Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumors, with morbidity and mortality of HCC
ranking sixth and third among all types of cancer, respectively
(1). Every year 110,000
individuals succumb to liver cancer in China, accounting for 45% of
liver cancer mortalities worldwide. HCC incidence in developing
countries is ~5–10 times higher than in developed countries,
including Europe and the United States. Epidemiological studies
have identified that the rate of HCC incidence and mortality ranks
second in the malignant cancers (2). HCC is characterized by a high degree
of malignancy, rapid development, poor prognosis and high
mortality. Surgery is currently the most effective treatment for
HCC. However, metastasis and recurrence are commonly observed in
60–70% of patients following radical surgical resection. At
present, there are no reliable serum markers for the diagnosis of
HCC, particularly early diagnosis, thus resulting in late
identification, high malignancy, rapid development, low cure rate,
poor prognosis and high mortality. Therefore, the identification of
highly sensitive and specific markers for predicting HCC
metastasis, recurrence and prognosis is of great interest to
researchers in biology, pharmacy and medicine.
Glypican-3 (GPC3) is a member of the heparan sulfate
proteoglycan family of proteins and has a basic structure
consisting of a core protein, a heparan sulfate chain and
glycosylphosphatidylinositol (GPI). GPC3 binds to the
exocytoplasmic surface of the cell membrane via the GPI anchor and
regulates cell morphology, adhesion, proliferation, migration,
survival and differentiation by receiving signals from receptors on
the cell surface (3,4). Previously, a number of studies have
demonstrated that GPC3 is important in the occurrence and
development of HCC (3–7). The protein has been found to be
expressed at high levels even in early-stage HCC tissues. By
contrast, no expression of GPC3 was detected in the livers of
healthy adults (6). GPC3 is
involved in the regulation of a number of cell physiological and
pathological processes by interaction with various ligands and
receptors, including cell adhesion molecules, matrix components,
growth factors, enzymes and enzyme inhibitors (7). GPC3 may also be involved in
inhibition and regulation of the growth of the majority of
mesodermal tissues and organs, however, the underlying molecular
mechanisms remain unknown. Taken together, GPC3 is of significant
potential for development as a biochemical marker for diagnosis of
liver cancer.
During development of HCC, GPC3 may function as a
promoting factor for tumor growth (8). Studies have demonstrated that GPC3
affects HCC cell growth by crosstalk with other signaling pathways
closely associated with the occurrence and development of tumors,
including Wnt (8), Hedgehog
(9), fibroblast growth factor
(FGF) (10), insulin-like growth
factor (IGF) (11,12), SMAD (10) and transforming growth factor-β
(13). However, the conclusions of
previous studies are inconsistent. For example, specific studies
have reported that GPC3 promotes cancer cell growth (8,11,13).
By contrast, Midorikawa et al(10) demonstrated that GPC3 was
overexpressed in HepG2 cells, where it interacted with FGF2 and
thus inhibited cell proliferation. Kwack et al(14) also reported that forced expression
of GPC3 reduced the growth of HCC cells. The inconsistencies
between these studies may be explained by the diversity of various
HCC cell lines. Therefore, the role of GPC3 in the development of
HCC and the underlying molecular mechanisms requires further
clarified. The aim of the present study was to investigate the role
of GPC3 in the occurrence and development of HCC. GPC3 recombinant
vector was transfected into Huh7 and SK-HEP-1 cells to upregulate
the expression of GPC3 and its biological effects on the cells.
Moreover, the effects of IGF2 and FGF2 on apoptotic cell death
induced by GPC3 overexpression in HCC cells were also examined. The
results indicate that overexpression of GPC3 inhibits occurrence
and development of HCC. GPC3 may act as a negative regulator of
IGF2 and FGF2 pathways.
Materials and methods
Cell culture
HCC cell lines, Huh7 and SK-HEP-1, were obtained
from the China Center for Type Culture Collection (Wuhan, China)
and cultured in Dulbecco’s modified Eagle’s medium (Gibco-BRL,
Carlsbad, CA, USA) containing 10% fetal calf serum. Cells were
maintained at 37°C in an atmosphere of humidified air with 5%
CO2 in a cell culture incubator.
Construction of GPC3 overexpression
vector
RNA was extracted from HCC cells using the TRIzol
Assay kit (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer’s instructions, followed by reverse
transcription PCR to amplify the coding region of GPC3. Then, the
products were digested with KpnI and EcoRI (Takara
Bio, Inc., Shiga, Japan), cloned into pcDNA3.1 vectors, sequenced
and verified. The primers used for GPC3 amplification were GPC3-F
(5′-cggggtaccgccaccatggccgggaccgtgcgcac-3′) and GPC3-R
(5′-ccggaattctcagtgcaccaggaagaagaagcacacca-3′).
