Introduction
Adrenomedullin (ADM), initially identified in human
pheochromocytoma, is a member of the calcitonin gene-related
peptide family (1). Synthesized as
a biologically inactive precursor protein of 185 amino acid
residues, ADM is converted to a Gly-extended intermediate form and
released as a mature amide structure peptide of 52 amino acid
residues (2). Upon secretion, it
exerts multiple autocrine/paracrine effects by binding to the
G-protein-coupled calcitonin receptor-like receptor (CRLR)
(3). ADM participates in a wide
range of physiological and pathological events, including cell
growth, vasorelaxation, angiogenesis and apoptosis (4). There is accumulating evidence that
ADM acts as a tumor growth factor: i) cancer tissues express higher
levels of ADM transcript and protein than non-cancerous tissues
(5); ii) ADM expression is
upregulated by hypoxia, a situation that tumor tissues frequently
experience; and iii) ADM protects against tumor cell death by the
upregulation of Bcl-2 or the downregulation of pro-apoptotic
factors, including Bax or Bid (6).
The malignant growth of tumors depends on multi-step
processes, including escape from host immune surveillance, rapid
cell proliferation, neovascularization and metastasis (7). The evasion of apoptosis is one of the
basic multi-step processes that leads to malignant progression
(7,8). For example, tumor cells downregulate
surface receptors that trigger apoptotic signals or secrete
survival factors, including transforming growth factor-β1 (9,10).
Although ADM has been shown to be a tumor survival factor in
several tumor tissues, including breast (6) and endometrial cancer tissues
(11), its role in
gastrointestinal cancers has not yet been studied.
In this study, we investigated whether ADM acts as a
tumor growth factor in gastrointestinal tumors. We found that ADM
expression is increased in colon cancers and that high ADM
expression correlates with poor patient survival. Our results
indicate that ADM functions as a tumor progression factor in colon
cancers.
Materials and methods
Tissue specimens
Surgical specimens, including lymph nodes, were
collected from 84 cases of colon adenocarcinoma and 72 cases of
stomach adenocarcinoma. Representative areas of the tumors, proven
by frozen sections, and matched macroscopically uninvolved mucosae
were snap-frozen in liquid nitrogen immediately and stored at −80°C
until analyzed. None of the patients had received chemo-, radio- or
immunotherapy prior to resection. The histopathological
characteristics of the examined cases of colon and stomach cancers
are summarized in Tables I and
II, respectively. Patient samples
were collected according to the institutional review board-approved
protocol.
 | Table IPathological data for adenocarcinomas
of the colon. |
Table I
Pathological data for adenocarcinomas
of the colon.
| Stage | Grade | No. of cases (%) |
|---|
|
|---|
| I | II | III |
|---|
| I | 9 | 3 | 0 | 12 (14.3) |
| II | 7 | 16 | 5 | 28 (33.3) |
| III | 6 | 13 | 10 | 29 (34.5) |
| IV | 3 | 7 | 5 | 15 (17.9) |
| Total | 25 | 39 | 20 | 84 (100) |
 | Table IIPathological data for adenocarcinomas
of the stomach. |
Table II
Pathological data for adenocarcinomas
of the stomach.
| Depth of
invasion | Grade | No. of cases (%) |
|---|
|
|---|
| I | II | III |
|---|
| Submucosa | 10 | 6 | 0 | 16 (22.2) |
| Muscle | 5 | 11 | 6 | 22 (30.6) |
| Serosa | 3 | 14 | 17 | 34 (47.2) |
| Total | 18 | 31 | 23 | 72 (100) |
Semi-quantitative RT-PCR
The RNA extraction, cDNA synthesis and quantitative
evaluation of the PCR products were performed as described
previously (12). The primers used
for ADM were sense (5′-agt ttc gaa aga agt gga at-3′) and
anti-sense (5′-gac gtt gtc ctt gtc ctt at-3′). PCR was performed
for 32 cycles of 95°C for 45 sec, 56°C for 45 sec and 72°C for 1
min. ADM expression was normalized to that of the housekeeping
gene, GAPDH, by densitometry (Bio-Rad, Hercules, CA, USA). The
relative ADM expression levels in normal tissues and
adenocarcinomas are presented as the ratio of ADM/GAPDH. The tumors
were classified according to ADM expression level into high
expression, within normal range and low expression groups in which
the ADM/GAPDH ratios were >1.5, 0.5–1.5 and <0.5 times their
values in the matched normal tissue, respectively (12).
