Introduction
Hypertension is a major health problem that leads to
a range of diseases. Hypertension is capable of promoting vascular
remodeling, which could be the main reason for increased peripheral
vascular resistance and high blood pressure levels. Hypertensive
vascular remodeling is associated with structural, functional and
biochemical adjustments of endothelial cells, and it involves the
degradation and reorganization of the extracellular matrix (ECM)
scaffold, as well as hypertrophy and hyperplasia of the vascular
smooth muscle cells (VSMCs), all of which contribute to a thickened
vessel wall and augmented vascular stiffness.
Vascular cells (endothelial cells, smooth muscle
cells and fibroblasts) are critical in vascular remodeling, and
many studies suggested that the endothelium senses the hemodynamic
changes and initiates the reorganization of the preexisting
cellular and extracellular components. This remodeling involves
cellular proliferation, apoptosis, migration, cell organization and
matrix-integrin interactions throughout the layered structure of
the vessel (1). Langille and
O’Donnell demonstrated that the endothelium, or a substance
produced by the endothelium, was essential for remodeling toward a
smaller lumen after a long-term flow reduction (2).
The latest in vitro and in vivo
studies have demonstrated that the transforming growth factor-β 1
(TGF-β1) isoform showed fundamental significance during vascular
development, atherogenesis, neointima proliferation and vessel
remodeling. The underlying mechanism may be related with its
effects on regulating ECM synthesis, cell cycle progression,
apoptosis, differentiation and migration (3–5). It
has also been proven that gene expression of TGF-β1 may be
associated with EC remodeling development (6).
Digitalis, which has been used in clinical practice
for over 100 years, has a positive inotropic effect in myocardial
cells. Ouabain, a digitalis compound, works as an endogenous
regulator of blood pressure and Na+, K+-ATPase activity (7). Recent observations indicate that
chronic ouabain treatment gives rise to hypertension (8) and hypertensive vascular remodeling
(9).
The grape seed proanthocyanidin extract (GSPE) has
been reported to be effective in treating arteriosclerosis
(10), while little is known about
its effects on systolic blood pressure and vascular remodeling.
In this study, the effects of GSPE on the blood
pressure and vascular remodeling were examined by treating
ouabain-induced hypertensive rats with GSPE (250 mg/kg·d), in
tandem with the measurement of the systolic blood pressure and
vascular remodeling parameters. The expression of nitric oxide (NO)
and endothelin-1 (ET-1) in the thoracic aorta were examined by
ELISA, and the mRNA and protein levels of TGF-β1 were respectively
detected by real-time PCR and western blotting.
Materials and methods
Animals
A total of 30 male Sprague-Dawley (SD) rats (5–6
weeks old, weighing 180–220 g, supplied by the Experimental Animal
Center of Shandong University, China) were housed in a 12:12-h
light-dark cycle at 24°C, and had free access to tap water and
standard rat chow ad libitum for 7 days to allow for
acclimatization prior to entering the study. All protocols were
approved by the Institutional Animal Care and Use Committee of the
Qilu Hospital, Shandong University.
Treatment
The 30 rats were randomized into three groups, with
10 rats in each group, treated with nitric sodium (NS), ouabain,
GSPE and ouabain, respectively, and thus named the NS group, the O
group and the GO group. Rats in the O group were administered
ouabain (Sigma Chemical Co., St. Louis, MO, USA) at a dose of 27.8
μg·kg−1·d−1 by intraperitoneal (ip)
injection in the early morning of each day for 5 consecutive weeks.
Rats in the GO group were administered oral GSPE at a dosage of 250
mg/kg·d, as well as ouabain at the same dose as the O group. Rats
in the NS group were administered 0.9% saline (1 ml·kg-1·d-1) ip
and 1 ml 0.9% NS orally for the same duration.
Systolic blood pressure (SBP)
measurement
The SBP of all animals was measured using an
indirect tail-cuff plethysmographic (TCP) method with a rat tail BP
monitor (RBP-I, Clinical Medicine Institute, Beijing Sino-Japan
Friendship Hospital, China). The rats were kept calm and conscious
until pulsatory signals from the arteria caudilis were displayed
steadily. At least 10 determinations were made on each rat and the
mean of 6 readings within a 5–10 mmHg range was taken as the SBP of
the rat (11). The systolic and
diastolic blood pressures were measured for 30 min with a pressure
transducer (model 1050BP, UFI, Inc., Morro Bay, CA, USA) and then
recorded using an interface and software for computer data
acquisition (model MP100A, BIOPAC System, Inc. Santa Barbara, CA,
USA). The SBP was measured once a week until the end of the
experiment (six times in total).
Tissue collection
Following five weeks of treatment, the rats were
sacrificed by decapitation. Thoracic aortas were rapidly excised
and dissected. For morphological and immunohistochemical
examination, aorta were fixed in buffered 10% neutral formalin. For
electron microscopy, thoracic aorta were trimmed to 1×1×1 mm and
fixed in 2.5% glutaraldehyde solution. For analysis of TGF-β1
expression, the aorta were instantly frozen in liquid nitrogen and
maintained at −80°C prior to use.
Hematoxylin and eosin (HE) staining
Specimens of thoracic aorta were fixed into buffered
10% neutral formalin for 48 h, dehydrated in graded ethanol
solutions, and then embedded in paraffin. The paraffin-embedded
specimens were sectioned at 5 μm. The morphological changes
were examined by light microscopy following HE staining.