Transfection of plasmid
Cells were seeded into 6-well plates at
1×105 cells/ml and incubated for 24 h. Plasmid
transfection was performed using Lipofectamine 2000 (Invitrogen
Life Technologies) according to the manufacturer’s instructions,
when the cell confluence reached ~70%. The concentration of plasmid
for transfection was 4 μg/well. Following 72 h, transfected cells
were analyzed by real-time PCR and western blot analysis. For
growth factor response assays, following transfection with
GPC3-overexpression plasmid for 24 h, cells were incubated in
serum-free medium in the presence of 10 ng/ml FGF2 or IGF2 (R&D
Systems, Minneapolis, MN, USA) for 48 h. Cells were then subjected
to detection of cell apoptosis.
Fluorescence quantitative PCR
Total RNA was extracted from the cells using TRIzol
and cDNA was prepared using 1 μg RNA. Quantitative PCR was
performed using SYBR-Green PCR Master Mix (Toyobo Co., Inc., Osaka,
Japan), with 100 ng cDNA contained in a 20-μl reaction mixture.
Primer sequences are presented in Table I. The reaction included 1 cycle of
95°C for 5 min and 40 cycles of 95°C for 30 sec, 55°C for 30 sec
and 72°C for 30 sec. Three independent experiments were conducted
for each sample. Data were analyzed by comparing 2-ΔΔCt
values.
 | Table IPrimers for quantitative PCR. |
Table I
Primers for quantitative PCR.
| Gene | Primer
sequences | Product size
(bp) | Accession no. |
|---|
| GAPDH | F:
5′-GGTATCGTGGAAGGACTC-3′
R: 5′-GTAGAGGCAGGGATGATG-3′ | 128 | NM_002046 |
| GPC3 | F:
5′-GAGACTGCGGTGATGATGAAG-3′
R: 5′-TCGGAGTTGCCTGCTGAC-3′ | 147 | NM_001164617 |
Western blot analysis
Total cellular proteins were extracted by incubating
cells in lysis buffer. Protein concentrations in the cell lysates
were determined using the bicinchoninic acid assay (Pierce
Biotechnology, Inc., Rockford, IL, USA). SDS-PAGE was performed on
8% glycine gels (Bio-Rad, Hercules, CA, USA), loading equal
concentrations of protein per lane. Following electrophoresis,
separated proteins were transferred to nitrocellulose membranes and
blocked with 5% non-fat milk in TBST buffer for 1 h. Membranes were
incubated with GPC3 and GAPDH antibodies (1:1,000; Novus
Biologicals, LLC, Littleton, CO, USA) in 5% non-fat milk overnight
at 4°C and then anti-rabbit IgG monoclonal antibody conjugated with
horseradish peroxidase (1:2,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA) for 1 h at room temperature. Protein bands were
detected using the West Femto system (Pierce Biotechnology,
Inc.).
Annexin V-FLUOS apoptosis analysis
Cells were collected following transfection for 72 h
and the translocation of phosphatidylserine in treated cells was
detected using the Annexin V-FLUOS staining kit (Roche Diagnostics
GmbH, Mannheim, Germany). Briefly, cells were suspended in 500 μl
binding buffer and incubated at room temperature in the dark for 15
min following labeling with 5 μl Annexin V-fluorescein
isothiocyanate (FITC) and 5 μl propidium iodide. The stained cells
were then analyzed by flow cytometry.
EdU incorporation assay
EdU incorporation assay was performed using an EdU
Apollo DNA in vitro kit (Guangzhou RiboBio Co., Ltd.,
Guangzhou, China) following the manufacturer’s instructions.
Briefly, cells were incubated with 100 μl EdU (50 μM)/well for 2 h.
Following supernatant removal, cells were fixed for 15–30 min at
room temperature using 100 μl fixing buffer (4% polyformaldehyde
containing PBS). Following fixation, cells were incubated with 2
mg/ml glycine for 10 min followed by washing with PBS. The cells
were then permeated with 100 μl/well permeabilization buffer (0.5%
Triton X-100 containing PBS) and incubated with 100 μl
Apollo® Solution (1X) for 30 min at room temperature in
the dark. Subsequently, incubation with 100 μl Hoechst 33342
solution (1X) was conducted for 30 min at room temperature in the
dark. Samples were washed prior to observation under fluorescence
microscopy.
Transwell Matrigel Invasion assay
Invasion of cells was evaluated by Transwell
Matrigel Invasion assay. Briefly, 200 μl cells following
transfection (1×106 cells/ml) and 600 μl complete medium
were added to the upper and lower compartments of the chamber,
respectively. Following incubation for 48 h, cells migrating to the
lower side of the filter were fixed with 4% paraformaldehyde for 15
min at room temperature, washed with PBS, stained with crystal
violet and then observed under a confocal microscope.
Statistical analysis
Experiments were performed in at least triplicate
and results are expressed as mean ± SD. Statistical analysis was
performed using SPSS v13 (SPSS Inc., Chicago, IL, USA). P<0.05
or <0.01 were considered to indicate a statistically significant
difference.
