Introduction
Meniscus has limited potential of self-repair, and
menisci injury may lead to long-term degenerative joint changes
(1,2). In addition to other therapies,
meniscal regeneration using tissue engineering techniques has been
attempted, based on the loading and culture of suitable cells into
appropriate scaffolds (3,4). With regards to the cell source,
meniscal chondrocytes, mesenchymal cells and pluripotential
fibroblasts have all been identified as possible sources for the
repair of meniscal tissue (5–7).
Compared with the cell sources mentioned above, myoblasts are a
promising source for meniscal engineering, as they are relatively
abundant and easily accessible with minimal donor site morbidity.
Myoblasts may also be good candidates for tissue engineering as
they have a higher cell yield and proliferate rapidly during in
vitro expansion (8).
With regards to the scaffold, a variety of natural
or artificial biomaterials have been investigated for use in
tissue-engineered meniscus. Due to the poor biomechanical
properties and the rapid degradation of fibrin and alginate,
polymer scaffolds with a stable, biodegradable and permeable pore
network were used to support cell attachment, proliferation and
nutrient exchange and to provide stability (5,9).
In this study, non-woven PLGA scaffolds were seeded
with myoblasts and transplanted into subcutaneous pockets of 24
combined immunodeficiency (SCID) mice. It was hypothesized that
such cell-seeded constructs would demonstrate a cartilage-like
morphology with expression of chondrocyte-specific molecules, while
also conserving sufficient biomechanical characteristics, after 12
weeks in vivo.
Materials and methods
Isolation, culture and induction of
myoblasts
Canine myoblasts were isolated and cultured as
previously described (10).
Primary cells were seeded on a culture dish at a density of
5×105 cells/cm2 in Dulbecco’s modified
Eagle’s medium (DMEM; Gibco-BRL, Grand Island, NY, USA) containing
10% fetal bovine serum (FBS; Gibco-BRL), 300 μg/ml of L-glutamine,
50 μg/ml vitamin C, 100 U/ml penicillin G, 100 μg/ml streptomycin
and amphotericin B 0.25 μg/ml (all from Sigma, St. Louis, MO, USA).
After medium change, cultured myoblasts were subjected to
chondrogenic induction with culture medium containing 50 ng/ml
CDMP-2 and 20 ng/ml TGF-β1 (Sigma). The myoblasts were cultured at
37°C in a humidified atmosphere of 95% air and 5% CO2.
The medium was changed every third day, and this washed out all
non-adherent cells. Cells were subcultured at a density of
1.0×104 cells/cm2 and treated with 0.25%
trypsin plus 0.02% EDTA (Gibco-BRL) when they reached 80%
confluence. The study was approved by the ethics committee of
Tongji University, Shanghai, China.
Immunocytochemistry assay of collagen
II
To determine the in vitro chondrogenic
induction effect, induced myoblasts were examined for type II
collagen expression using immunocytochemical staining. Briefly,
cells were incubated at 37°C for 1 h with mouse anti-collagen-II
monoclonal antibody (IgG1; BD Biosciences Clontech, Franklin Lakes,
NJ, USA) diluted in phosphate-buffered saline (PBS, 1:200),
followed by incubation with 1:100 diluted horseradish peroxidase
(HRP)-conjugated anti-mouse antibody (Dako, Carpinteria, CA, USA)
for 30 min and color development with diaminobenzidine
tetrahydrochloride (DAB). Normal menisci served as positive
controls.
Preparation of PLGA scaffold and cell
seeding
A non-woven copolymer scaffold of L-lactide and
glycolide (90/10, PLGA) in the form of fibers was generously
provided by Shanghai Ju Rui Biomaterials Co., Inc., (China). The
scaffolds were cylindrical, with a diameter of 10 mm and a
thickness of 2 mm. The pore sizes of the non-woven fibers were on
average 75 μm, the pore volume accounted for 97% of the total
volume, and the filament diameter was 13 μm. The PLGA constructs
were treated using the low-pressure plasma technique at the end of
the production process. A partially ionized gas reacted with the
surface of the scaffolds and formed reactive particles. Prior to
cell seeding, the scaffolds were immersed in DMEM/F12 medium
containing 10% (v/v) FBS for 12 h to enhance cell adhesion onto the
scaffold.
Chondrogenically induced myoblasts at passage 3
(1.5×107 in 0.3 ml) were harvested and placed onto PLGA
scaffolds, respectively, to form cell-scaffold constructs and the
constructs were cultured at 37°C in a humidified atmosphere of 5%
CO2 for 5 h, which allowed the complete adhesion of
myoblasts to the scaffold. The cell-PLGA constructs in inductive
media were subsequently cultured in vitro for 14 days.
Medium was changed three times a week. As an experimental control,
scaffolds with non-induced myoblasts were cultured for the same
lengths of time.
Surgical procedure
Six-week-old athymic nude mice were used in this
study, and were obtained from the Agricultural Institute of
Shanghai Jiaotong University, China. Animal care and experimental
procedures were in accordance with the guidelines of the
Administrative Panel on Laboratory Animal Care of China. Under
general intraperitoneal anesthesia and after disinfection of the
back of each of the 24 mice, two subcutaneous pockets were bluntly
created through a 1.5-cm incision in the back. A construct (induced
cell-PLGA construct or non-induced cell-PLGA construct control) was
inserted in each subcutaneous pocket. The wound was closed using a
single interrupted suture. No animal died during the experimental
period. The animals were sacrificed and the implants were harvested
at 8 and 12 weeks, respectively.
