Introduction
According to various cancer statistics over the past
decade, gastric cancer remains the fourth most common human
epithelial malignancy and is the second leading cause of
cancer-related mortality worldwide, with particularly high
incidences and mortality rates in eastern Asia (1,2).
There are 400,000 new cases of gastric cancer and 300,000
mortalities annually in China (3).
Metastasis is the biggest obstacle to the treatment and
satisfactory prognosis of gastric cancer. Therefore, the
exploration of new therapeutic targets to prevent the metastasis of
gastric cancer is urgently required.
An increasing amount of evidence has suggested that
microRNAs (miRNAs), which mediate the post-transcriptional
regulation of gene expression, control tumorigenesis and cancer
metastasis (4,5). The overexpression of oncogenic miRNAs
or the underexpression of tumor suppressor miRNAs play critical
roles in cancer metastasis.
The dysregulation and dysfunction of miR-146a has
been reported to be involved in the development and progression of
various types of cancer (4,6–13).
We previously found that the expression of miR-146a is
downregulated in gastric cancer, and that the downregulation of
miR-146a is associated with tumor size, cell differentiation and
poor prognosis (14). In addition,
miR-146a has been identified as a metastasis-suppressor miRNA in
breast cancer and pancreatic cancer (9–11).
Recent studies have shown that lower levels of miR-146a are
associated with lymph node metastasis and venous invasion in
gastric cancer (15,16). However, the mechanisms underlying
this process remain poorly understood.
In the current study, the role of miR-146a in
gastric cancer metastasis was investigated. The results from a
wound-healing assay and a Transwell assay in vitro and a
metastasis formation assay in vivo demonstrated that
miR-146a suppresses gastric cancer cell invasion and metastasis.
Furthermore, a luciferase reporter assay and western blot analysis
were used to confirm that miR-146a functions as a metastatic
suppressor in gastric cancer by targeting the L1 cell adhesion
molecule (L1CAM) directly.
Materials and methods
Cell lines and culture conditions
The human gastric cancer cell line, MKN-45, was
obtained from the Shanghai Institute of Cell Biology (Shanghai,
China). The cells were propagated in RPMI-1640 medium (Invitrogen,
Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (FBS).
The cells were cultured at 37°C in a water-saturated atmosphere
with 5% CO2.
Transient overexpression of miR-146a
miR-146a mimics and negative control mimics were
obtained from Shanghai GenePharma Co. Ltd. (Shanghai, China)
(14). miR-146a mimics and
negative control mimics were transfected into MKN-45 cells with
siPORT™ NeoFX™ Transfection Agent (Ambion, Austin, TX, USA)
according the manufacturer’s instructions, at a final concentration
of 50 nM.
Scratch wound healing assay
A scratch wound healing assay was performed as
previously described (17).
Briefly, transfected cells in 6-well plates were cultured until
cells reached confluency, and were then starved overnight. Cell
layers were wounded using a 200 μl pipette tip and cultured for
another 48 h. Photographs were taken at the 0-, 24- and 48-h
time-points.
Cell migration and invasion assay
A Transwell cell migration and Matrigel invasion
assay were carried out to investigate the impact of miR-146a on the
migratory and invasive ability of MKN-45 cells. For migration
detection, transfected cells were placed in a Transwell chamber at
2×104 cells/well. The lower Transwell chamber contained
10% FBS for use as a chemoattractant. For the invasion assay, the
bottom of the culture inserts (8-mm pores) were coated with 30 μl
of the mixture containing serum-free RPMI-1640 and Matrigel (1:8;
BD Biosciences, Bedford, MA, USA). The Matrigel was allowed to
solidify at 37°C overnight. After solidification, cells
(2×104 cells/well) were re-seeded onto the upper
chamber. After 24 h, the cells that had migrated or invaded through
the membrane were fixed with 95% alcohol and stained with crystal
violet. The number of migrated cells or invaded cells was
quantified by counting 5 independent symmetrical visual fields
under the microscope.