Results
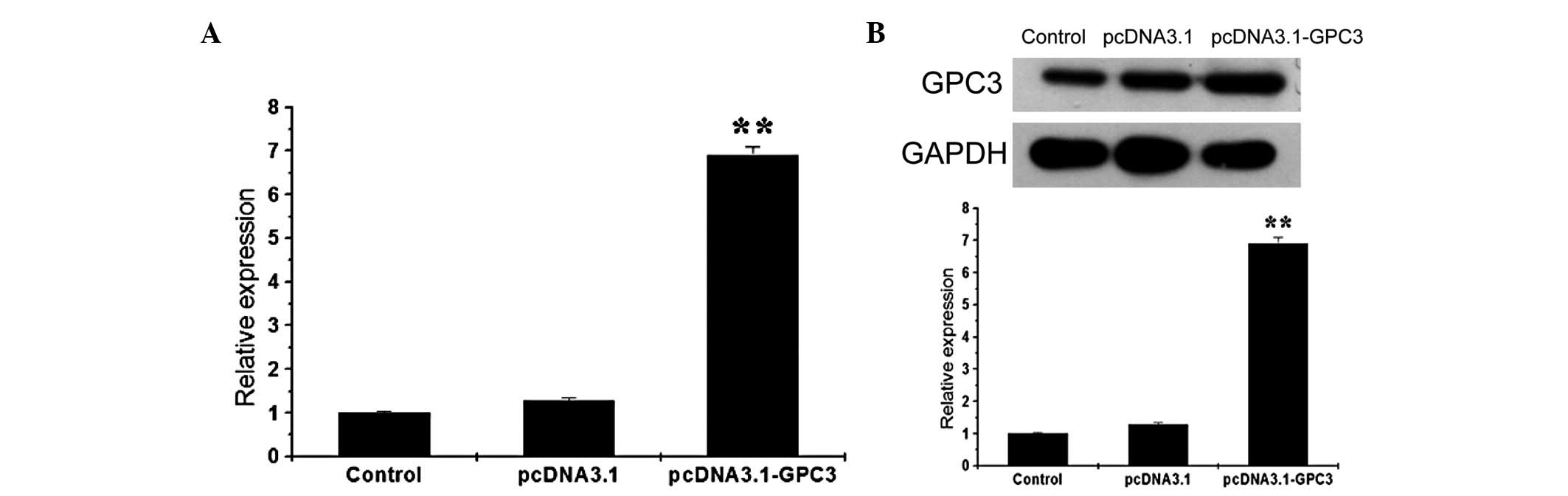
Overexpression of GPC3 in HCC cells
Following successful amplification of the coding
region of GPC3 by PCR, the product was double digested and cloned
into a pcDNA3.1 vector with KpnI and EcoRI, followed
by sequencing and verification. The GPC3 overexpression vector was
transfected into Huh7 cells. Following 72 h, cells were collected
and subjected to fluorescence quantitative PCR and western blot
analysis. The expression of GPC3 mRNA in Huh7 cells was found to be
significantly increased following transfection, which was ~7 times
that of the control group (Fig.
1). Consequently, GPC3 protein expression was also enhanced
following transfection. Similarly, GPC3 in SK-HEP-1 cells at mRNA
and protein levels was also increased following GPC3 overexpression
(data not shown).
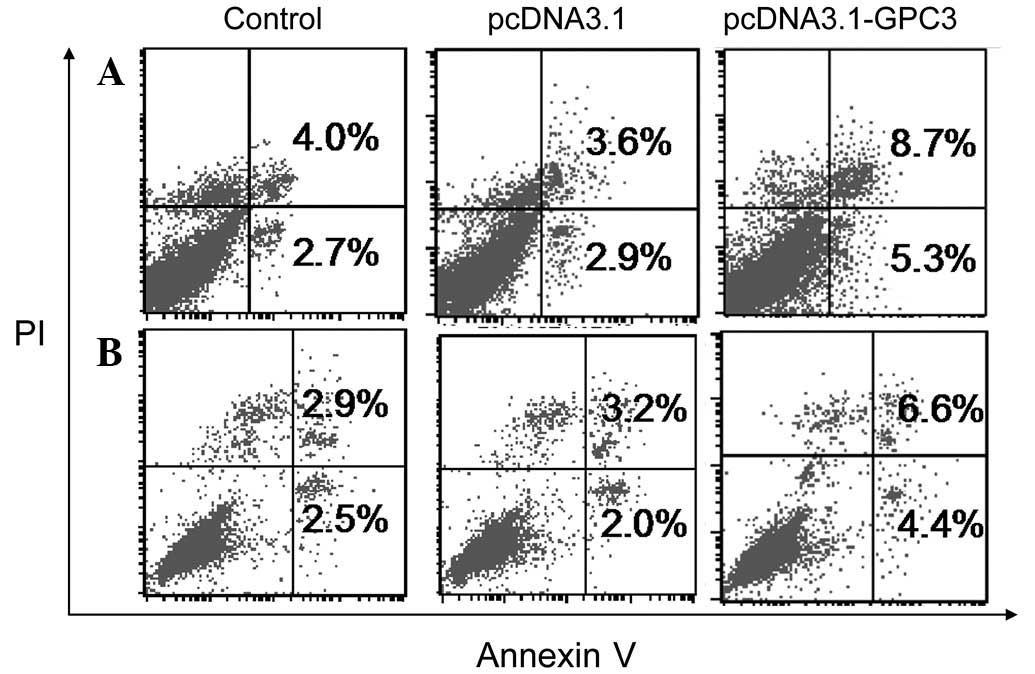
Effect of GPC3 on apoptosis of HCC cell
lines
Resistance to apoptosis is a common characteristic
of cancer cells. Therefore, the role of GPC3 in HCC development and
malignance was examined by evaluating the effect of GPC3
overexpression on HCC cell apoptosis as detected by Annexin V-FLUOS
flow cytometric analysis. As demonstrated in Fig. 2, overexpression of GPC3 was found
to significantly induce early and late apoptosis and total
apoptotic cell death in Huh7 and SK-HEP-1 cells. The different
levels of apoptosis observed in the two cell lines indicated that
the effects of GPC3 overexpression were cell-type specific. Taken
together, the results demonstrate that overexpression of GPC3
promotes apoptosis in HCC cells. Therefore, overexpression of GPC3
in liver cancer may inhibit the occurrence and development of
HCC.