Ultrastructural examination
Thoracic aorta were trimmed into tissue blocks of 1
mm3 on ice and immediately put into the 2.5%
glutaraldehyde fixation solution at 4°C for 2 h followed by
postfixation in 1% osmium tetroxide (in 0.1 M phosphate buffer) for
2 h at 4°C. The samples were then dehydrated in a graded ethanol
series with acetone, permeated and embedded in epoxide resin.
Semi-thin sections of approximately 75 nm were prepared, stained
with uranyl acetate and lead citrate, and then observed with an
H-800 transmission electron microscope (TEM; Hitachi Electronic
Instruments, Tokyo, Japan).
ELISA
Animals were treated as above, and thoracic aortas
were excised and stored at −70°C until assay. The amount of NO and
ET-1 was measured using a colorimetric method (optical density)
after detection of the protein with a kit purchased from Shanghai
Jianglaibio Ltd. Co. (Shanghai, China) and Enzo Life Sciences, Inc.
(NY, USA), respectively.
Total RNA isolation and cDNA synthesis of
TGF-β1
Total RNA isolation from tissues was performed with
TRIzol reagent (Omega Bio-Tek, Norcross, GA, USA) according to the
manufacturer’s instructions. All total RNA samples were subjected
to DNase I treatment (DNase I; Fermentas, Burlington, ON, Canada)
and stored in RNase-free double distilled water at −80°C. RNA
quantity and purity were determined by spectrophotometry. The
integrity of RNA molecules was monitored by 1% agarose gel
electrophoresis, and specimens with well-pronounced rRNA bands were
selected for reactions. The first strand of cDNA was synthesized as
follows (First Strand cDNA Synthesis kit, Fermentas): a mixture of
1 μg of total RNA, 1 μl oligo (dT)18 primer and
DEPC-treated water to 12 μl was heated for 5 min at 65°C and
chilled on ice; then 4 μl of 5× reaction buffer, 1 μl
of RNase inhibitor (20 U/μl), 2 μl of dNTP mix (10mM)
and 1 μl of reverse transcriptase (200 U/μl) were
added and incubated for 60 min at 42°C, followed by 5 min at 70°C
to inactivate the reverse transcriptase, and the mixture was then
stored at −20°C for mRNA expression analysis.
Real-time quantitative PCR analysis of
TGF-β1
Real-time quantitative PCR (qPCR) was performed by
RealMaster Green (Tiangen, Beijing, China). The total reaction
volume was 20 μl (1.5 μl of cDNA template, 8
μl 2.5× real master mix, 1 μl 20× SYBR solution, 9
μl double distilled water and 0.25 μl 5 mM each of
the forward and reverse primers). The real-time quantitative PCR
program was 95°C for 1 min, followed by 35 cycles of 95°C for 5
sec, 58°C for 15 sec and 68°C for 20 sec. Melting curve analysis
and 2% agarose gel electrophoresis were used to confirm the
specificity of each product, the efficiency of PCR was determined
by analysis of two-fold or five-fold serial dilutions of cDNA and
designed to detect all the signals in the spanning region. The
efficiencies were close to 100%, allowing the use of the
2−ΔΔCT method for calculation of relative gene
expression. All qPCR was conducted with negative controls. The mRNA
expression levels of TGF-β1 in the thoracic aorta were examined by
qPCR. Each sample was performed in triplicate and the data were
normalized to β-actin expression. The primer sequences are listed
in Table I.
 | Table IPrimer sequences for detection of PCNA
mRNA transcripts. |
Table I
Primer sequences for detection of PCNA
mRNA transcripts.
| Gene | Sense (5′-3′) | Anti-sense
(5′-3′) | Length (bp) |
|---|
| β-actin |
GAAGTGTGACGTTGACAT |
ACATCTGCTGGAAGGTG | 245 |
| TGF-β1 |
AGAAGTCACCCGCGTGCTAAT |
CACTGCTTCCCGAATGTCTGA | 144 |
Western blot analysis of TGF-β1 protein
expression
Total protein was extracted from the frozen thoracic
aorta tissues using RIPA lysis buffer (1% Triton X-100, 1%
deoxycholate, 0.1% SDS) and 1 mM PVMF. Following ultrasonication
for 5 min, extracts were centrifuged at 12,000 × g for 15 min at
4°C, and the supernatants containing protein were retained. The
protein concentrations in the samples were measured with the BCA
method (Beyotime® Institute of Biochemistry, China). In
total, 50 μg of protein samples were resolved by
electrophoresis on a 12% SDS-polyacrylamide gel (Bio-Rad, Hercules,
CA, USA). Proteins were transferred onto a polyvinylidene
difluoride (PVDF) membrane. After blocking with 5% skimmed
milk/TBST for 1 h, the membranes were incubated overnight with
primary antibodies against TGF-β1 (mouse monoclonal, 1:250,
Abcam®, Hong Kong), and then stripped and incubated with
the respective peroxidase-conjugated AffiniPure goat
anti-rabbit/mouse IgG (1:10000, ZSGB-Bio). The bands were
visualized using the enhanced chemiluminescence system (ECL) and
analyzed densitometrically using Image J software. In the meantime,
PVDF membranes were probed with β-actin as an internal control to
ensure equal loading.