Construction of miR-146a expression
vectors and cell transfection
The pcDNA6.2-GW/EmGFP-miR plasmid vector
(Invitrogen) was used to construct a miR-146a overexpressing
plasmid. A DNA fragment with mature miR-146a or a negative control
mismatched sequence was chemically synthesized and inserted into
this vector. The nucleotide sequences of the inserts were verified
to be in the correct sequence and orientation for expression in the
new construct by DNA sequencing. The stable transfection of the
appropriate miRNA plasmids was carried out using Lipofectamine 2000
(Invitrogen) according to the manufacturer’s instructions.
Successfully transfected cell clones were obtained in a selection
medium containing 3.5 μg/ml blasticidin to generate 2 stable
monoclonal cell lines (the miR-146a stable cell line,
MKN-45-miR-146a, and the control stable cell line,
MKN-45-miR-negative control). Real-time PCR was performed as
previously described to detect the expression levels of miR-146a
after stable transfection (14).
In vivo metastasis assays
For in vivo metastasis assays,
MKN-45-miR-146a cells or MKN-45-miR-negative control
(MKN-45-miR-NC) cells were transplanted into nude mice (5-week-old
BALB/c-nu/nu, 10 per group, 1×106 cells for each mouse)
through the lateral tail vein. Mice were sacrificed after 10 weeks.
All animal experiments were performed in full compliance with
previously established guidelines approved by the Animal Care
Committee at Drum Tower Hospital. The lungs were dissected and
subjected to hematoxylin & eosin (H&E) staining. The number
of metastases in the lungs was examined histologically.
Luciferase reporter assay
For the luciferase reporter experiment, a 3′-UTR
segment containing the miR-146a-binding site of L1CAM was
chemically synthesized and inserted into the pGL3-control vector
(Promega, Madison, WI, USA) at the XbaI site immediately
downstream from the luciferase stop codon. DNA segments with
scrambled target sites (L1CAM) designed to interfere with seed
sequence recognition were also cloned to serve as a control for
specificity. In total, 800 ng of the firefly luciferase reporter
vector and 80 ng of the control vector containing Renilla
luciferase, pRL-TK (Promega), were transfected in combination with
50 pM miR-146a or negative control using Lipofectamine 2000 in
24-well plates according to the manufacturer’s instructions.
Luciferase activities were measured 24 h after transfection using
the Dual Luciferase Reporter assay system (Promega) on a Centro LB
960 (Berthold, Bad Wildbad, Germany). Three independent experiments
were performed in triplicate.
Western blot analysis
For protein extraction, confluent cells were washed
with cold PBS twice, denatured in lysis buffer (20 mM Tris-HCl, 200
mM NaCl, 0.2% Nonidet P-40, 0.5% Triton X-100 and protease
inhibitors) and boiled at 100°C for 10 min. Equal amounts of
protein extracts were separated on 10% polyacrylamide gels by using
standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE), then transferred onto PVDF membranes (Bio-Rad,
Hercules, CA, USA). Following blocking in Tris-buffered saline
(TBS) with 0.1% Triton X-100 and 5% milk, the membranes were
incubated with mouse anti-human L1CAM monoclonal antibody (Santa
Cruz, CA, USA) and anti-β-actin (Sigma-Aldrich, St. Louis, MO, USA)
at 4°C overnight. Following washing, the membranes were incubated
with secondary goat anti-mouse antibody conjugated to horseradish
peroxidase (Sigma-Aldrich) at room temperature for 1 h. Signal
detection was carried out by an enhanced chemiluminescence (ECL)
system (Amersham Pharmacia Biotech).
Statistical analysis
Each experiment was repeated at least 3 times. All
results are expressed as the means ± standard deviation (SD). The
difference between the means was analyzed using the Student’s
t-test or the χ2 test. All statistical analyses were
performed using SPSS 16.0 software (Chicago, IL, USA). Differences
were considered significant when p<0.05.