Immunoblot analysis
Total proteins were extracted from the tumor tissues
or normal counterpart mucosae and quantitated by the biuret method.
A 30-μg sample of protein from each tissue was loaded onto 12%
SDS-polyacrylamide gel and separated by electrophoresis before
transfer to nitrocellulose membranes. The blots were incubated with
goat anti-human ADM antibody (Bachem AG, Bubendorf, Switzerland) or
anti-human tubulin antibody (BD Pharmingen, San Diego, CA, USA),
followed by chemoluminescent (ECL)-based detection (Amersham
Pharmacia Biotech, Piscataway, NJ, USA). ADM expression was
quantitated by densitometry following adjustment for the expression
levels of tubulin. ADM expression levels in the normal and
adenocarcinoma tissues were calculated as the ratio of ADM/tubulin
expression. The samples were classified according to ADM expression
level into high expression, within normal range and low expression
groups in which the ADM/tubulin ratios were >1.5, 0.5–1.5 and
<0.5 times their values in the matched normal tissue,
respectively.
Immunohistochemistry and staining
evaluation
Formalin-fixed paraffin-embedded tissue sections
were deparaffinized, rehydrated and washed twice for 5 min in wash
buffer (50 mM Tris/HCl, pH 7.6, 50 mM NaCl). Endogenous peroxidase
was quenched with 3% hydrogen peroxide in methanol for 5 min. The
slides were washed as before and then incubated in blocking buffer
for 1 h. This was followed by incubation with 1:100 diluted
anti-human ADM antibody for 1 h. The slides were washed twice and
further incubated with biotinylated secondary antibody followed by
avidin-conjugated horseradish peroxidase. The slides were
visualized using the DAB substrate-chromogen system (Dako,
Carpinteria, CA, USA) and counterstained with hematoxylin.
Immunohistochemical staining was evaluated by an arbitrary
quantitative scoring system using an image analyzer. The fields
were scored on a scale of 0–4 according to the area of staining as
follows: 0, none; 1, ≤25%; 2, 26–50%; 3, 51–75%; and 4, ≥76%. For
each case, the mean score (sum of scores for each field/fields
counted) was calculated.
Statistical analysis
The significance of the association between the ADM
expression levels and clinical and pathological parameters was
evaluated by a one-way ANOVA multiple comparison test (Tukey and
Tamhane) or Student’s t-test, and P<0.05 was considered to
indicate a statistically significant result. Kaplan-Meier survival
curves were constructed for patients whose tumors were classified
as having a high or low expression of ADM and the curves were
compared by the log-rank test.