Statistical analysis
All data analyses were performed using
SPSS® version 11.5 (SPSS® Inc., Chicago, IL,
USA) for Windows®. The data were shown as the means ±
SD. An independent sample t-test was used to compare continuous
data between the two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Blood pressure
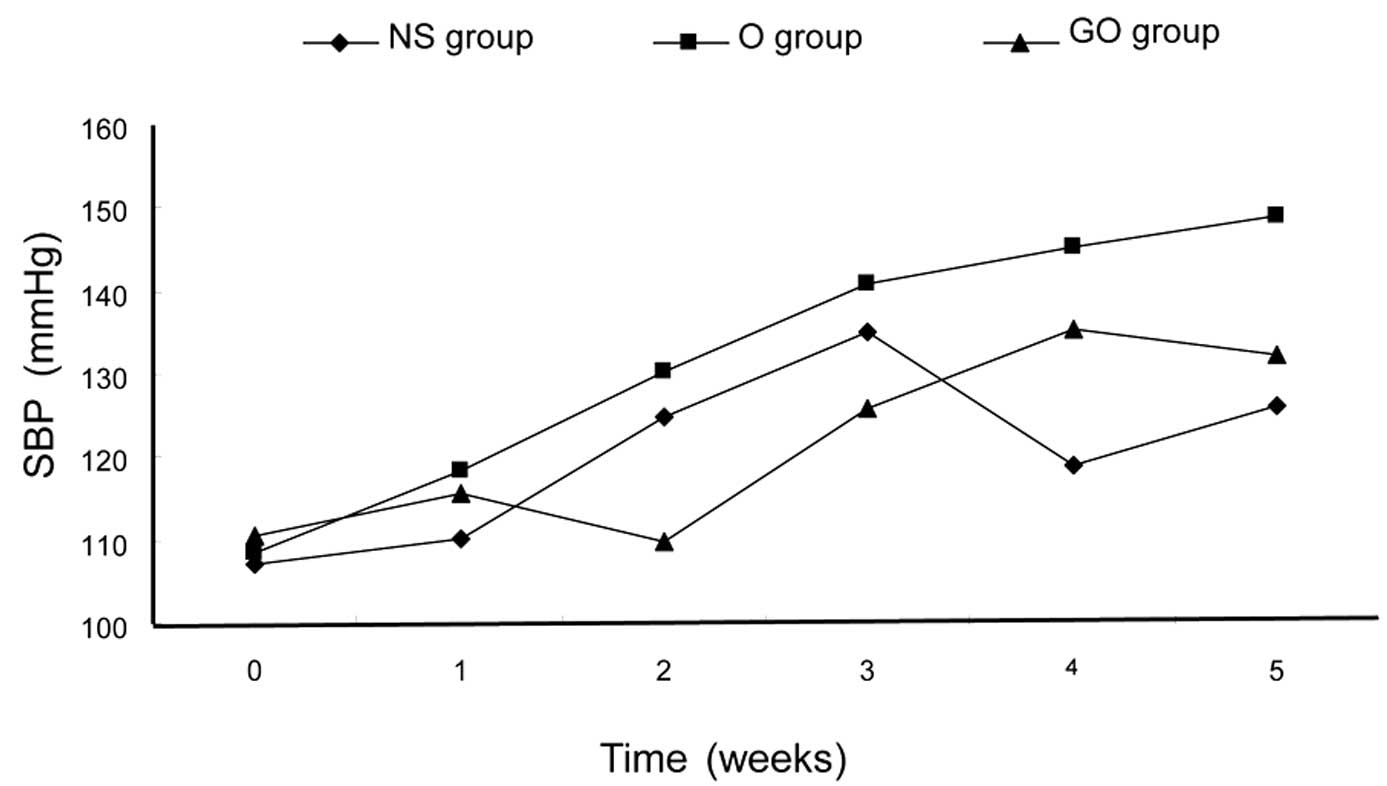
Over a 5-week treatment period, the mean SBPs of the
three groups were 108.1, 150.3 and 111.8 mmHg, respectively. There
was no significant difference between the GO group and the control
group (P>0.05; Fig. 1).
However, in the O group, the systolic blood pressure was much
higher than that of the GO and NS groups (P<0.05). At the end of
the treatment, no significant difference in body weight among the
three groups was observed (329±14, 335±9 and 317±10 g, P>0.05).
Fig. 1 shows the changes in SBP in
the three experimental groups over a 5-week period. SBP was
assessed using the TCP measurements in each group. The blood
pressure was the same in each group at the baseline (P>0.05),
However, after 5 weeks, GSPE-treated animals (GO group) showed
significantly decreased SBP compared with those in the O group
(P<0.01).