Results
miR-146a suppresses gastric cancer
migration and invasion in vitro
A wound-healing assay was performed to examine the
effect of overexpression of miR-146a on cell migration. It was
found that MKN-45 cells transfected with miR-146a mimics closed the
scratch wound more slowly than cells transfected with negative
controls (Fig. 1A). We also
estimated the effects of miR-146a on the migration and invasion of
MKN-45 cells using a Transwell cell migration and Matrigel invasion
assay. The data demonstrated that the overexpression of miR-146a
markedly inhibited the migration and invasion of MKN-45 cells. The
number of MKN-45 cells transfected with miR-146a mimics (292±29,
P<0.01) that had migrated through the membrane without Matrigel
was significantly lower than that of MKN-45 cells transfected with
the negative controls (72±15) (Fig.
1B). A similar result was found with the invaded cells; the
number of MKN-45 cells transfected with miR-146a mimics (249±16,
P<0.01) passing through the Matrigel was significantly lower
than that of MKN-45 cells transfected with the negative controls
(77±10) (Fig. 1C).
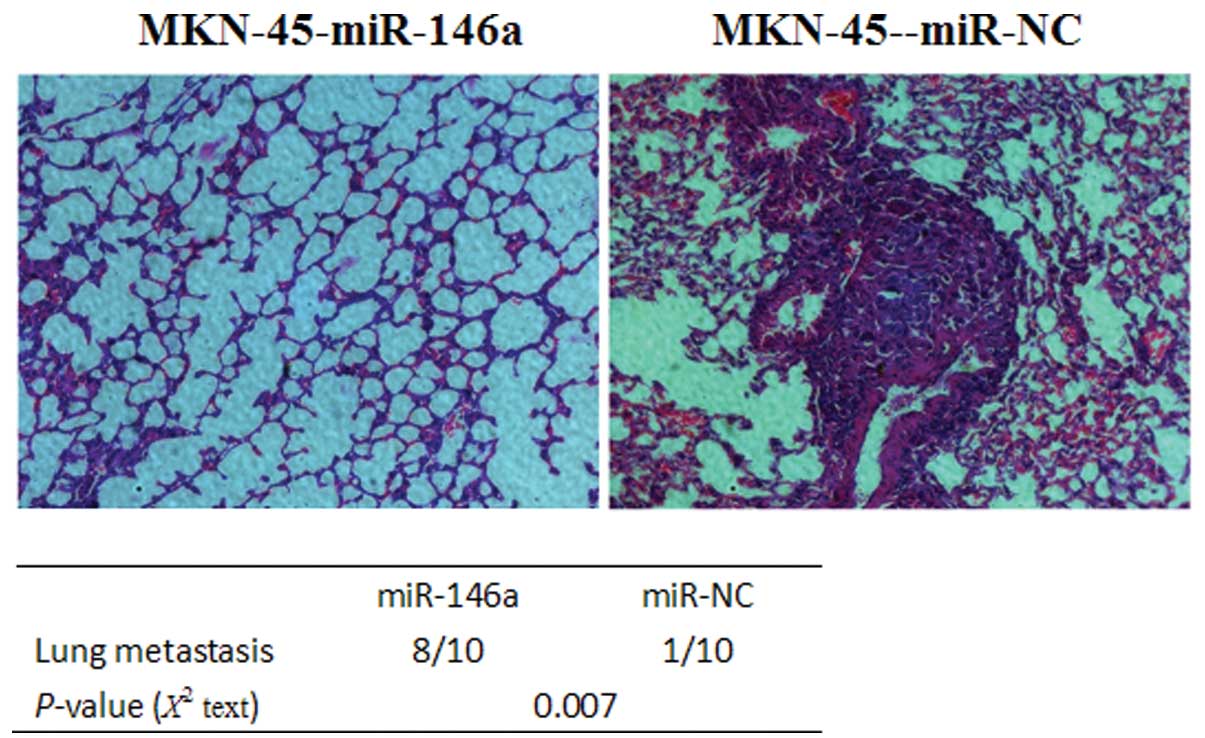
miR-146a suppresses gastric cancer cell
metastasis in vivo
To further explore the effects of miR-146a on tumor
metastasis in vivo, plasmids stably expressing miR-146a or
the negative control were constructed (Fig. 2A). miR-146a or negative control
plasmids were then stably transfected into human gastric cancer
MKN-45 cells. As shown in Fig. 2B,
miR-146a levels were higher in the cells stably expressing miR-146a
than in the cells transfected with the negative control.