Histological and immunohistochemical
analyses
Eight and 12 weeks after implantation, the implants
were retrieved and analyzed histologically and
immunohistochemically, respectively. For histological analyses,
specimens were fixed in 10% (v/v) buffered formalin, dehydrated
with a series of graded alcohol and embedded in paraffin. Tissue
sections (4-μm thick) were stained with hematoxylin and eosin for
morphological analysis.
Expression of collagen type II was detected using
monoclonal antibodies (Dako). Briefly, after deparaffinization,
sections were predigested with trypsin at 37°C for 30 min to
facilitate antibody access, endogenous peroxidase was removed by
the treatment of 0.3% H2O2 in methanol at
room temperature for 30 min, and non-specific antibody binding was
blocked by incubation of sections in 10% normal goat serum at 37°C
for 30 min. Mouse anti-canine collagen type II diluted 1:100 in
0.01 M PBS (pH 7.4) was applied as a primary antibody at 4°C
overnight. Sections were then incubated with the secondary
antibody, rabbit anti-mouse immunoglobulin (Dako) for 60 min,
followed by application of mouse PAP kit (Dako). Collagen type II
was visualized by the reactions with 0.05% diaminobenzidine
containing 0.01% H2O2.
Analysis of mRNA for extracellular
matrices and collagen with reverse transcriptase-polymerase chain
reaction
RNA samples were obtained after 12 weeks and
transcribed into cDNA by real-time reverse transcriptase-polymerase
chain reaction (RT-PCR) for gene expression of type I collagen,
type II collagen and aggrecan. After explantation, the constructs
were digested with Ultraturax™ and total mRNA was prepared using
TRIzol reagent according to the manufacturer’s instructions
(Gibco-BRL). Total RNA (1 μg) was treated with 1 unit of
deoxyribonuclease I (DNase I; Gibco-BRL) in order to digest genomic
DNA contamination. Random-primed cDNA synthesis was performed using
1 μg of DNase I-treated total RNA and 50 units of StrataScript
reverse transcriptase according to the manufacturer’s instructions
(Stratagene, La Jolla, CA, USA). TaqMan™ PCR assays were performed
in 96-well optical plates on an ABI Prism 7700 Sequence Detection
system (Applied Biosystems, Forster City, CA, USA) using ABsolute
QPCR ROX Mix (Abgene, Hamburg, Germany) according to the
manufacturer’s instructions. The thermal cycling conditions were
95°C for 15 min followed by 40 cycles at 95°C for 15 sec and 60°C
for 1 min. The PCR products were analyzed by electrophoresis in 2%
agarose gels and stained with ethidium bromide. The mRNAs analyzed
were collagen I (681 bp), collagen II (447 bp), aggrecan (321 bp)
and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (211 bp).
Primer sequences for GAPDH, aggrecan, collagen I and collagen II
were as follows: GAPDH, sense 5′-CCTCTATGCCAACACAGTGC-3′ and
antisense 5′-GTACTCCTGCTTGCTGATCC-3′; aggrecan, sense 5′-TAG
AGAAGAAGAGGGGTTAGG-3′ and antisense 5′-AGCACT AGGAGCCAGGGTTAT-3′;
collagen I, sense 5′-ATGCCC AAGACTACCAGTGG-3′ and antisense
5′-TCCTGG AAGCTCTTCTCAGT-3′; collagen II, sense 5′-TTTCCC
AGGTCAAGATGGTC-3′ and antisense 5′-CTTCAGCAC CTGTCTCACCA-3′.
Biomechanical analysis of engineered
cartilage
Eight and 12 weeks after implantation, the
biomechanical properties of the engineered cartilage were tested by
measuring the compressive modulus. Briefly, the specimens were
harvested and trimmed to fit in a test chamber (5 mm diameter) of a
biomechanical analyzer (Instron, Canton, MA, USA). A constant
compressive strain rate of 1 mm/min was used until the maximal
force of 450 N was reached, and thus a force-displacement curve was
obtained. The compressive moduli of tested tissues were
automatically calculated by the machine and further verified by
manual calculation with the formula: ΔP/A × L/ΔL
(ΔP, the compressive force margin of the 2 points on the
linear segment of the load-displacement curve before the first
break point; ΔL, the displacement margin of the
corresponding two abovementioned points; A, the area of
tested tissue; L, the thickness of tested tissue). In
addition, the normal canine menisci were tested as a control. ANOVA
analysis was applied and P<0.05 was considered to indicate a
statistically significant result.
Glycosaminoglycan (GAG) quantification of
engineered cartilage
GAG quantitative analysis of 8- and 12-week
specimens was performed. Briefly, the specimens were minced and
triturated to prepare the protein solution. A series of reagents
was added step by step to ensure the specific binding of Alcian
blue and polysulfated molecules of GAGs in engineered cartilage.
All GAGs were precipitated specifically in guanidine-HCl by using a
low pH in combination with detergent and a high salt concentration.
The precipitate was dissolved in a mixture of guanidine-HCl and
propanol. For quantification, the absorbance was recorded in a
microplate reader with a 600-nm filter, and a linear standard curve
between 0.5 and 20 μg was generated by adding known amounts of
proteoglycans. The results were analyzed using the ANOVA test. A
P-value <0.05 was considered statistically significant. Since no
neocartilage was generated we did not analyse the quantitive GAG in
the control group. The positive control was also tested as a normal
meniscus.
Statistical analysis
The presence of type II collagen in the repaired
meniscus tissue from the different groups was noted and
statistically compared using the Fisher’s exact test. Compressive
modulus and GAG content in the different groups were analyzed with
ANOVA. For all evaluations, the level of statistical significance
was set at a probability value of <0.05.