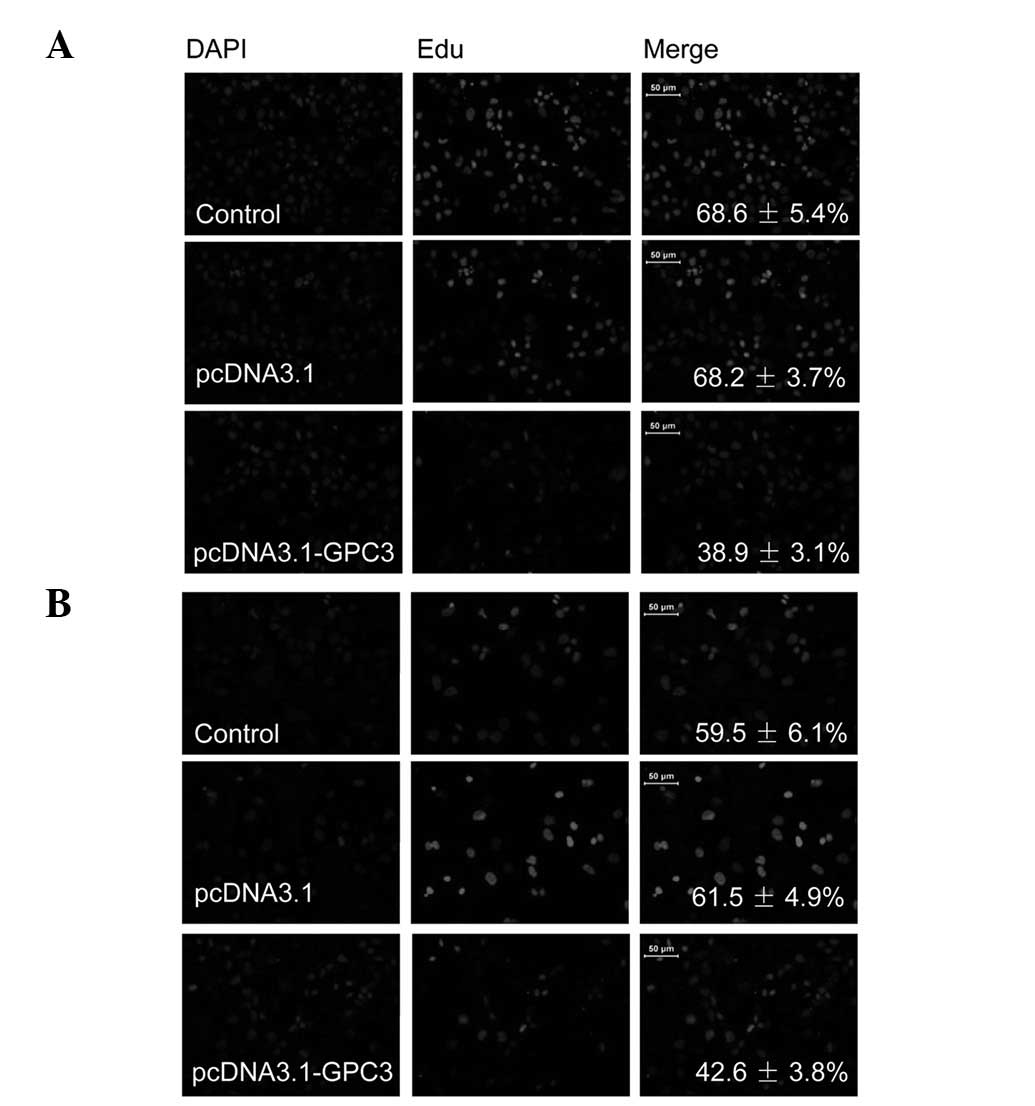
Role of GPC3 in HCC cell
proliferation
The EdU incorporation assay, a sensitive and
specific method, was employed to determine the effect of GPC3 on
HCC cell proliferation. As revealed in Fig. 3A, the number of EdU-positive Huh7
cells transfected with GPC3 overexpression vectors was
significantly reduced compared with those transfected with pcDNA3.1
blank vector. A similar reduction at lower levels was also observed
in SK-HEP-1 cells (Fig. 3B). These
results indicate that overexpression of GPC3 inhibits proliferation
of HCC cells.