MKN-45-miR-146a cells or MKN-45-miR-NC cells were transplanted into
nude mice through the lateral tail vein. Histological analysis of
the lungs of mice confirmed that miR-146a suppressed lung
metastasis formation. Lung metastasis of gastric cancer was
apparent in mice injected with MKN-45-miR-NC cells (Fig. 3). By contrast, few metastatic
tumors were detected in the mice injected with MKN-45-miR-146a
(Fig. 3). Our results indicate
that miR-146a is a negative regulator for gastric cancer
metastasis.
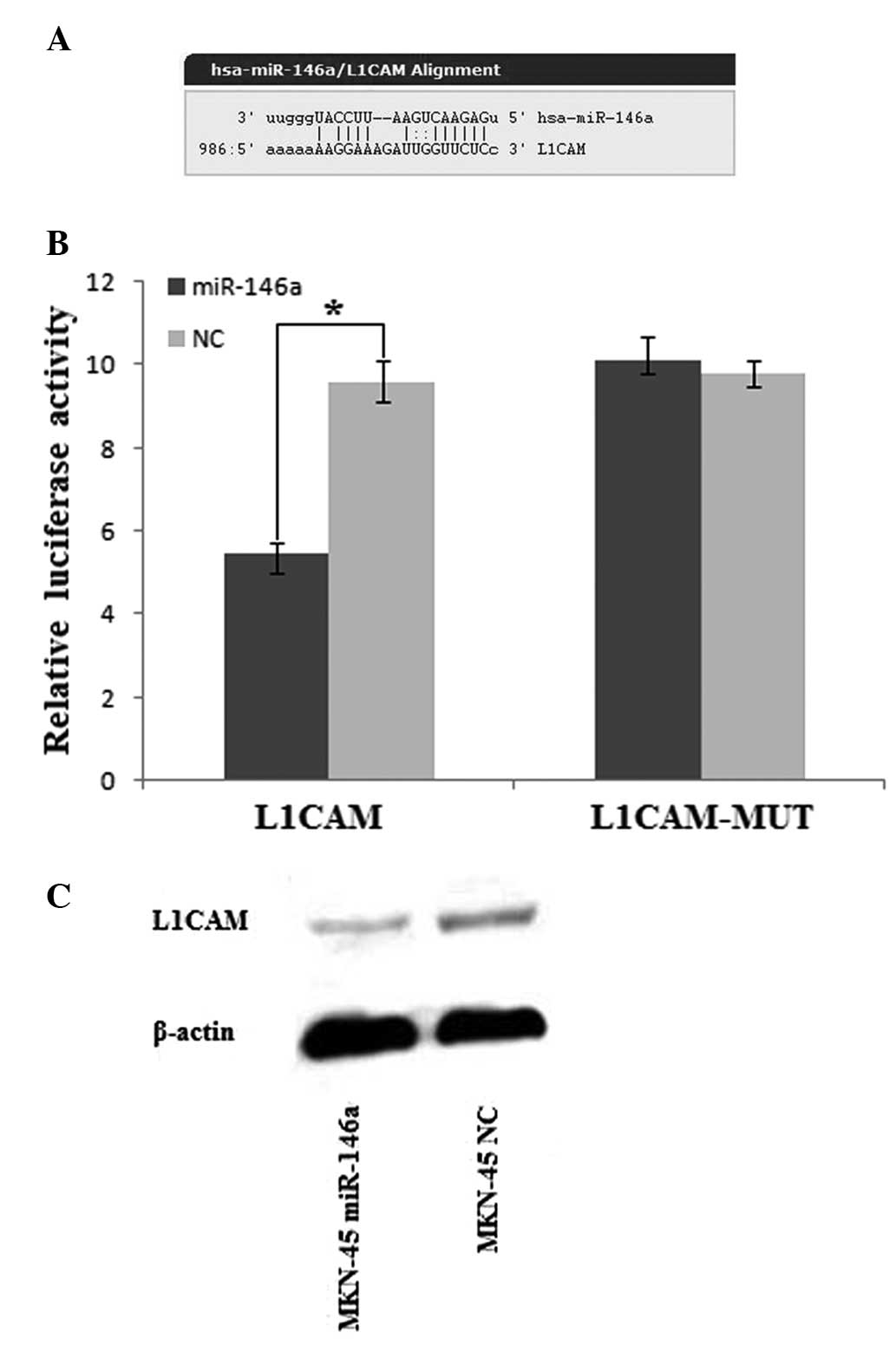
miR-146a post-transcriptionally reduces
L1CAM expression by directly targeting its 3′UTR
For miRNA target prediction, we used microrna.org
online software (http://www.microrna.org/microrna/getMirnaForm.do).
Among a total of 6,798 genes that were potentially targeted by
miR-146a, it was found that L1CAM may contribute to the metastasis
of gastric cancer (Fig. 4A). The
overexpression of L1CAM has been correlated with tumor progression
and metastasis of a number of types of cancer, including gastric
cancer (18,19). To confirm that L1CAM is a direct
target of miR-146a, we constructed the luciferase reporter,
pGL3-L1CAM-3′UTR. A scrambled target site (pGL3-L1CAM-MUT) was also
constructed as the control for sequence specificity. All the
reporters were transfected in MKN-45 cells. In MKN-45 cells, a
significant decrease in relative luciferase activity was noted when
pGL3-L1CAM-3′UTR was co-transfected with the mature miR-146a mimic
but not with the negative control (Fig. 4B). On the contrary, there was no
significant difference of the relative luciferase activity of the
pGL3-L1CAM-MUT reporter between MKN-45 cells transfected with