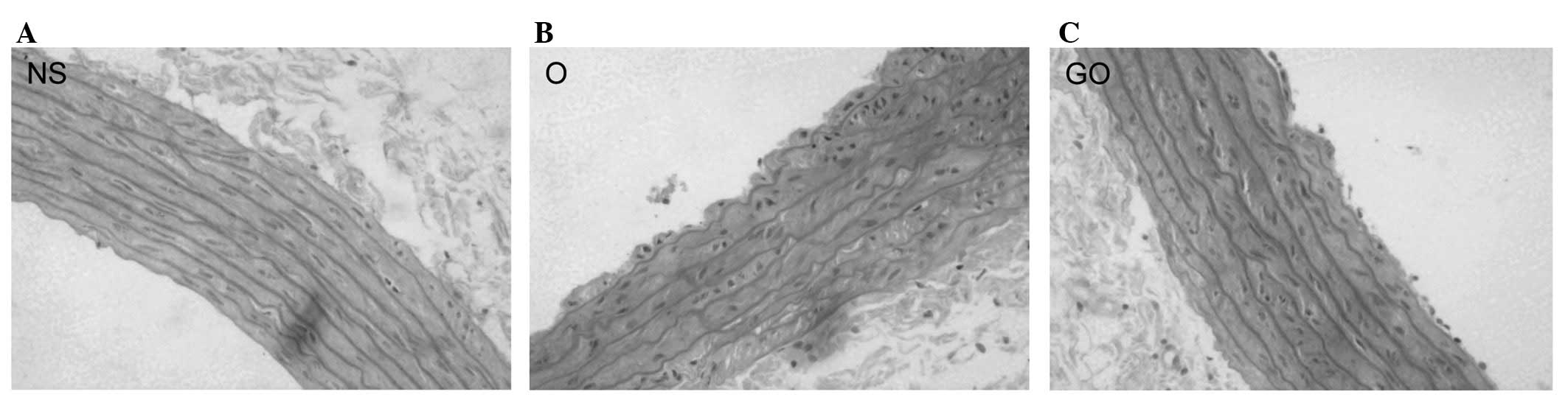
HE staining of aorta
Fig. 2 shows
histological sections of the thoracic aorta stained with HE. The
arrangement of the elastic fibers of aortas from rats in the NS
group was normal and there was no hyperplasia of collagen in the
vessel wall (Fig. 2A). The aortic
wall in the O group rats thickened, with hyperplastic collagen
fibers in the media and with decreased, disordered and even
ruptured elastic fibers (Fig. 2B).
Aortic elastic fibers in the GO group were fairly ordered. Collagen
fibers were almost normal compared to that in the O group (Fig. 2C).
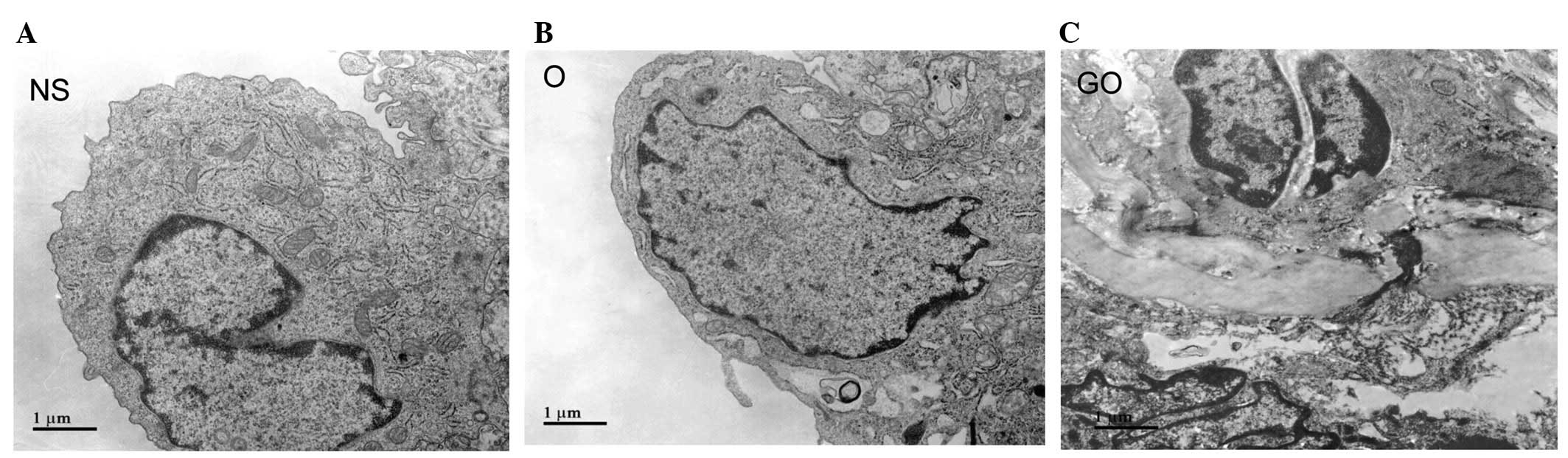
Ultrastructural changes of the thoracic
aorta
Fig. 3 shows
transmission electron photomicrographs of the thoracic aorta in the
three groups. The NS group showed a normal tight junction and gap
junction structure between the endothelial cells. The majority of
heterochromatin was distributed in the circumference of the
nucleus. In the O group, the morphology of endothelial cells in the
thoracic aorta changed, with vacuolated cytoplasm and enlarged
endoplasmic reticulum, and with no or decreased myofilaments.
Nuclear chromatin was dense and observed in lumps of different
sizes, which were mainly located in the nuclear membrane. In the GO
group, elastin fibers among the endothelial cells increased, with
irregular arrangement, and were partly disrupted. Nucleolemma
introcession was observed.