Results
ADM transcript expression in
gastrointestinal tumors
The 84 colon and 72 stomach cancer specimens with
matched uninvolved mucosal tissues were analyzed by RT-PCR to
determine the mRNA expression levels of ADM. The RT-PCR assays were
controlled by equalization of input RNA for each sample and
comparable amplification efficiencies were validated by the
uniformity of control GAPDH RT-PCR product yields. ADM transcripts
were present in the normal and cancerous tissues of the stomach and
colon. However, the mean ADM/GAPDH mRNA ratio was significantly
higher in the colon cancer tissues than in the normal colonic
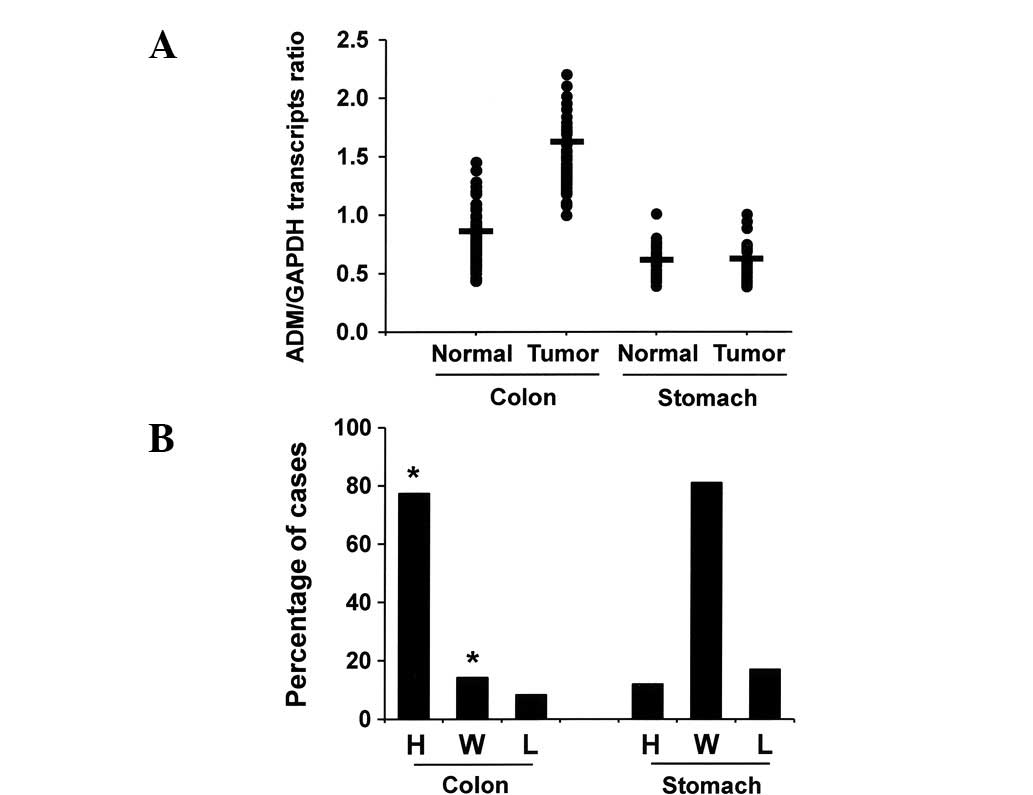
mucosae (Fig. 1A). Based on the
quantitative scales described in the methods section, 77.4% (65/84)
of colonic adenocarcinomas had high expression of ADM, 14.3%
(12/84) had within normal range expression of ADM and 8.3% (7/84)
had low expression of ADM (Fig.
1B). In contrast, we found no significant differences in the
ADM transcript levels between normal gastric mucosae and stomach
cancer tissues. These data indicate that ADM mRNA expression levels
are increased in colon cancers compared with matched normal mucosal
tissue (*P<0.05).
Expression levels of ADM protein in
gastrointestinal tumors
We analyzed the expression levels of ADM protein in
the same stomach and colon cancer samples and uninvolved matched
mucosal tissue by western blot analysis. Equivalent amounts of
protein were loaded and validated by western blotting for tubulin.