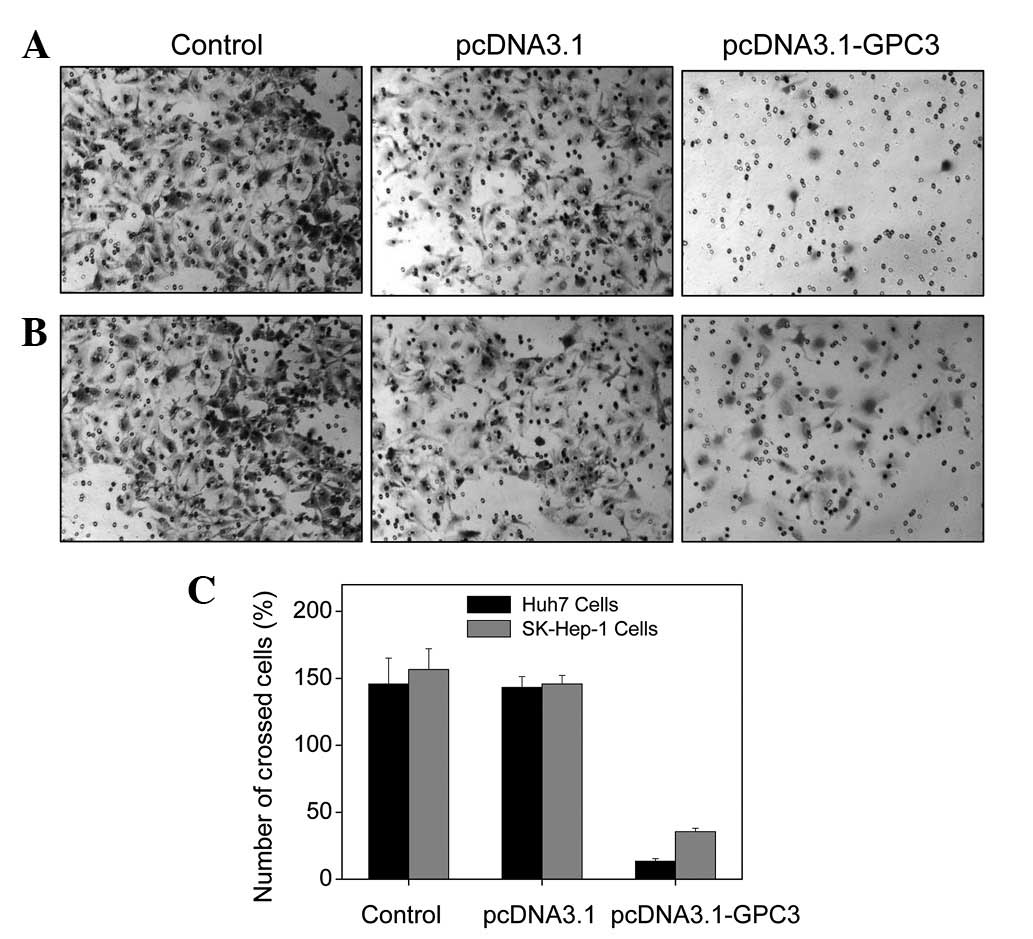
Effect of GPC3 expression on HCC cell
invasion
Malignant cancer cells may acquire the ability to
invade and metastasize. In the present study, the Transwell assay
was used to determine the effect of GPC3 expression on HCC cell
invasion. As demonstrated in Fig.
4A, invasion of Huh7 cells was significantly inhibited
following overexpression of GPC3. Similar reductions in cell
invasion at lower levels were also observed in SK-HEP-1 cells
(Fig. 4B). These results indicate
that GPC3 is a negative regulator of HCC cell invasion.