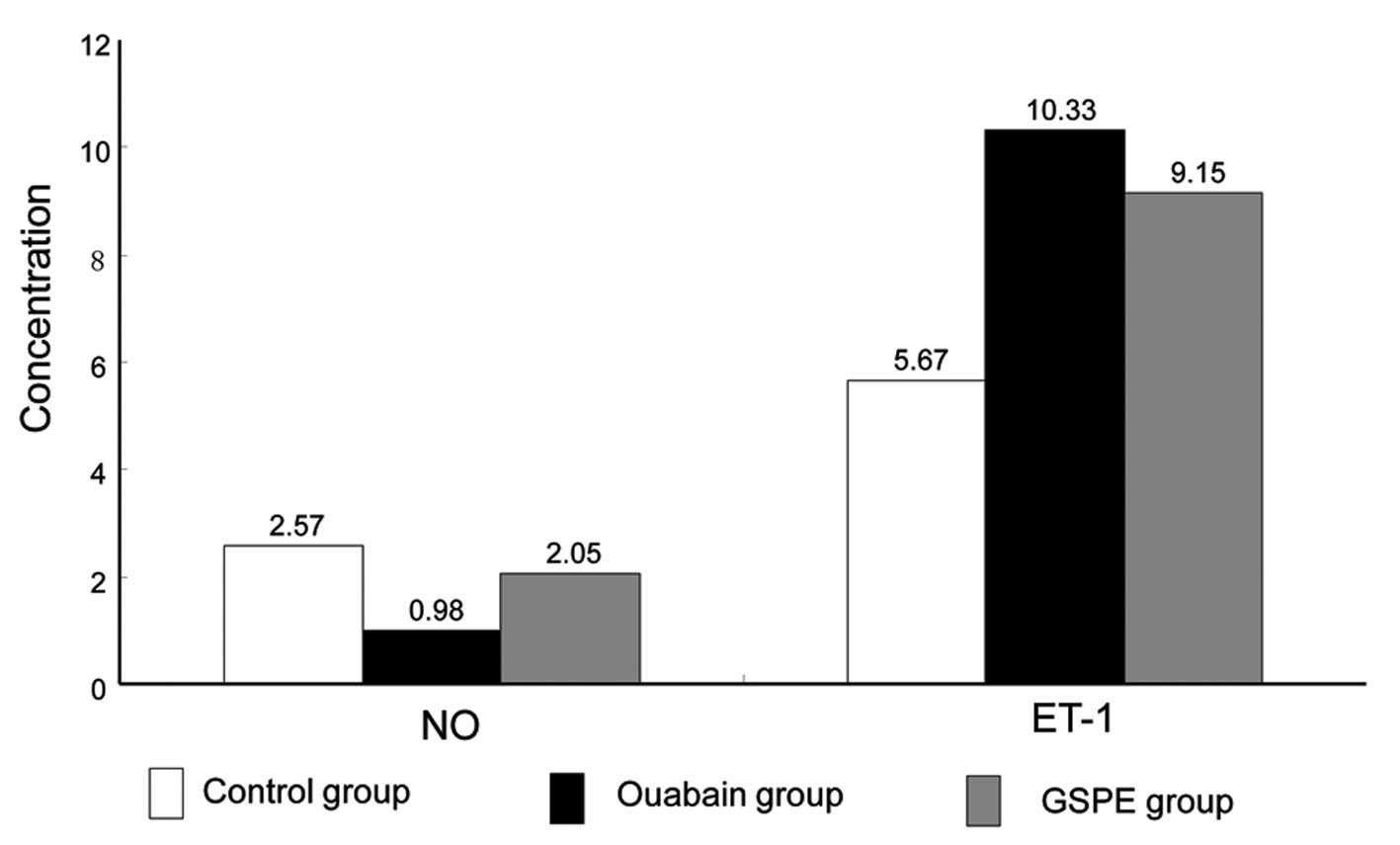
Expression of NO and ET-1
Fig. 4 shows the
concentration of NO and ET-1 in the thoracic aorta from the three
groups. Compared with the NS group, the concentration of NO in the
thoracic aorta in the O group decreased significantly (0.98 vs.
2.57 pg/mgprot, P<0.01); while GSPE treatment increased NO
production when comparing the GO group with the O group (2.57 vs.
0.98 pg/mgprot, P<0.01). However, the ET-1 expression increased
greatly in the O group in comparison to that in the NS group (10.33
vs. 5.67 pg/ml, P<0.01), which was capable of being reversed by
GSPE treatment (10.33 vs. 9.15 pg/ml, P<0.01).
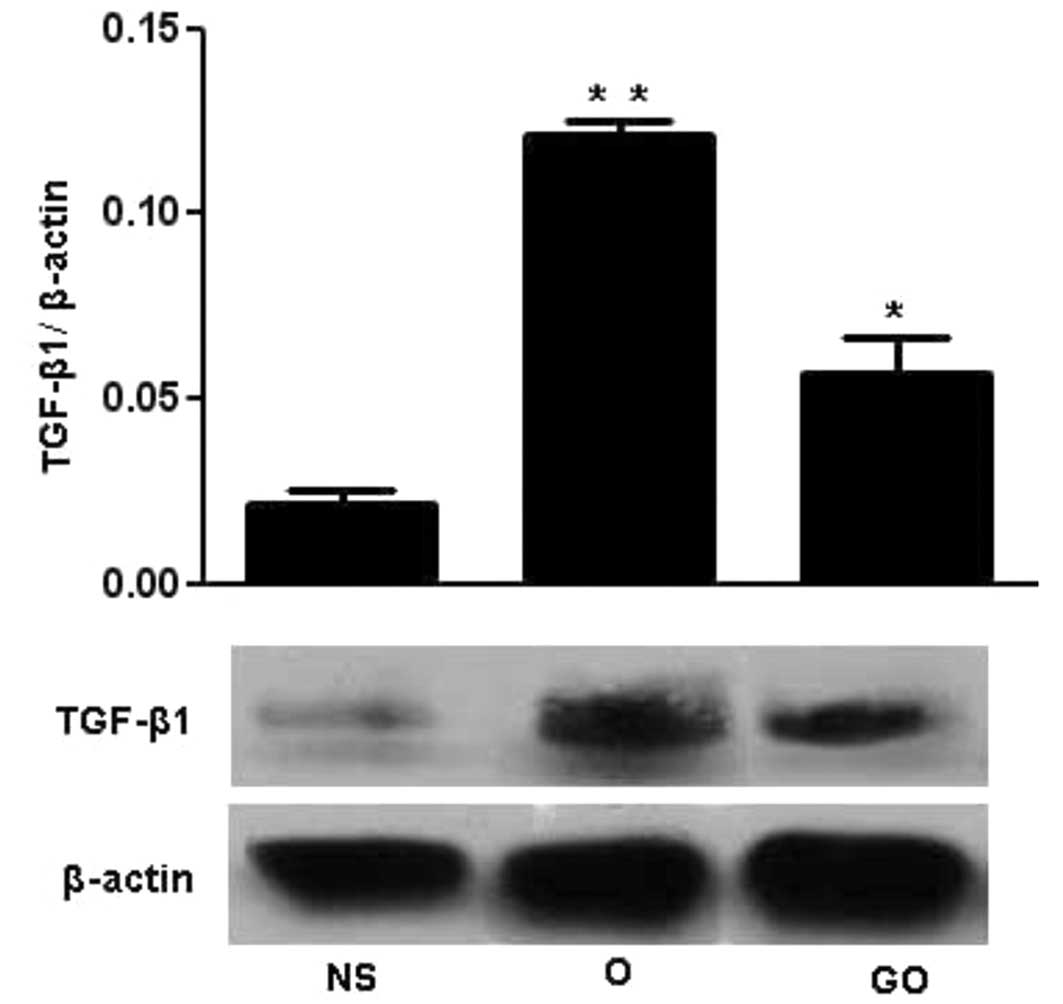
Protein expression of TGF-β1
Fig. 5 shows
western blot analyses of TGF-β1 expression in the three groups.
Lysates of the aorta cells treated with or without GSPE were
analyzed by western blotting using TGF-β1 antibody with β-actin as
an internal control. TGF-β1 expression in thoracic aortas in the O
group was significantly increased compared to that in the NS group,
and GSPE could decrease the expression of TGF-β1 compared with that
in the O group.
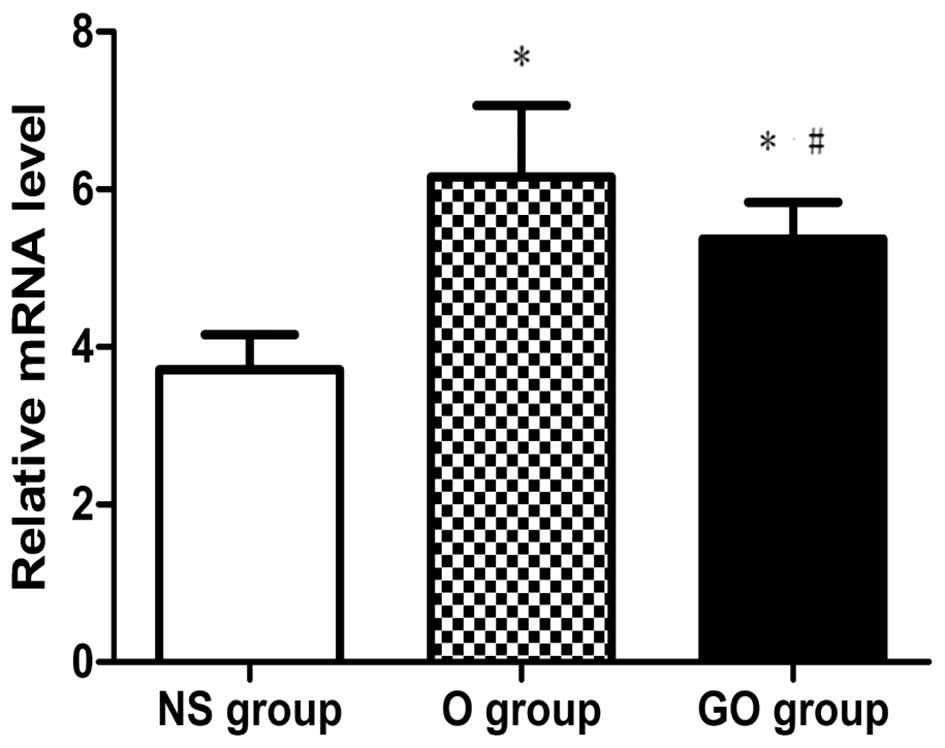
Real-time quantitative PCR analysis of
TGF-β1
Fig. 6 shows the
mRNA expression of TGF-β1 in the three groups. Total RNA was
isolated from the thoracic aorta in NS, O and GO group rats and
subjected to RT-PCR. 18S rRNA gene expression was used as an
internal control. The results revealed that the mRNA expression of
TGF-β1 in the O group was significantly increased compared to that
in the NS group, and GSPE was capable of decreasing the mRNA level
of TGF-β1 significantly compared with that in the O group.
Discussion
Hypertension is a major factor promoting vascular
remodeling, which leads to vascular stiffness. Vascular remodeling
involves degradation and reorganization of the ECM scaffold, as
well as hypertrophy and/or hyperplasia of the vascular smooth
muscle cells (VSMCs). It was reported that VSMCs and cardiac
hypertrophy were found before high blood pressure in the
spontaneously hypertensive rat (SHR) without correlation with blood
pressure levels (12).
Digitalis has a positive inotropic effect in
myocardial cells, and it has been used in clinical practice for
over 100 years. Ouabain, as a digitalis compound, is an endogenous
regulator of blood pressure and Na+, K+-ATPase activity (7). Recent studies suggested that chronic
ouabain treatment produced hypertension (8) and hypertensive vascular remodeling
(9).