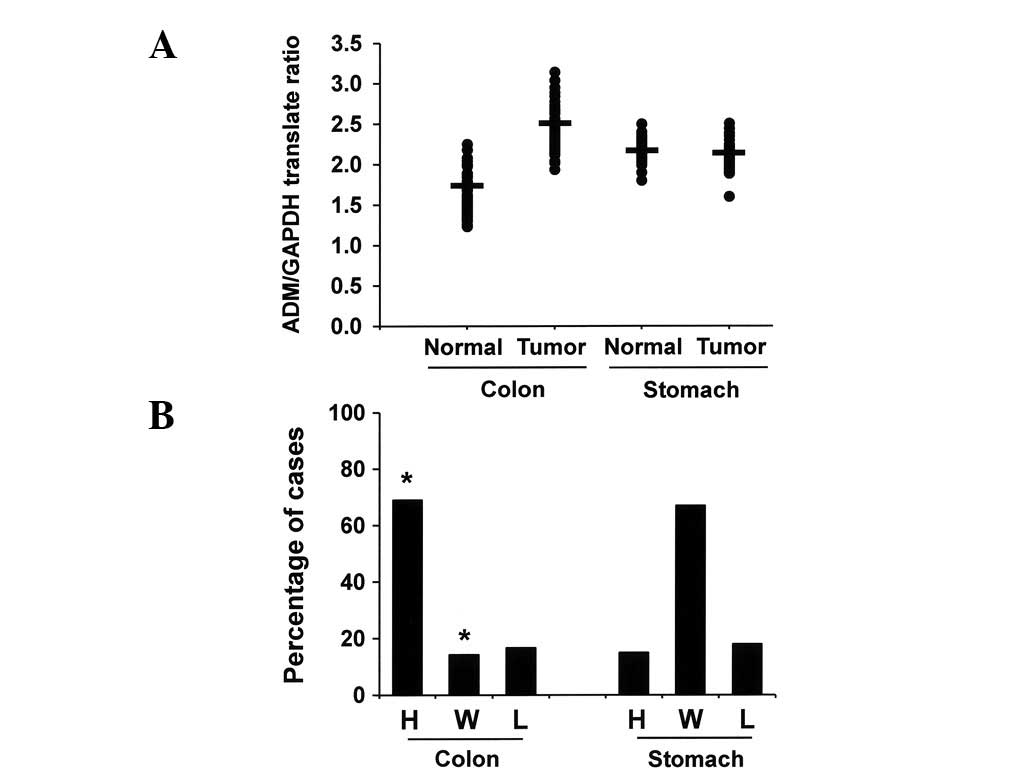
The mean ADM/tubulin protein ratio was significantly higher in the
colon cancers than in the uninvolved colonic mucosae (Fig. 2A). Sixty-nine percent (58/84),
14.3% (12/84) and 16.7% (14/84) of colonic adenocarcinomas
expressed ADM at high levels, within the normal range and at low
levels, respectively (Fig. 2B). In
contrast, we did not observe a significant difference in the ADM
expression levels between normal gastric mucosae and stomach cancer
tissues. These results indicate that the expression of ADM protein
in colon cancer tissues is significantly higher than in matched
normal mucosal tissue (*P<0.05).
Correlation of ADM expression scores with
pathological parameters
We analyzed the expression levels of ADM in colon
cancers according to pathological parameters. To quantitatively
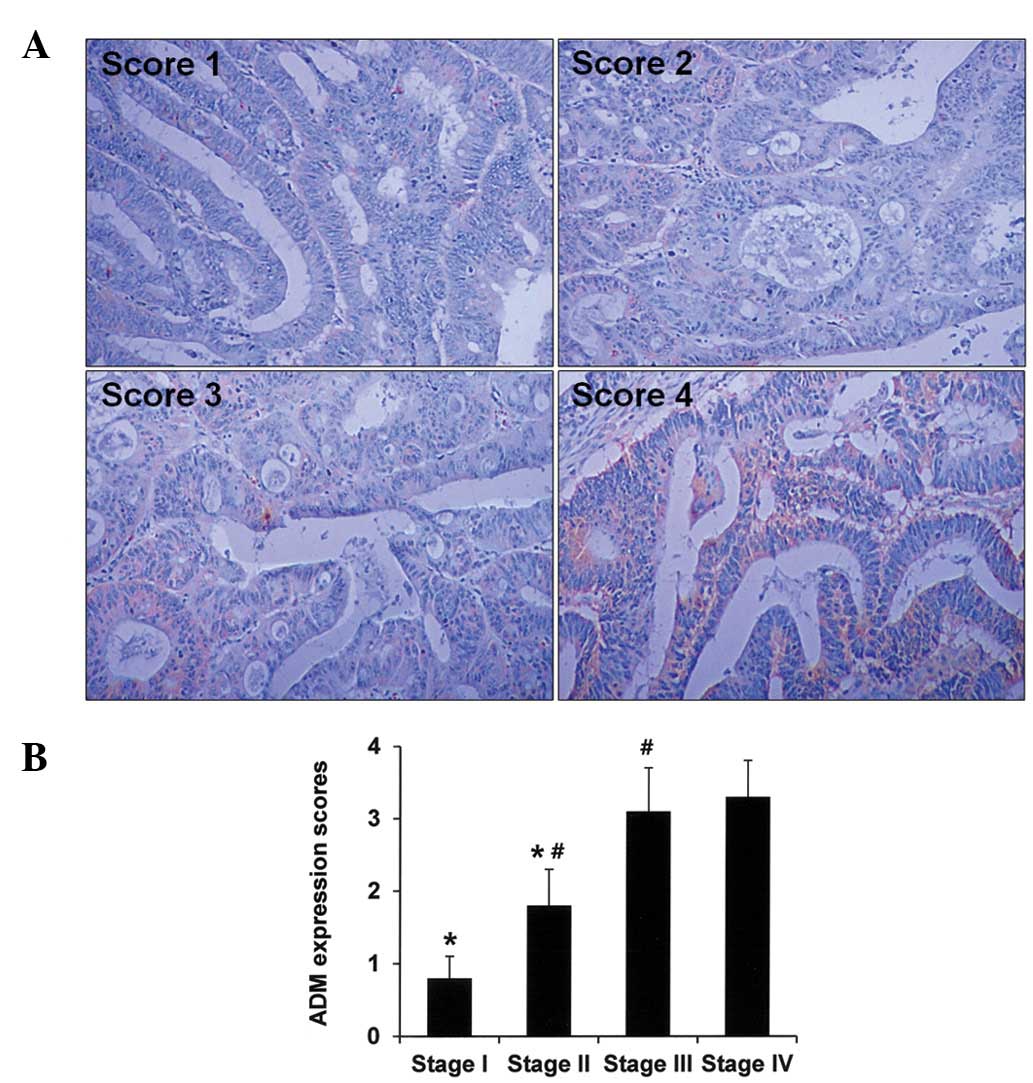
evaluate ADM expression, tumor tissue sections were stained
immunohistochemically and staining intensities were scored as
described in the Materials and methods section. Representative
immunohistochemical staining results are shown in Fig. 3A. We observed that ADM expression
scores increased according to colon cancer stage
(*P<0.05), suggesting that ADM expression is
associated with colon cancer progression (Fig. 3B). However, other pathological
parameters, including tumor size and the histological grade of the
colon cancer did not correlate with the ADM expression scores (data
not shown). We then analyzed ADM expression according to clinical