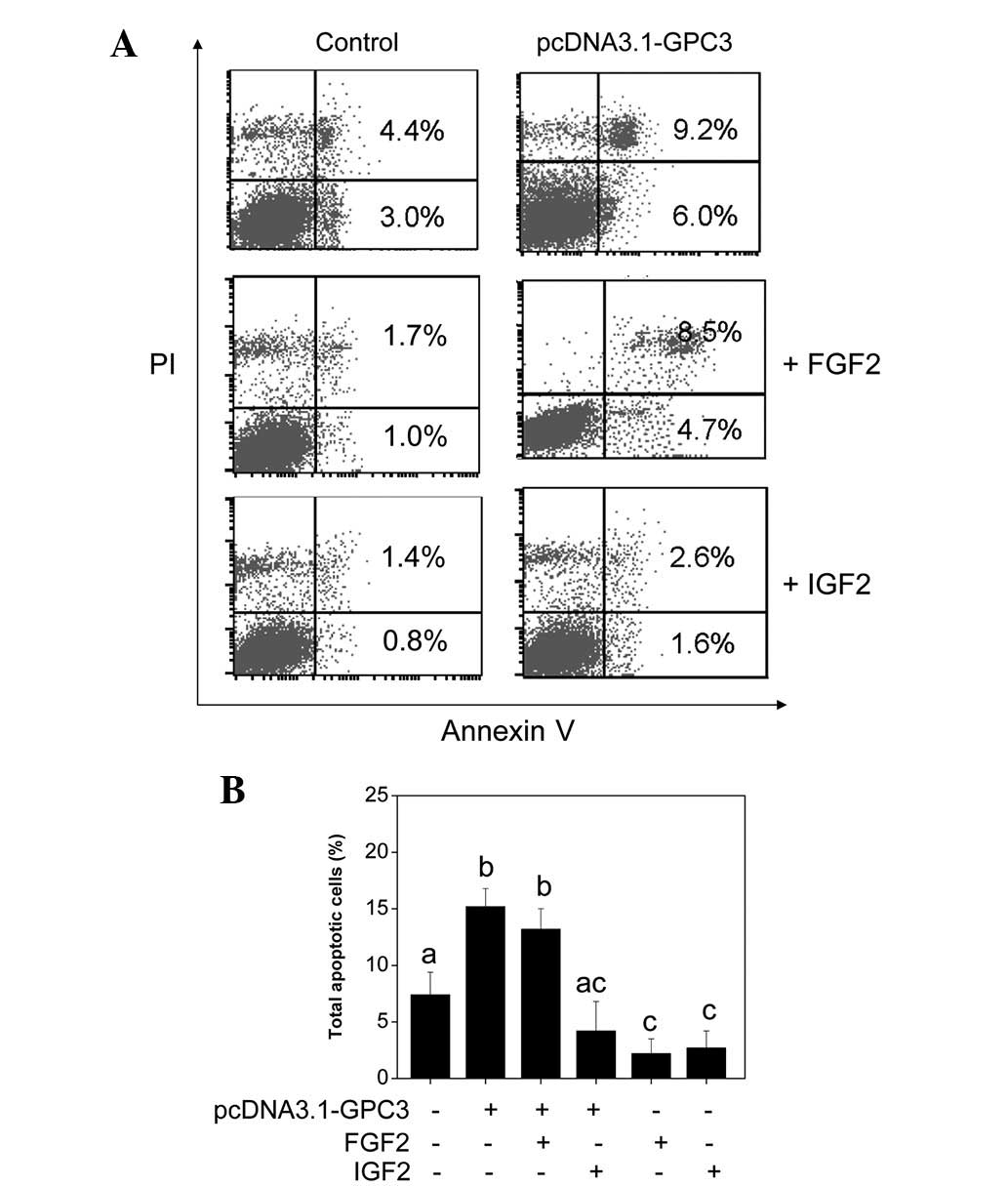
Effect of IGF2 and FGF2 on HCC cell
apoptosis induced by GPC3 overexpression
To further examine the crosstalk between GPC3 and
IGF2 and FGF2, growth factor response assays were conducted.
Following transfection with the GPC3-overexpression plasmid, cells
were subsequently incubated in serum-free medium in the presence of
10 ng/ml FGF2 or IGF2 for 48 h. Cells were then subjected to cell
apoptosis detection. As shown in Fig.
5A, the early and late phase apoptosis in Huh7 cells induced by
GPC3 overexpression was inhibited by cotreatment with FGF2 and IGF2
at various levels. The antagonistic effects of IGF2 were observed
to be significantly higher than that of FGF2. Results are
consistent with the hypothesis of crosstalk between GPC3 and IGF2
and FGF2 pathways. GPC3 may act as a negative regulator of the IGF2
and FGF2 pathways.
Discussion
At present, there are no reliable serum markers for
the diagnosis of HCC, particularly early diagnosis, thus resulting
in late identification, high malignancy, rapid development, low
cure rate, poor prognosis and high mortality. Therefore, the
identification of highly sensitive and specific markers for
prediction of HCC metastasis, recurrence and prognosis is an
important aim of a number of biological, pharmaceutical and medical
studies. Numerous studies have hypothesized that GPC3 is important
in the occurrence and development of HCC and is of significant
potential for development as a biochemical marker for diagnosis of
liver cancer (3–7).
To determine the effects of GPC3 in HCC, the GPC3
expression vector was transfected into Huh7 and SK-HEP-1 cells and
real-time PCR and western blot analysis were used to examine the
changes of GPC3 at mRNA and protein levels in these cell lines. A
number of previous studies have found that overexpression of GPC3
in HepG2 cells decreased cell apoptosis and increased cell
proliferation and invasion activity. By contrast, knockdown of GPC3
resulted in converse effects (8,11,13,15,16).