Results
Morphological and immunohistochemical
analysis
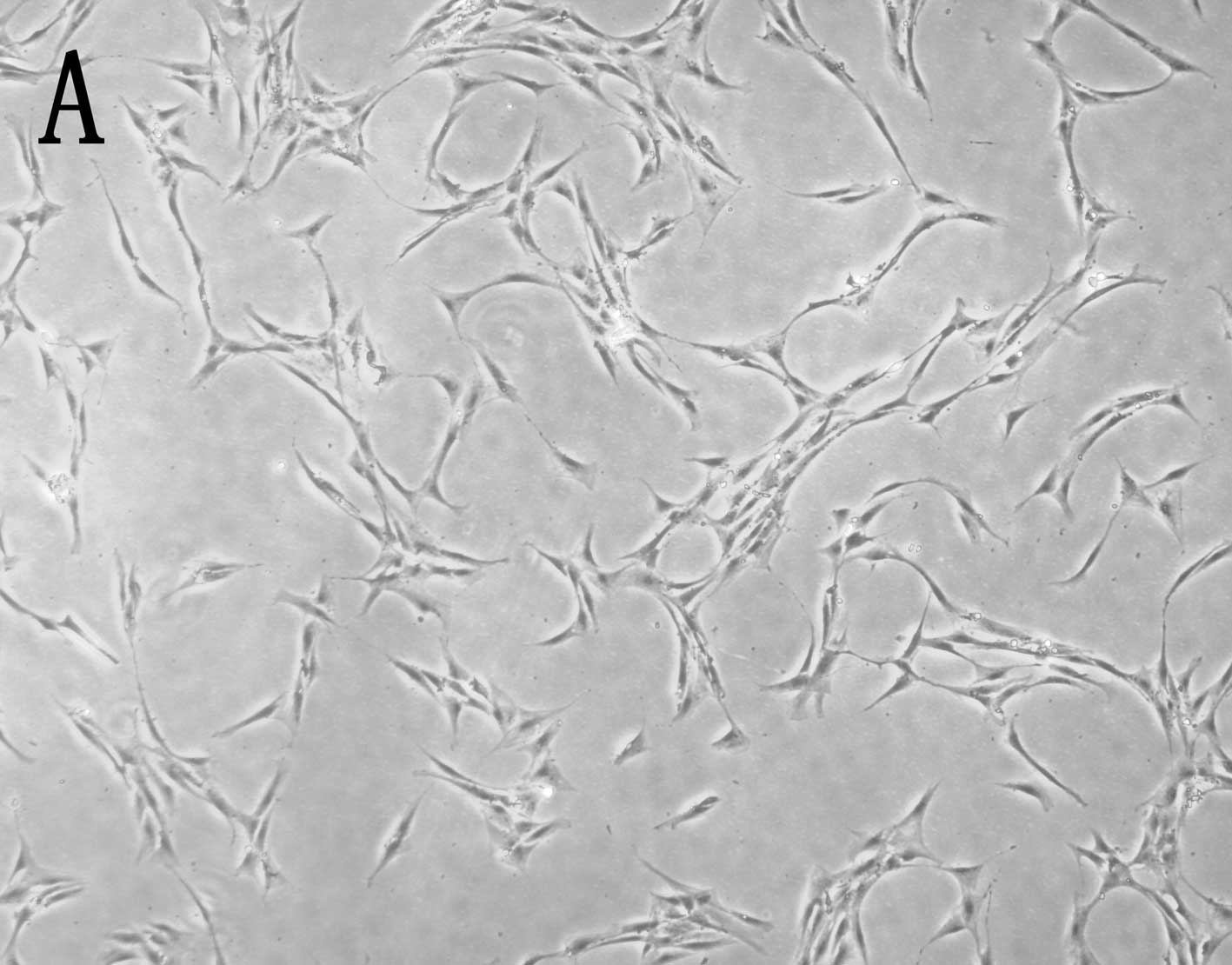
As shown in Fig. 1,
myoblasts induced with CDMP-2 and TGF-β1 underwent a morphological
change after chondrogenic induction, approaching the shape of
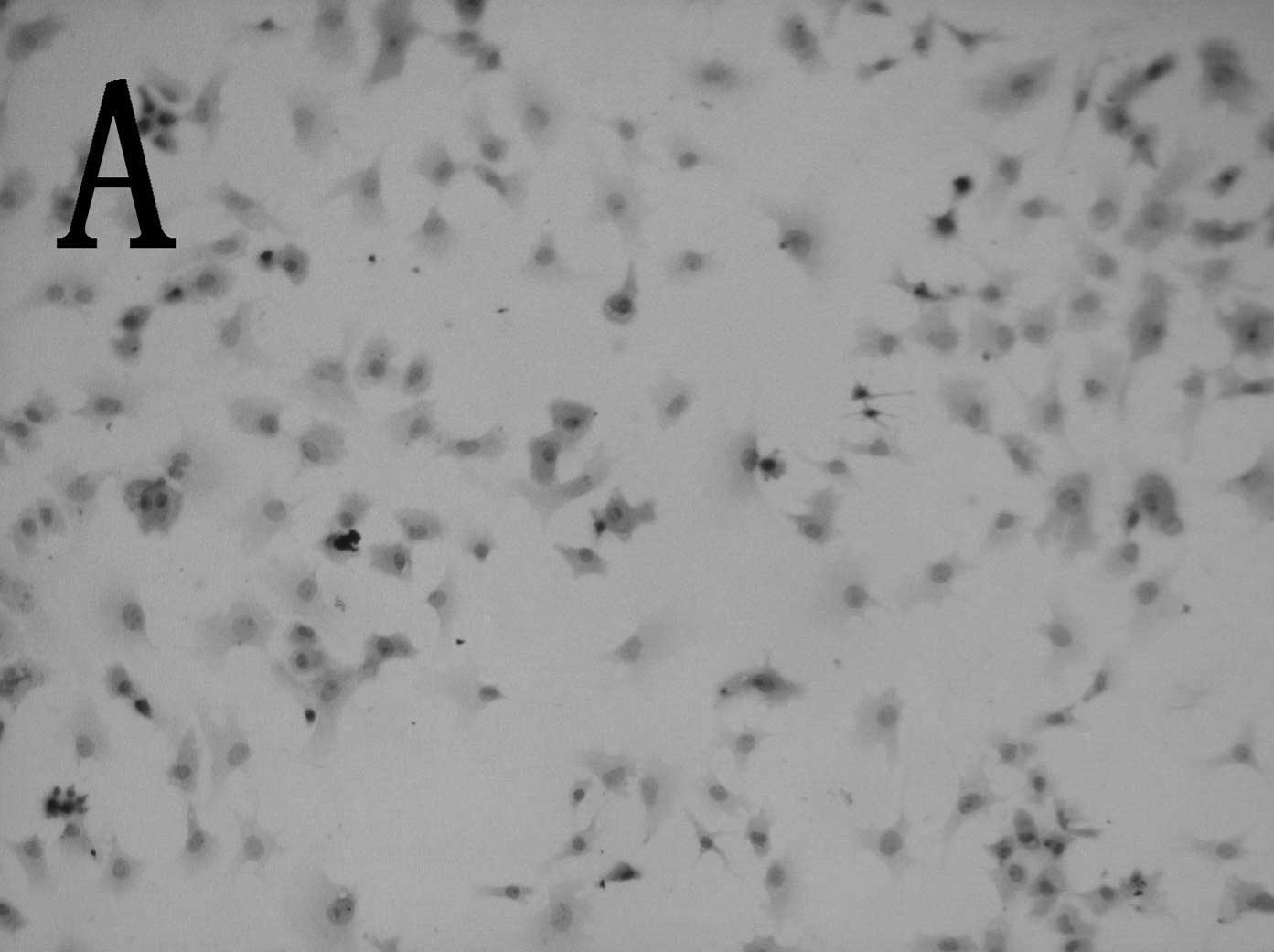
native chondrocytes. In addition, the induced cells showed
significantly enhanced collagen II expression compared to control
cells (Fig. 2).
Gross morphology of engineered
cartilage
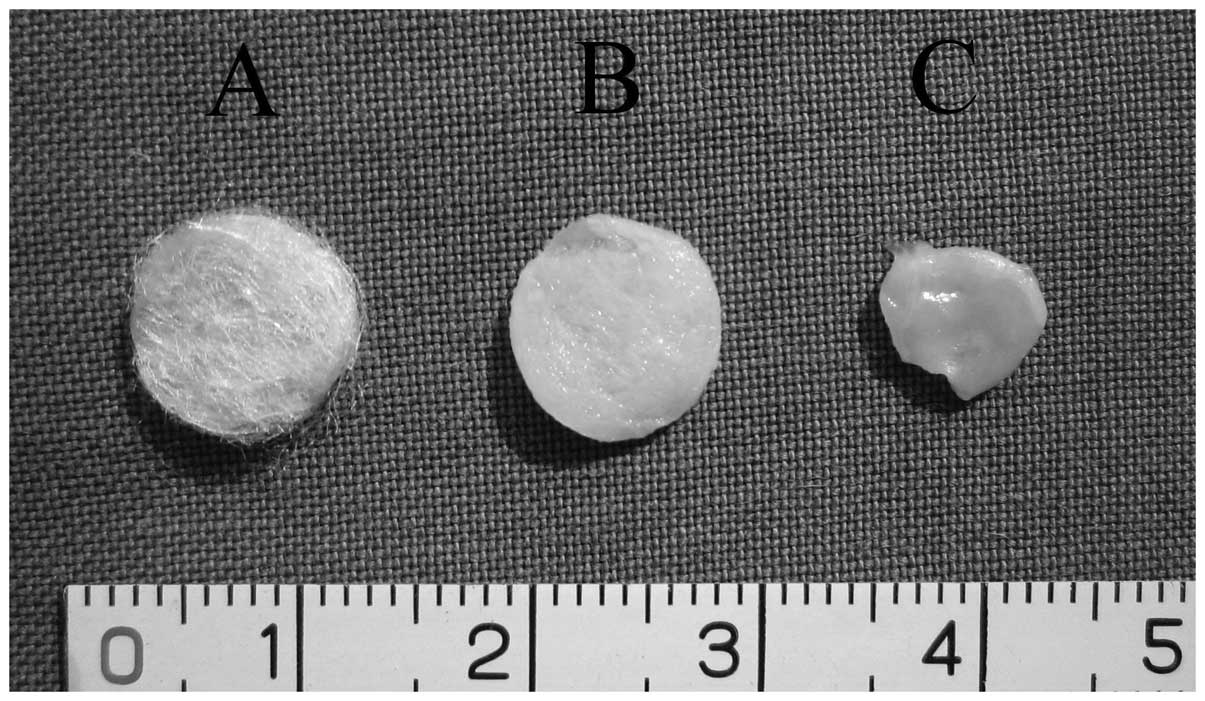
Specimens harvested after 8 weeks of in vivo
culture showed that all induced cell-PLGA constructs maintained the
approximate original scaffold shape and size, and had a compact
consistency (Fig. 3). The
non-induced cell-PLGA constructs showed a failure to maintain the
shape and size of the original scaffold. Twelve-week specimens
showed a more cartilaginous appearance. However, no construct was
identified in the control group, indicating that the scaffold was
degraded completely at 12 weeks.