survival rates. Information regarding survival was available for 54
of the colon cancer patients. Overall survival analysis revealed
that patients with tumors with lower expression of ADM (ADM
expression score <2) had longer survival times than patients who
had tumors with higher expression of ADM (ADM expression score
>2, P<0.05; Fig. 4). In
contrast, we found no correlations between the ADM expression
levels and pathological parameters or survival rates in the stomach
cancer patients (data not shown).
Discussion
ADM is a pluripotent hormone secreted by many
tissues in the body and it regulates a variety of physiological
activities (13). It also
participates in many pathological processes, including
cardiovascular and inflammatory disorders, diabetes and cancer
(7). Cancer cells that grow
rapidly and aggressively have the following general
characteristics: growth factor production, insensitivity to growth
inhibition signals and the ability to evade apoptosis (8). Several reports have suggested that
ADM plays a role in cancer progression by aggravating the molecular
and physiological features of malignant cells (7). Examples of tumors where ADM plays
this role include brain tumors (14), lung cancers (15) and breast cancers (6). Furthermore, a study has shown that
in vitro tumor growth was blocked by a polyclonal antibody
specific for ADM, which competed with ADM for cellular receptors
(16). In our study, we provided
clinicopathological data that supports the hypothesis that ADM is
involved in colon cancer progression.