For example, knockdown of GPC3 in MHCC97-H cells using
GPC3-targeted shRNA resulted in the inhibition of cell
proliferation and invasion activity (15). Sun et al(13) revealed that transfection of Huh7
and HepG2 cells with GPC3-specific siRNA effectively inhibited cell
proliferation. Cheng et al(11) reported that NIH3T3 cells
transfected with a GPC3 expression vector revealed overexpression
of GPC3 and excessive cell proliferation. When PLC-PRF-5 HCC cells
with low GPC3 expression levels were transformed into cells
expressing high levels of GPC3, their growth rates were greatly
increased (11). In addition,
knockout of the GPC3 gene in Huh7 cells resulted in the inhibition
of growth, while GPC3 activated the IGF signaling pathway and
promoted growth of HCC cells (11). With respect to the underlying
mechanisms through which GPC3 activated HCC cell proliferation,
Capurro et al(8) indicated
that GPC3 increased the signaling molecules in the classical Wnt
pathway through autocrine/paracrine, while mutant GPC3 inhibited
the Wnt pathway and inhibited cancer cell growth. Kittaka et
al(16) demonstrated that GPC3
is an important protein in activation of the integrin signaling
pathway, promoting the proliferation and invasion activity of HCC
cells through associated signaling pathways.
In the present study, overexpression of GPC3 was
found to result in inhibitory effects on Huh7 and SK-HEP-1 cells.
Overexpression of GPC3 effectively increased cell apoptosis and
inhibited cell proliferation and invasion in the cell lines. These
results were consistent with previous reports (14,17,18).
Farooq et al(17) reported
that blocking endogenous GPC3 expression with an antisense
transcript promoted the growth of HepG2 and Hep3B hepatoma cells,
indicating that GPC3 is an inhibitor of cell proliferation. Sung
et al(18) revealed that
antisense-mediated knockdown of GPC3 in HepG2 cells significantly
promoted cell growth through pathways independent of IGF2.
Furthermore, Kwack et al(14) demonstrated that forced expression
of GPC3 effectively reduced HCC cell growth and FGF2-mediated cell
proliferation was inhibited by GPC3. In addition, the authors
reported that adhesion of HCC cells to collagen type I and
fibronectin was decreased by GPC3, whereas cell migration and
invasion were stimulated (14).
The growth inhibition of GPC3 overexpression on Huh7
and SK-HEP-1 HCC cells was similar to the action of GPC3 in healthy
tissues. Previous studies have demonstrated that GPC3 is important
for formation and development of tissue and organs during the
embryo and fetus periods. GPC3 largely functions as a negative
regulator of excessive growth of tissues and organs to regulate the
overall body size (19). Liu et
al(20) found that expression
of GPC3 increased 2 days after hepatectomy, peaking at day 5. When
expression levels of GPC3 peaked in healthy liver cells, cell
division and proliferation began to decline. Therefore, it was
concluded that GPC3 functioned as a negative regulator of liver
regeneration and hepatocyte proliferation with the involvement of
CD81. Further studies on GPC3 transgenic mice found that
overexpression of GPC3 suppressed hepatocyte proliferation and
liver regeneration (21). Lin
et al(22) also revealed
that hepatocyte-targeted overexpression of GPC3 in GPC3 trangenic
mice was important for regulation of liver size and termination of
hepatocyte proliferation induced by the xenobiotic mitogens
phenobarbital and TCPOBOP. Mutation and loss of function of GPC3
led to excessive growth and Simpson-Golabi-Behmel syndrome
(23), which is characterized by
excessive growth of the liver and other organs and increased risk
of cancer, particularly HCC and Wilms tumor (24). Zittermann et al(25) found that sGPC3-expressing cells
exhibited a lower proliferation rate. In addition, sGPC3 was
identified to significantly inhibit in vivo growth of Huh6,
HepG2 and Huh7 HCC cells. Moreover, this study revealed that sGPC3
blocked Wnt signaling in Huh6- and Huh7-derived tumors and Erk1/2
and Akt phosphorylation in tumors generated by Huh7 and HepG2
cells, respectively. Significant anti-angiogenic effects of GPC3 in
Huh7 and HepG2-derived tumors were observed. Therefore, the authors
concluded that sGPC3 inhibited HCC tumorigenicity by blocking the
activity of several pro-tumorigenic growth factors. The
observations of Feng et al(26) are consistent with this
conclusion.
FGFs are a family of broad-spectrum growth factors
affecting a plethora of cellular activities. The interaction of at
least 23 ligands, 4 receptors and multiple coreceptors provides a
significant complexity to a signaling system capable of effecting a
multitude of responses (27). FGF2
is produced by epithelial and cancer cells and is involved in
developmental processes and the regulation of differentiation,
proliferation and migration. FGF2 is a critical factor for
embryonic stem cell growth in culture without induction of
differentiation. Signaling cascades activated through FGF2 binding
to the FGF receptor include the ras-raf-MAPK, PLCγ/PKC and PI3K/AKT
pathways (28). IGF2 is a potent
cellular mitogen primarily produced by the liver and is frequently
overexpressed in tumors. IGF2 binds to the IGF-I receptor,
activating the AKT, mTOR, ERK and JNK pathways (29). Aberrant levels of IGF2 are
associated with Wilms tumor, Beckwith-Wiedmann syndrome and
colorectal cancer (29). Crosstalk
between GPC3 and FGF2 and IGF2 has also been reported in previous
studies (4,6,7,22–24,30).
Farooq et al reported that blocking endogenous GPC3
expression with an antisense transcript promoted the growth of
HepG2 and Hep3B cell lines (17).
