parkin mediated mitophagy for the
treatment of Parkinson's disease. Neurosci Lett. 705:7–13. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Matsuda N, Sato S, Shiba K, Okatsu K,
Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, et al:
PINK1 stabilized by mitochondrial depolarization recruits Parkin to
damaged mitochondria and activates latent Parkin for mitophagy. J
Cell Biol. 189:211–221. 2010. View Article : Google Scholar : PubMed/NCBI
|