First, we determined ADM expression levels in
colonic and gastric cancers as compared with matched normal mucosal
tissues. We found significantly higher expression levels of ADM
mRNA and protein in colon cancers than in uninvolved mucosae,
suggesting that ADM plays a role in colon cancer. ADM ligand and
receptor expression in colonic mucosae and cancers have been
reported previously (17–20), but tumors and matched normal
mucosal tissues were not compared in these previous studies. ADM
expression is regulated by hypoxia-inducible factor-1α (HIF-1α), a
major transactivator in response to hypoxia (21). Hypoxia driven by the rapid
proliferation of cancer cells may induce HIF-1α accumulation and,
in turn, may upregulate ADM expression. However, higher expression
levels of ADM in tumors originating from non-solid hollow viscera,
such as the colon, suggest that unknown factors other than hypoxia
are involved in ADM regulation. Although the molecular mechanisms
underlying the involvement of ADM in tumor progression are not
clear, putative pathways include: i) upregulation of Bcl-2
(11); ii) stimulation of
neovascularization (22); iii)
activation of the phosphatidylinositol 3-kinase/Akt pathway
(23); and iv) modulation of
immune responses (24). Whatever
the mechanisms, our data clearly show that colon cancers express
significantly higher levels of ADM than uninvolved mucosae. In
contrast, ADM was not overexpressed in stomach cancers. This
suggests that stomach cancer progression may be fundamentally
different from that of colon cancer. Correlations between ADM
expression levels and pathological parameters further support our
contention that ADM expression has biological significance in colon
cancer. Current prognostic assessment of colon cancer is based on
the AJCC TNM staging system. The survival data presented here
indicate that determination of ADM expression levels may provide
useful additional prognostic information.
In conclusion, we have shown that ADM expression
levels are significantly higher in colon cancer tissues than in
uninvolved colonic mucosae and that this higher expression is
correlated with tumor stage and clinical survival rate.
Collectively, these data indicate that ADM is involved in colon
cancer progression.
Acknowledgements
This research was supported by the Kyung Hee
University Research Fund in 2011 (KHU-20100136).
References
|
1
|
Kitamura K, Kangawa K, Kawamoto M, Ichiki
Y, Nakamura S, Matsuo H and Eto T: Adrenomedullin: a novel
hypotensive peptide isolated from human pheochromocytoma. Biochem
Biophys Res Commun. 192:553–560. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eipper BA, Stoffers DA and Mains RE: The
biosynthesis of neuropeptides: peptide α-amidation (Review). Annu
Rev Neurosci. 15:57–85. 1992.
|
|
3
|
Kamitani S, Asakawa M, Shimekake Y,
Kuwasako K, Nakahara K and Sakata T: The RAMP2/CRLR complex is a
functional adrenomedullin receptor in human endothelial and
vascular smooth muscle cells. FEBS Lett. 448:111–114. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hinson JP, Kapas S and Smith DM:
Adrenomedullin, a multifunctional regulatory peptide (Review).
Endocr Rev. 21:138–167. 2000.PubMed/NCBI
|
|
5
|
Li Z, Takeuchi S, Otani T and Maruo T:
Implications of adrenomedullin expression in the invasion of
squamous cell carcinoma of the uterine cervix. Int J Clin Oncol.
6:263–270. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martínez A, Vos M, Guédez L, Kaur G, Chen
Z, Garayoa M, Pío R, Moody T, Stetler-Stevenson WG, Kleinman HK and
Cuttitta F: The effects of adrenomedullin overexpression in breast
tumor cells. J Natl Cancer Inst. 94:1226–1237. 2002.PubMed/NCBI
|
|
7
|
Zudaire E, Martínez A and Cuttitta F:
Adrenomedullin and cancer (Review). Regul Pept. 112:175–183. 2003.
View Article : Google Scholar
|
|
8
|
Hanahan D and Weinberg RA: The hallmarks
of cancer (Review). Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
9
|
Möller P, Koretz K, Leithäuser F,
Brüderlein S, Henne C, Quentmeier A and Krammer PH: Expression of
APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in
normal and neoplastic colon epithelium. Int J Cancer. 57:371–377.