miR-146a mimics and MKN-45 cells transfected with negative controls
(Fig. 4B). These results suggest
that miR-146a downregulates L1CAM expression by directly targeting
its 3′UTR. In support of these results, we next examined the L1CAM
protein level in MKN-45 cells transfected with miR-146a mimics or
negative controls by western blot analysis. A clear reduction in
the level of the endogenous L1CAM protein in miR-146a-transfected
MKN-45 cells was observed compared to the negative
control-transfected cells normalized to an endogenous reference
β-actin protein (Fig. 4C). These
results demonstrate that miR-146a targets L1CAM in gastric
cancer.
Discussion
Previous reports have shown that miR-146a may
function as a tumor suppressor or oncogene, depending on the tumor
type. The downregulation of miR-146a is a frequent event in various
types of tumors, such as natural killer T-cell lymphoma, glioma,
prostate and gastric cancer (7,10,11,14,15).
On the contrary, miR-146a is upregulated in cervical cancer and
anaplastic thyroid carcinoma and functions as an oncogene (6,13).
The role of miR-146a in gastric cancer is inconsistent. We, and
others, have shown previously that miR-146a is downregulated in
gastric cancer and may function as a tumor suppressor (14–16),
while there is a contradictory result that miR-146a is upregulated
in gastric cancer (20). In this
study, we further show that the overexpression of miR-146a
suppressed gastric cancer MKN-45 cell invasion and metastasis in
vitro and in vivo. L1CAM was identified as a direct
target of miR-146a. The data from the current study suggest that
miR-146a acts as a potential metastasis suppressor in gastric
cancer.