Histological and immunohistochemical
assessment of engineered cartilage
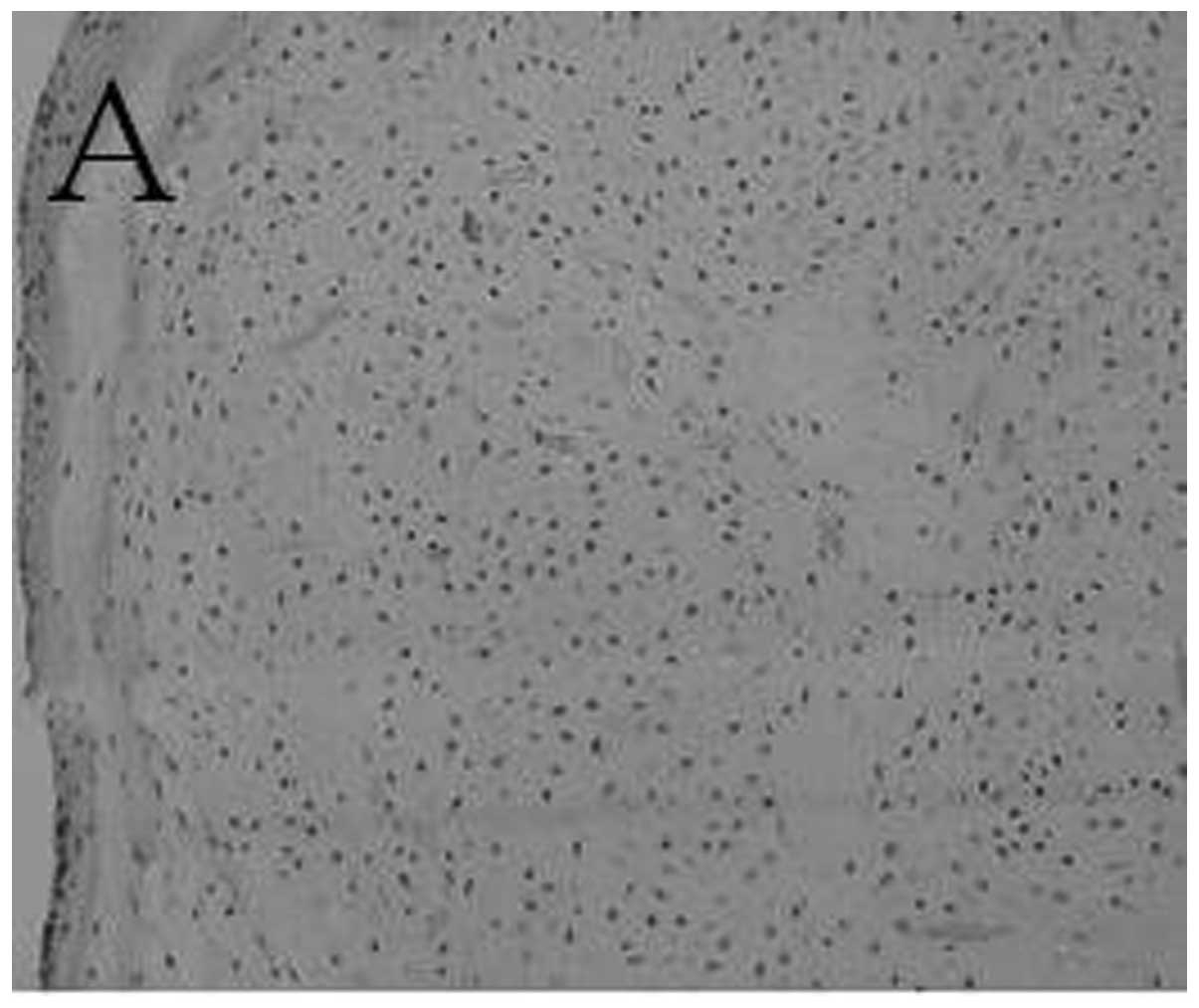
A histological section of engineered cartilage at 8
weeks had the appearance of fibrocartilage with
fibrochondrocytic-like cells (Fig.
4A). In the control group, only a band of fibrous tissue was
observed (Fig. 4B). The engineered
cartilage at 12 weeks had histological structures more similar to
those of normal cartilage than those at 8 weeks (Fig. 4C).
Immunohistological staining indicated that
engineered cartilage was positively stained for type II collagen at
8 weeks post-implantation (Fig.
5A). With the maturation of engineered cartilage, the
expression and distribution of collagen II was found to be similar
to that of the neighboring native meniscus at 12 weeks
post-implantation (Fig. 5C).
However, no expression of collagen II was observed in all specimens
of the control group at 8 weeks (Fig.
5B).