Elevated levels of endogenous ouabain or a closely
related isomer are involved in rat and human hypertension and in
associated cardiovascular complications. Several findings indicated
that endogenous ouabain, in addition to directly influencing blood
pressure, may be involved in the development of cardiovascular
complications (cardiac hypertrophy, heart failure and myocardial
infarction) associated with hypertension. Endogenous ouabain may
therefore play an important role in vivo as a
prohypertrophic hormone and thus may affect cardiovascular function
and structure, as it is responsible for cardiac remodeling which
contributes to an increased risk of morbid events (8,13–15).
Furthermore, exogenous ouabain induced hypertension when
chronically administered to normotensive rats (16,17).
Our study showed that a five-week ouabain
administration could induce hypertension effectively. Moreover,
histological studies showed that ouabain significantly promoted
neointimal hyperplasia and VSMC migration when compared with those
of the control group.
GSPEs are a group of polyphenolic bioflavonoids
exhibiting multiple pharmacological activity and therapeutic
potential (4,18). GSPEs have been reported to protect
against oxidant injury during ischemia/reperfusion in the rat heart
(19–21). Although previous studies have
implicated antioxidant effects of GSPE (19–21),
none of them revealed the effect of GSPE on endothelial function
and hypertension.
In 2009, a study demonstrated that GSPE was capable
of lowering blood pressure in subjects with metabolic syndrome and
they also found that the phenolic compounds in the extract are
absorbed and that its antioxidant properties were capable of
reducing the concentration of Ox-LDL in plasma (22).
Endothelium-derived relaxing factors such as NO and
prostacyclin usually act in coordination with endothelium-derived
constricting factors such as ET-1, thromboxane and serotonin to
accommodate changes in the cardiac output and to keep the blood
pressure relatively constant. The imbalance of these
endothelium-derived factors may elevate vasomotor tone, promote
VSMC proliferation and induce vascular remodeling.
NO and ET-1 are key regulators of vasodilatory
actions. NO acts as a second messenger for the actions of a number
of growth factors, peptides, coagulation factors and hormones, and
is a powerful regulator of vascular function. Endothelium-derived
NO is a powerful regulator of vascular function, and it appears
that the abnormalities in the production or actions of NO lead to
endothelial dysfunction and abnormal vascular remodeling (1). ET-1 is the dominant vasoconstrictive
factor. Studies have noted that aortic ET-1 content is
significantly increased in DOCA-salt hypertensive rats compared
with that in age-matched control rats (23). It has been proposed that this
hypertension is due to an imbalance between endogenous
vasoconstrictors and the diminished vasodilating effect of NO.
Several candidates for endogenous vasoconstrictors that may
contribute to sustained hypertension induced by NO blockade have
been reported (24–26).
To evaluate the effect of GSPE on endothelial
function, ELISA was carried out to examine the concentration of NO
and ET-1 in the thoracic aorta.
Through detecting the concentration of NO and ET-1
in the thoracic aorta, we proved that ouabain impaired the balance
between NO and ET-1, two major vaso-active substances, which may
contribute to damaged endothelial function. GSPE increased NO
production and decreased ET-1 expression, resulting in improved
endothelial function and better vasodilation.
Improved endothelial function is capable of
inhibiting vascular remodeling. Therefore, we subsequently detected
another molecule (transforming growth factor-β 1, TGF-β1) that is
critical in mediating vascular remodeling. The latest in
vitro and in vivo studies also demonstrated that TGF-β1
is of fundamental importance during vascular development,
atherogenesis, neointima proliferation and vessel remodeling. The
possible mechanism may be due to its regulation of ECM synthesis,
cell cycle progression, apoptosis, differentiation and migration
(3–5). A previous study showed that gene
expression of TGF-β1 may be associated with its development
(6). A large number of studies
reveal that increased mRNA levels of TGF-β1 were observed in
myocardial remodeling (27).
In the present study, we proved that ouabain could
induce TGF-β1 expression at the mRNA and protein level, resulting
in vascular remodeling. This result was consistent with the
previous reports. GSPE could efficiently inhibit this harmful
pathway and therefore block the vascular remodeling induced by
ouabain.
In conclusion, our present study suggested that GSPE
could decrease blood pressure efficiently and reverse vascular
remodeling in ouabain-induced hypertensive rats. This may be
attributed to the regulation of NO and ET-1 balance and the
suppression of TGF-β1 expression by GSPE. Therefore, GSPE may be a
potential anti-hypertensive agent for patients with hypertensive
vascular diseases.
Acknowledgements
This study was supported by a grant from the
National Nature Science Foundation of China (30700884) and the
Shandong Provincial Scientific and Technological Project
(2010GGC10294, BS2009SW015).
References
|
1
|
Rudic RD and Sessa WC: Nitric oxide in
endothelial dysfunction and vascular remodeling: clinical
correlates and experimental links. Am J Hum Genet. 64:673–677.