Our findings are consistent with those from recent
reports showing that miR-146a suppresses the metastasis of many
types of cancer including pancreatic, breast and prostate cancer
(4,7,9,12).
More recently, Kogo et al reported that the ectopic
expression of miR-146a inhibits the migration and invasion of
gastric cancer in vitro (15). These results collectively suggest
that miR-146a functions as a metastasis-suppressor miRNA in gastric
cancer.
miR-146a is commonly lost in metastatic prostate
cancer and the downregulation of miR-146a may be the late event in
the progression of prostate cancer (5,7). Lin
et al found that miR-146a was expressed markedly in
non-cancerous prostatic epithelium and gradually disappeared with
cancer progression (7). If the
loss of miR-146a expression is also a late event in the progression
of gastric cancer, based on our results that miR-146a is a
metastasis-suppressor miRNA in gastric cancer, the loss of miR-146a
expression may lead to increased invasiveness and metastatic
ability in gastric cancer. Therefore, the expression of miR-146a
may correlate with the metastatic ability and clinical stage of
gastric cancer. The tissue samples used in the different studies
perhaps account for the contradictory results; certain studies have
shown that miR-146a expression is downregulated, while others have
shown that it is upregulated. Indeed, Kogo et al also
reported that the miR-146a level was associated with the clinical
stage (TNM) (15). We speculate
that miR-146a may exert its bidirectional function in gastric
cancer and that miR-146a expression may be lost gradually in the
late stage of gastric cancer, thus promoting cancer metastasis. A
larger number of tissue samples are still required to confirm this
in future studies. Although the expression of miR-146a is
contradictory in gastric cancer, there is no disagreement as to the
function of miR-146a as a metastasis-suppressor. miR-146a may be a
new target for suppressing tumor metastasis.
Previous studies have found that miR-146a inhibits
tumor metastasis by directly targeting the epidermal growth factor
receptor (EGFR), interleukin 1 receptor-associated kinase 1
(IRAK1), TNF receptor-associated factor 6 (TRAF6), Notch1 and
Rho-associated, coiled-coil-containing protein kinase 1 (ROCK1) in
pancreatic, breast, glioma and prostate cancer (4,9,10,12).
Kogo et al reported that miR-146a inhibits migration and
invasion and downregulates EGFR and IRAK1 expression in
vitro (15). In the current
study, we found a novel direct target of miR-146a, L1CAM. We used a
luciferase reporter assay and western blot analysis to confirm that
L1CAM is a target of miR-146a in gastric cancer cells. L1CAM is
initially identified in the nervous system and is a cell adhesion
molecule which belongs to the immunoglobulin superfamily of cell
adhesion molecules (IgCAM) (21).
It has been observed exclusively in the invasion front of
colorectal cancer (22). L1CAM
expression in gastric cancer is a relatively strong prognostic
factor for patients with pathologically confirmed T3-stage cancer
(19).
In conclusion, the results from the present study
show that miR-146a suppresses gastric cancer cell invasion and
metastasis, which was in part due to the downregulation of L1CAM,
the molecular target that is commonly associated with gastric
cancer metastasis and prognosis. Therefore, miR-146a may have the
therapeutic potential to suppress gastric cancer invasion and
metastasis.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (No. 81101580; 81071815), Jiangsu
Province Key Medical Center Foundation and Scientific and
Technological Innovation Plan Fund of Postgraduate from Jiangsu
Province (No. 5X22013084).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Leung WK, Wu MS, Kakugawa Y, et al:
Screening for gastric cancer in Asia: current evidence and
practice. Lancet Oncol. 9:279–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006.