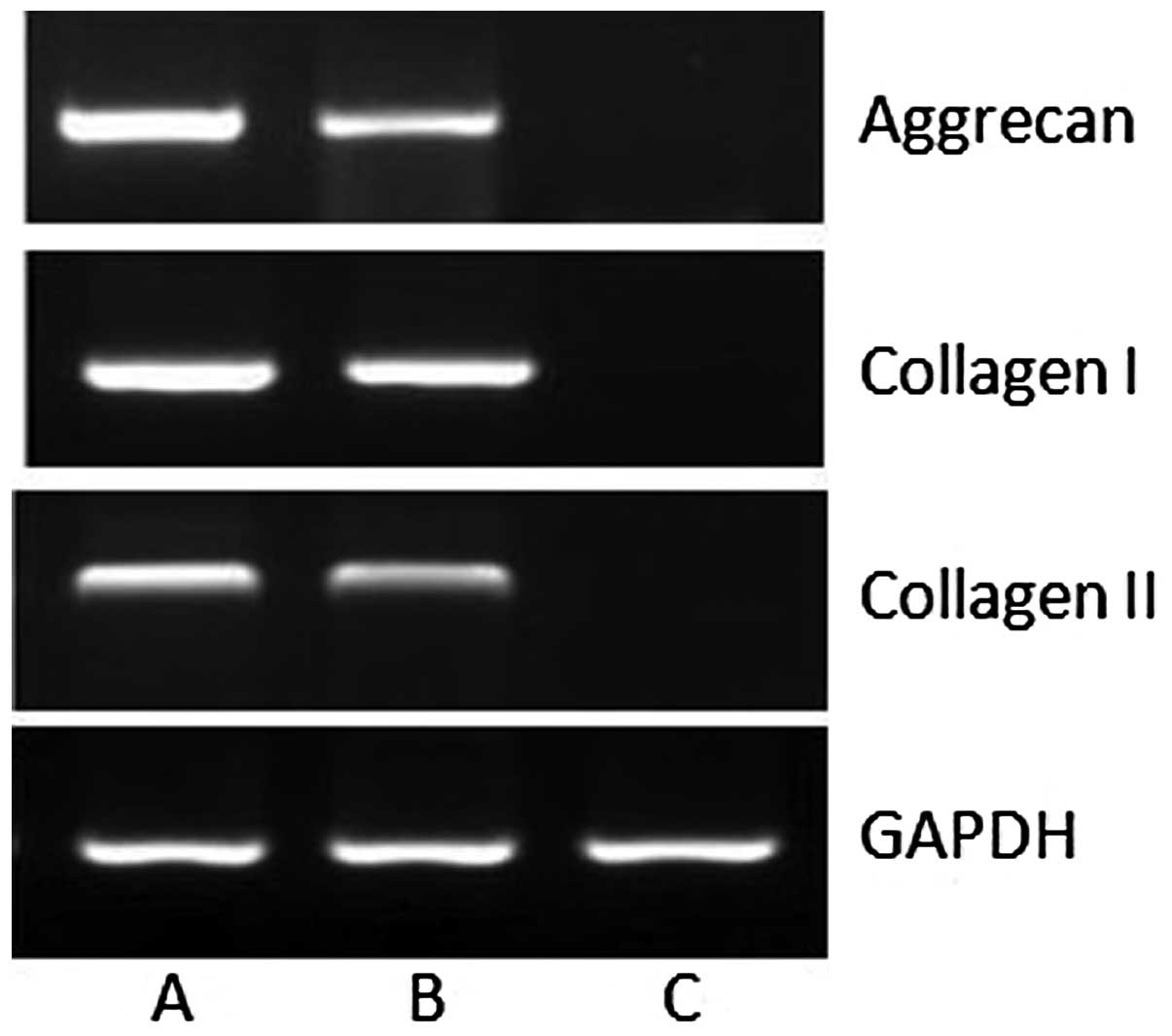
Expression of cartilage-specific genes by
RT-PCR
To investigate the chondrogenic differentiation of
the engineered cartilage, mRNA expression of type I collagen, type
II collagen and aggrecan was assessed using RT-PCR. At 12 weeks,
expression of mRNAs for type I collagen, type II collagen and
aggrecan was detected. By contrast, none of the assessed genes were
expressed in the control tissue (Fig.
6). Gene expression of the normal meniscus was assessed as a
positive control.
Biomechanical properties of engineered
cartilage
As summarized in Table
I, the compressive moduli increased with length of
transplantation time. At 8 weeks, the moduli reached 50.41 and
23.16% of the normal meniscus level, respectively, in the
experimental and control groups, and the experimental group
increased further to 85.72% at 12 weeks. Statistical analysis
demonstrated that the compressive modulus was higher in the induced
group (Exp) than in the non-induced group (Ctrl) (P<0.05),
indicating that in vitro chondrogenic induction is helpful
for improving biomechanical properties of myoblast-engineered
cartilage.
 | Table IThe compressive moduli of repaired
cartilage. |
Table I
The compressive moduli of repaired
cartilage.
| 8 weeks (n=12) | 12 weeks (n=12) |
|---|
|
|
|
|---|
| Groups | Moduli (MPa) | Percentage of normal
(%) | Moduli (MPa) | Percentage of normal
(%) |
|---|
| Normal | 27.12±0.69 | | 28.92±1.35 | |
| Exp | 13.67±1.830 | 50.41±2.93 | 24.79±2.78 | 85.72±4.23 |
| Ctrl | 6.28±1.52 | 23.16±2.49 | | |
GAG content quantification
The GAG deposition of the engineered cartilage was
further quantified by biochemical analysis and also compared with
that of the normal meniscus. As shown in Table II, it was found that the amount of
GAG in the experimental group at 8 weeks reached 47.39% of that in
the normal meniscus, as compared with 23.26% for the control group
(P<0.05). However, with the maturation of the engineered
cartilage to 12 weeks, GAG content was increased, reaching 91.35%
of that in the normal meniscus (P<0.05). A significant
difference was also observed between the amount of GAG in the
engineered cartilage at 8 and 12 weeks (P<0.05).
 | Table IIGAG contents (wet weight) of repaired
cartilage. |
Table II
GAG contents (wet weight) of repaired
cartilage.
| 8 weeks (n=12) | 12 weeks (n=12) |
|---|
|
|
|
|---|
| Groups | GAG content
(mg/g) | Percentage of normal
(%) | GAG content
(mg/g) | Percentage of normal
(%) |
|---|
| Normal | 12.07±0.37 | | 12.95±1.23 | |
| Exp | 5.72±0.87 | 47.39±2.37 | 11.83±3.05 | 91.35±5.27 |
| Ctrl | 2.81±1.03 | 23.26±4.76 | | |
Discussion
Meniscal injury is one of the most common injuries
to the knee. The menisci are important for normal knee function. A
meniscus injury increases the risk of subsequent development of
degenerative changes in the knee (11). Repairing meniscus injuries remains
a challenge in sports medicine, since meniscus usually does not
regenerate by itself following injury. The emergence of a tissue
engineering technique may provide a satisfactory solution to the
problem.