1994.PubMed/NCBI
|
|
10
|
Rich JN, Borton AJ and Wang X:
Transforming growth factor-b signaling in cancer (Review). Microsc
Res Tech. 52:363–373. 2001. View Article : Google Scholar
|
|
11
|
Oehler MK, Norbury C, Hague S, Rees MC and
Bicknell R: Adrenomedullin inhibits hypoxic cell death by
upregulation of Bcl-2 in endometrial cancer cells: a possible
promotion mechanism for tumour growth. Oncogene. 20:2937–2945.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ryu BK, Lee MG, Chi SG, Kim YW and Park
JH: Increased expression of cFLIP(L) in colonic adenocarcinoma. J
Pathol. 194:15–19. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hinson JP, Thomson LM and Kapas S:
Adrenomedullin and CGRP receptors mediate different effects in the
rat adrenal cortex. Endocr Res. 24:725–728. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Satoh F, Takahashi K, Murakami O, Totsune
K, Sone M, Ohneda M, Abe K, Miura Y, Hayashi Y and Sasano H:
Adrenomedullin in human brain, adrenal glands and tumor tissues of
pheochromocytoma, ganglioneuroblastoma and neuroblastoma. J Clin
Endocrinol Metab. 80:1750–1752. 1995.
|
|
15
|
Martinez A, Miller MJ, Unsworth EJ,
Siegfried JM and Cuttitta F: Expression of adrenomedullin in normal
human lung and in pulmonary tumors. Endocrinology. 136:4099–4105.
1995.PubMed/NCBI
|
|
16
|
Ouafik L, Sauze S, Boudouresque F, Chinot
O, Delfino C, Fina F, Vuaroqueaux V, Dussert C, Palmari J, Dufour
H, et al: Neutralization of adrenomedullin inhibits the growth of
human glioblastoma cell lines in vitro and suppresses tumor
xenograft growth in vivo. Am J Pathol. 160:1279–1292. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miller MJ, Martínez A, Unsworth EJ, Thiele
CJ, Moody TW, Elsasser T and Cuttitta F: Adrenomedullin expression
in human tumor cell lines. Its potential role as an autocrine
growth factor. J Biol Chem. 271:23345–23351. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakayama M, Takahashi K, Murakami O,
Shirato K and Shibahara S: Induction of adrenomedullin by hypoxia
and cobalt chloride in human colorectal carcinoma cells. Biochem
Biophys Res Commun. 243:514–517. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kitani M, Sakata J, Asada Y, Kitamura K
and Eto T: Distribution and expression of adrenomedullin in human
gastrointestinal tissue. Ann Clin Biochem. 35:643–648. 1998.
View Article : Google Scholar
|
|
20
|
Marutsuka K, Nawa Y, Asada Y, Hara S,
Kitamura K, Eto T and Sumiyoshi A: Adrenomedullin and
proadrenomudullin N-terminal 20 peptide (PAMP) are present in human
colonic epithelia and exert an antimicrobial effect. Exp Physiol.
86:543–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garayoa M, Martínez A, Lee S, Pio R, An
WG, Neckers L, Trepel J, Montuenga LM, Ryan H, Johnson R, et al:
Hypoxia-inducible factor-1 (HIF-1) up-regulates adrenomedullin
expression in human tumor cell lines during oxygen deprivation: a
possible promotion mechanism of carcinogenesis. Mol Endocrinol.
14:848–862. 2000. View Article : Google Scholar
|
|
22
|
Zhao Y, Hague S, Manek S, Zhang L,
Bicknell R and Rees MC: PCR display identifies tamoxifen induction
of the novel angiogenic factor adrenomedullin by an non estrogenic
mechanism in the human endometrium. Oncogene. 16:409–415. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim W, Moon SO, Sung MJ, Kim SH, Lee S,
Kim HJ, Koh GY and Park SK: Protective effect of adrenomedullin in
mannitol-induced apoptosis. Apoptosis. 7:527–536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kamoi H, Kanazawa H, Hirata K, Kurihara N,
Yano Y and Otani S: Adrenomedullin inhibits the secretion of
cytokine-induced neurtophil chemoattractant, a member of the
interleukin-8 family, from rat alveolar macrophages. Biochem
Biophys Res Commun. 211:1031–1035. 1995. View Article : Google Scholar : PubMed/NCBI
|