|
|
4
|
Bhaumik D, Scott GK, Schokrpur S, Patil
CK, Campisi J and Benz CC: Expression of microRNA-146 suppresses
NF-κB activity with reduction of metastatic potential in breast
cancer cells. Oncogene. 27:5643–5647. 2008.PubMed/NCBI
|
|
5
|
Nicoloso MS, Spizzo R, Shimizu M, Rossi S
and Calin GA: MicroRNAs - the micro steering wheel of tumour
metastases. Nat Rev Cancer. 9:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang X, Tang S, Le SY, et al: Aberrant
expression of oncogenic and tumor-suppressive microRNAs in cervical
cancer is required for cancer cell growth. PLoS One. 3:e25572008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin SL, Chiang A, Chang D and Ying SY:
Loss of mir-146a function in hormone-refractory prostate cancer.
RNA. 14:417–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jazdzewski K, Murray EL, Franssila K,
Jarzab B, Schoenberg DR and de la Chapelle A: Common SNP in
pre-miR-146a decreases mature miR expression and predisposes to
papillary thyroid carcinoma. Proc Natl Acad Sci USA. 105:7269–7274.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hurst DR, Edmonds MD, Scott GK, Benz CC,
Vaidya KS and Welch DR: Breast cancer metastasis suppressor 1
up-regulates miR-146, which suppresses breast cancer metastasis.
Cancer Res. 69:1279–1283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mei J, Bachoo R and Zhang CL:
MicroRNA-146a inhibits glioma development by targeting Notch1. Mol
Cell Biol. 31:3584–3592. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paik JH, Jang JY, Jeon YK, et al:
MicroRNA-146a downregulates NFκB activity via targeting TRAF6 and
functions as a tumor suppressor having strong prognostic
implications in NK/T cell lymphoma. Clin Cancer Res. 17:4761–4771.
2011.PubMed/NCBI
|
|
12
|
Li Y, Vandenboom TG 2nd, Wang Z, et al:
miR-146a suppresses invasion of pancreatic cancer cells. Cancer
Res. 70:1486–1495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pacifico F, Crescenzi E, Mellone S, et al:
Nuclear factor-κB contributes to anaplastic thyroid carcinomas
through up-regulation of miR-146a. J Clin Endocrinol Metab.
95:1421–1430. 2010.
|
|
14
|
Hou Z, Xie L, Yu L, Qian X and Liu B:
MicroRNA-146a is down-regulated in gastric cancer and regulates
cell proliferation and apoptosis. Med Oncol. 29:886–892. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kogo R, Mimori K, Tanaka F, Komune S and
Mori M: Clinical significance of miR-146a in gastric cancer cases.
Clin Cancer Res. 17:4277–4284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tchernitsa O, Kasajima A, Schafer R, et
al: Systematic evaluation of the miRNA-ome and its downstream
effects on mRNA expression identifies gastric cancer progression. J
Pathol. 222:310–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: a convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raveh S, Gavert N and Ben-Ze’ev A: L1 cell
adhesion molecule (L1CAM) in invasive tumors. Cancer Lett.
282:137–145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kodera Y, Nakanishi H, Ito S, et al:
Expression of L1 cell adhesion molecule is a significant prognostic
factor in pT3-stage gastric cancer. Anticancer Res. 29:4033–4039.
2009.PubMed/NCBI
|
|
20
|
Xiao B, Zhu ED, Li N, et al: Increased
miR-146a in gastric cancer directly targets SMAD4 and is involved
in modulating cell proliferation and apoptosis. Oncol Rep.
27:559–566. 2012.PubMed/NCBI
|
|
21
|
Rathjen FG and Schachner M:
Immunocytological and biochemical characterization of a new
neuronal cell surface component (L1 antigen) which is involved in
cell adhesion. EMBO J. 3:1–10. 1984.PubMed/NCBI
|
|
22
|
Gavert N, Conacci-Sorrell M, Gast D, et
al: L1, a novel target of beta-catenin signaling, transforms cells
and is expressed at the invasive front of colon cancers. J Cell
Biol. 168:633–642. 2005. View Article : Google Scholar : PubMed/NCBI
|