Tissue engineering is based on a unique combination
of exogenous cells, specific stimuli and matrix scaffold in an
in vitro or in vivo environment. In the present
study, myoblasts were used. Myoblasts, which are adult stem cells
and include skeletal muscle cell precursors, have been shown to
possess multipotential mesenchymal stem cell activity and are
capable of forming chondrocytes, osteocytes and adipocytes as well
as myocytes (12–14). Previous reports suggest that a
degree of plasticity remains prior to terminal myoblastic
differentiation. Therefore, myoblasts may prove useful for the
development of new therapeutic approaches aimed at the regeneration
of damaged or diseased tissues. Myoblasts have been investigated as
a candidate cell source for tissue engineering (15–17).
In comparison with other stem cell sources, myoblasts represent a
more promising source for cartilage engineering, as they are
relatively abundant and easily accessible, with minimal donor site
morbidity (18,19). Studies have shown that myoblasts
have a higher cell yield and more rapid proliferation ability
during in vitro expansion (8,20).
In this study, we observed a chondrogenic response with a dose of
50 ng/ml CDMP-2 and 20 ng/ml TGF-β1 provided continuously with each
medium change. Chondrogenic differentiation, analyzed using
immunohistochemistry and gene expression profiles, was observed.
This suggests that key events responsible for the commitment of
myoblasts to the chondrogenic lineage occur during the early
initial period of cell growth and proliferation.
PGA is a commonly used synthetic polymer in
cartilage tissue engineering. In order to maintain its dimensional
stability and enhance its mechanical properties, fibrous PGA meshes
are coated with solutions of PLA. Evaporation of the solvent for
PLA leads to the formation of PLGA composites with specific shapes.
The feasibility of using a PLGA composite as a scaffold to engineer
cartilage tissue has been documented in a number of studies
(21,22). It was also shown that adhesion and
proliferation of chondrocytes on PGA fibers was significantly
suppressed when an increased amount of PLA was added (21,22).
Therefore, the concentration of PLA solution to be added needs to
be lowered but still sufficient to function as glue to maintain the
structural stability of the PGA 3D scaffold. In the present study,
1.5% PLA in dichloromethane was used. Any further lowering would
cause an unstable configuration of the scaffold. Scanning electron
microscopy revealed that PLA at this concentration is capable of
wrapping PGA fibers together and that the shape of scaffold was
maintained when they were kept in culture medium for as long as 5
weeks (22).
The main advantage of using a PLGA scaffold for
meniscal tissue engineering is its suitable degradation rate, which
matches the kinetics of new meniscal formation in
vivo(5). The degradation of
non-woven PGA scaffold is reported to be complete over a period of
2 months in vivo(23). In
the present study, no undegraded PLGA fibers were observed
histologically in the experimental or control groups at 12 weeks
post-implantation. Due to its rapid degradation, PLGA scaffold was
found to accelerate chondrogenesis of constructs prepared from
dedifferentiated chondrocytes and PLGA, as the accumulation of the
deposited cartilage-specific extracellular matrix (ECM) and
expression of marker genes both in vitro and in vivo
were significantly enhanced compared with those of constructs
prepared from PGA. It was also proposed that early degradation of
PLGA fibers may have a positive effect on chondrogenesis by leaving
new spaces for cells to further fill in and produce new
intercellular matrix, which in turn may facilitate the formation of
more cell-matrix and cell-cell contact.
In this study, the specimens showed a cartilaginous
appearance. However, no construct was identified in all control
groups after 8 weeks of in vivo culture. As shown by the
histological analysis, the section of engineered cartilage had the
appearance of fibrocartilage with fibrochondrocytic-like cells at 8
and 12 weeks. In the control group, only a band of fibrous tissue
was observed. Immunohistological staining indicated that engineered
cartilage was positively stained for type II collagen compared with
the control group at 8 and 12 weeks post-implantation. At 12 weeks,
expression of mRNAs for type I collagen, type II collagen and
aggrecan was detected by RT-PCR. By contrast, no assessed genes
were expressed in the control tissue. In addition, the
biomechanical properties (compressive modulus) and biochemical
composition (GAG quantification) of engineered cartilage at 12
weeks post-implantation were similar to those of corresponding
normal cartilage, which indicated that the engineered cartilage was
formed both structurally and functionally.
In conclusion, we showed chondrogenic
differentiation of myoblasts seeded into PLGA scaffolds following
implantation in a subcutaneous pocket of nude mice. Further studies
are required to investigate implanted myoblast-seeded PLGA
scaffolds in a joint, including biomechanical stress for the
evaluation of a possible positive stimulus by mechanical loading
after implantation.
Acknowledgements
This study was supported by the Shanghai Natural
Science Foundation (Grant No. 09ZR1425500) and Research Grant for
Health Science and Technology of Pudong Health Bureau of Shanghai
(Grant No. PW2009B-2).