1999. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Langille BL and O’Donnell F: Reductions in
arterial diameter produced by chronic decreases in blood flow are
endothelium-dependent. Science. 231:405–407. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ghosh J, Murphy MO, Turner N, Khwaja N,
Halka A, Kielty CM and Walker MG: The role of transforming growth
factor beta1 in the vascular system. Cardiovasc Pathol. 14:28–36.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shao ZH, Becker LB, Vanden Hoek TL,
Schumacker PT, Li CQ, Zhao D, Wojcik K, Anderson T, Qin Y, Dey L
and Yuan CS: Grape seed proanthocyanidin extract attenuates oxidant
injury in cardiomyocytes. Pharmacol Res. 47:463–469. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heimark RL, Twardzik DR and Schwartz SM:
Inhibition of endothelial regeneration by type-beta transforming
growth factor from platelets. Science. 233:1078–1080. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li RK, Li G, Mickle DA, Weisel RD, Merante
F, Luss H, Rao V, Christakis GT and Williams WG: Overexpression of
transforming growth factor-beta1 and insulin-like growth factor-I
in patients with idiopathic hypertrophic cardiomyopathy.
Circulation. 96:874–881. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang BS and Leenen FH: Brain
renin-angiotensin system and ouabain-induced sympathetic
hyperactivity and hypertension in Wistar rats. Hypertension.
34:107–112. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hamlyn JM, Hamilton BP and Manunta P:
Endogenous ouabain, sodium balance and blood pressure: a review and
a hypothesis. J Hypertens. 14:151–167. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ren YP, Huang RW and Lu ZR: Ouabain at
pathological concentrations might induce damage in human vascular
endothelial cells. Acta Pharmacol Sin. 27:165–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamakoshi J, Kataoka S, Koga T and Ariga
T: Proanthocyanidin-rich extract from grape seeds attenuates the
development of aortic atherosclerosis in cholesterol-fed rabbits.
Atherosclerosis. 142:139–149. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duarte J, Pérez-Palencia R, Vargas F,
Ocete MA, Pérez-Vizcaino F, Zarzuelo A and Tamargo J:
Antihypertensive effects of the flavonoid quercetin in
spontaneously hypertensive rats. Br J Pharmacol. 133:117–124. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koprdová R, Cebová M and Kristek F:
Long-term effect of losartan administration on blood pressure,
heart and structure of coronary artery of young spontaneously
hypertensive rats. Physiol Res. 58:327–335. 2009.
|
|
13
|
Ferrandi M, Manunta P, Ferrari P and
Bianchi G: The endogenous ouabain: molecular basis of its role in
hypertension and cardiovascular complications. Front Biosci.
10:2472–2477. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schoner W and Scheiner-Bobis G: Endogenous
cardiac glycosides: hormones using the sodium pump as signal
transducer. Semin Nephrol. 25:343–351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hamlyn JM, Ringel R, Schaeffer J, Levinson
PD, Hamilton BP, Kowarski AA and Blaustein MP: A circulating
inhibitor of (Na+ + K+)ATPase associated with essential
hypertension. Nature. 300:650–652. 1982.
|
|
16
|
Manunta P, Rogowski AC, Hamilton BP and
Hamlyn JM: Ouabain-induced hypertension in the rat: relationships
among plasma and tissue ouabain and blood pressure. J Hypertens.
12:549–560. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xavier FE, Rossoni LV, Alonso MJ, Balfagón
G, Vassallo DV and Salaices M: Ouabain-induced hypertension alters
the participation of endothelial factors in alpha-adrenergic
responses differently in rat resistance and conductance mesenteric
arteries. Br J Pharmacol. 143:215–225. 2004. View Article : Google Scholar
|
|
18
|
Bagchi D, Bagchi M, Stohs S, Ray SD, Sen
CK and Preuss HG: Cellular protection with proanthocyanidins
derived from grape seeds. Ann N Y Acad Sci. 957:260–270. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sato M, Maulik G, Ray PS, Bagchi D and Das
DK: Cardioprotective effects of grape seed proanthocyanidin against
ischemic reperfusion injury. J Mol Cell Cardiol. 31:1289–1297.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato M, Ray PS, Maulik G, Maulik N,
Engelman RM, Bertelli AA, Bertelli A and Das DK: Myocardial
protection with red wine extract. J Cardiovasc Pharmacol.
35:263–268. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Facino RM, Carini M, Aldini G, Berti F,
Rossoni G, Bombardelli E and Morazzoni P: Diet enriched with
procyanidins enhances antioxidant activity and reduces myocardial
post-ischaemic damage in rats. Life Sci. 64:627–642. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sivaprakasapillai B, Edirisinghe I,
Randolph J, Steinberg F and Kappagoda T: Effect of grape seed
extract on blood pressure in subjects with the metabolic syndrome.
Metabolism. 58:1743–1746. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujita K, Matsumura Y, Kita S, Miyazaki Y,
Hisaki K, Takaoka M and Morimoto S: Role of endothelin-1 and the
ETA receptor in the maintenance of deoxycorticosterone
acetate-salt-induced hypertension. Br J Pharmacol. 114:925–930.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsuoka H, Nishida H, Nomura G, Van Vliet
BN and Toshima H: Hypertension induced by nitric oxide synthesis
inhibition is renal nerve dependent. Hypertension. 23:971–975.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pollock DM, Polakowski JS, Divish BJ and
Opgenorth TJ: Angiotensin blockade reverses hypertension during
long-term nitric oxide synthase inhibition. Hypertension.
21:660–666. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qiu C, Engels K and Baylis C: Angiotensin
II and alpha 1-adrenergic tone in chronic nitric oxide
blockade-induced hypertension. Am J Physiol. 266:R1470–1476.
1994.PubMed/NCBI
|
|
27
|
Bujak M and Frangogiannis NG: The role of
TGF-beta signaling in myocardial infarction and cardiac remodeling.
Cardiovasc Res. 74:184–195. 2007. View Article : Google Scholar : PubMed/NCBI
|