References
|
1
|
Hart R, Janecek M, Siska V, Kucera B and
Stipcák V: Correlation of long-term clinical and radiological
results after meniscectomies. Acta Chir Orthop Traumatol Cech.
72:304–307. 2005.PubMed/NCBI
|
|
2
|
van der Wal RJ, Thomassen BJ and van Arkel
ER: Long-term clinical outcome of open meniscal allograft
transplantation. Am J Sports Med. 37:2134–2139. 2009.PubMed/NCBI
|
|
3
|
Yamasaki T, Deie M, Shinomiya R, Yasunaga
Y, Yanada S and Ochi M: Transplantation of meniscus regenerated by
tissue engineering with a scaffold derived from a rat meniscus and
mesenchymal stromal cells derived from rat bone marrow. Artif
Organs. 32:519–524. 2008. View Article : Google Scholar
|
|
4
|
Webber RJ, York JL, Vanderschilden JL and
Hough AJ Jr: An organ culture model for assaying wound repair of
the fibrocartilaginous knee joint meniscus. Am J Sports Med.
17:393–400. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang SW, Son SM, Lee JS, Lee ES, Lee KY,
Park SG, Park JH and Kim BS: Regeneration of whole meniscus using
meniscal cells and polymer scaffolds in a rabbit total meniscectomy
model. J Biomed Mater Res A. 78:659–671. 2006. View Article : Google Scholar
|
|
6
|
Ishida K, Kuroda R, Miwa M, Tabata Y,
Hokugo A, Kawamoto T, Sasaki K, Doita M and Kurosaka M: The
regenerative effects of platelet-rich plasma on meniscal cells in
vitro and its in vivo application with biodegradable gelatin
hydrogel. Tissue Eng. 13:1103–1112. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peretti GM, Gill TJ, Xu JW, Randolph MA,
Morse KR and Zaleske DJ: Cell-based therapy for meniscal repair: a
large animal study. Am J Sports Med. 32:146–158. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu SH, Yang AH, Wei CF, Chiang HS and
Chancellor MB: Multi-potent differentiation of human purified
muscle-derived cells: potential for tissue regeneration. BJU Int.
105:1174–1180. 2009.PubMed/NCBI
|
|
9
|
Zhou G, Liu W, Cui L, Wang X, Liu T and
Cao Y: Repair of porcine articular osteochondral defects in
non-weightbearing areas with autologous bone marrow stromal cells.
Tissue Eng. 12:3209–3221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu W, Wang Y, Qiu G and Chen B:
Characterization of the purification and primary culture of adult
canine myoblasts in vitro. Mol Med Report. 3:463–468.
2010.PubMed/NCBI
|
|
11
|
Gu YL and Wang YB: Treatment of meniscal
injury: a current concept review. Chin J Traumatol. 13:370–376.
2010.PubMed/NCBI
|
|
12
|
Gu Y, Wang Y, Dai H, Lu L, Cheng Y and Zhu
W: Chondrogenic differentiation of canine myoblasts induced by
cartilage-derived morphogenetic protein-2 and transforming growth
factor-β1 in vitro. Mol Med Report. 5:767–772.
2012.PubMed/NCBI
|
|
13
|
Asakura A, Komaki M and Rudnicki M: Muscle
satellite cells are multipotential stem cells that exhibit
myogenic, osteogenic and adipogenic differentiation.
Differentiation. 68:245–253. 2001. View Article : Google Scholar
|
|
14
|
Matsushita T, Matsui N, Fujioka H, Kubo S,
Kuroda R, Kurosaka M and Yoshiya S: Expression of transcription
factor sox9 in rat L6 myoblastic cells. Connect Tissue Res.
45:164–173. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goldring K, Partridge T and Watt D: Muscle
stem cells. J Pathol. 197:457–467. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Day CS, Kasemkijwattana C, Menetrey J,
Floyd SS Jr, Booth D, Moreland MS, Fu FH and Huard J:
Myoblast-mediated gene transfer to the joint. J Orthop Res.
15:894–903. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koning M, Harmsen MC, van Luyn MJ and
Werker PM: Current opportunities and challenges in skeletal muscle
tissue engineering. J Tissue Eng Regen Med. 3:407–415. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh D, Nayak V and Kumar A:
Proliferation of myoblast skeletal cells on three-dimensional
supermacroporous cryogels. Int J Biol Sci. 6:371–381. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marsano A, Millward-Sadler SJ, Salter DM,
Adesida A, Hardingham T, Tognana E, Kon E, Chiari-Grisar C, Nehrer
S, Jakob M and Martin I: Differential cartilaginous tissue
formation by human synovial membrane, fat pad, meniscus cells and
articular chondrocytes. Osteoarthritis Cartilage. 15:48–58. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stern-Straeter J, Bran G, Riedel F, Sauter
A, Hörmann K and Goessler UR: Characterization of human myoblast
cultures for tissue engineering. Int J Mol Med. 21:49–56. 2008.
|
|
21
|
Moran JM, Pazzano D and Bonassar LJ:
Characterization of polylactic acid-polyglycolic acid composites
for cartilage tissue engineering. Tissue Eng. 9:63–70. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui L, Wu Y, Cen L, Zhou H, Yin S, Liu G,
Liu W and Cao Y: Repair of articular cartilage defect in non-weight
bearing areas using adipose derived stem cells loaded polyglycolic
acid mesh. Biomaterials. 30:2683–2693. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zwingmann J, Mehlhorn AT, Südkamp N, Stark
B, Dauner M and Schmal H: Chondrogenic differentiation of human
articular chondrocytes differs in biodegradable PGA/PLA scaffolds.
Tissue Eng. 13:2335–2343. 2007. View Article : Google Scholar : PubMed/NCBI
|