Sung et al also reported that antisense-mediated knockdown
of GPC3 in HepG2 cells significantly promoted the growth of
hepatoma cells and this growth promotion was independent of the
IGF2 signaling pathway (18). In
addition, Kwack et al found that FGF2-mediated cell
proliferation was inhibited by GPC3 (14). However, in a breast cancer cell
model, Peters et al(30)
identified that GPC3 inhibited invasion and migration of the cancer
cells. Additional studies in ovarian, lung, mesothelioma and breast
cancer cell models also demonstrated that, overexpression of GPC3
inhibits cancer cell proliferation (31–34).
Furthermore, these studies revealed that addition of IGF2
suppressed the apoptosis-inducing effects of GPC3. By contrast,
FGF2 demonstrated reduced effects (4,6,7). In
the current study, growth factor response assays were conducted to
examine the crosstalk between GPC3 and IGF2 and FGF2 in HCC cells.
The results indicate that early and late phase apoptosis in Huh7
cells induced by GPC3 overexpression was inhibited by cotreatment
of FGF2 and IGF2 at various levels. The antagonistic effect of IGF2
was significantly higher than that of FGF2 (Fig. 5). These results are consistent with
those of previous studies, which confirmed the crosstalk between
GPC3 and IGF2 and FGF2 pathways. Taken together, these observations
indicate that GPC3 functions as a negative regulator of the IGF2
and FGF2 pathways.
In conclusion, HCC cells are different from human
hepatoma cells, thus, the actions of GPC3 may be cell-type
specific. The distinct effects of GPC3 on various HCC cells may be
attributed to the varied expression levels of GPC3. These results
were consistent with the hypothesis that GPC3 inhibits cell
proliferation and invasion through induction of apoptosis at
specific concentrations. Taken together, these results suggest that
overexpression of GPC3 inhibits the occurrence and development of
HCC.
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuen MF and Lai CL: Serological markers of
liver cancer. Best Pract Res Clin Gastroenterol. 19:91–99. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu H, Li P, Zhai Y, et al: Diagnostic
value of glypican-3 in serum and liver for primary hepatocellular
carcinoma. World J Gastroenterol. 16:4410–4415. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akutsu N, Yamamoto H, Sasaki S, et al:
Association of glypican-3 expression with growth signaling
molecules in hepatocellular carcinoma. World J Gastroenterol.
16:3521–3528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan B, Wei JJ, Qian YM, et al: Expression
and clinicopathologic significance of glypican 3 in hepatocellular
carcinoma. Ann Diagn Pathol. 15:162–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iglesias BV, Centeno G, Pascuccelli H, et
al: Expression pattern of glypican-3 (GPC3) during human embryonic
and fetal development. Histol Histopathol. 23:1333–1340.
2008.PubMed/NCBI
|
|
7
|
Hippo Y, Watanabe K, Watanabe A, et al:
Identification of soluble NH2-terminal fragment of glypican-3 as a
serological marker for early-stage hepatocellular carcinoma. Cancer
Res. 64:2418–2423. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Capurro MI, Xiang YY, Lobe C and Filmus J:
Glypican-3 promotes the growth of hepatocellular carcinoma by
stimulating canonical Wnt signaling. Cancer Res. 65:6245–6254.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Capurro MI, Xu P, Shi W, Li F, Jia A and
Filmus J: Glypican-3 inhibits Hedgehog signaling during development
by competing with patched for Hedgehog binding. Dev Cell.
14:700–711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Midorikawa Y, Ishikawa S, Iwanari H, et
al: Glypican-3, overexpressed in hepatocellular carcinoma,
modulates FGF2 and BMP-7 signaling. Int J Cancer. 103:455–465.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng W, Tseng CJ, Lin TT, et al:
Glypican-3-mediated oncogenesis involves the insulin-like growth
factor-signaling pathway. Carcinogenesis. 29:1319–1326. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakurai M, Shibata K, Umezu T, et al:
Growth-suppressing function of glypican-3 (GPC3) via insulin like
growth factor II (IGF-II) signaling pathway in ovarian clear cell
carcinoma cells. Gynecol Oncol. 119:332–336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun CK, Chua MS, He J and So SK:
Suppression of glypican 3 inhibits growth of hepatocellular
carcinoma cells through up-regulation of TGF-β2. Neoplasia.
13:735–747. 2011.PubMed/NCBI
|
|
14
|
Kwack MH, Choi BY and Sung YK: Cellular
changes resulting from forced expression of glypican-3 in
hepatocellular carcinoma cells. Mol Cells. 21:224–228.
2006.PubMed/NCBI
|
|
15
|
Ruan J, Liu F, Chen X, et al: Inhibition
of glypican-3 expression via RNA interference influences the growth
and invasive ability of the MHCC97-H human hepatocellular carcinoma
cell line. Int J Mol Med. 28:497–503. 2011.PubMed/NCBI
|
|
16
|
Kittaka N, Takemasa I, Takeda Y, et al:
Molecular mapping of human hepatocellular carcinoma provides deeper
biological insight from genomic data. Eur J Cancer. 44:885–897.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Farooq M, Hwang SY, Park MK, Kim JC, Kim
MK and Sung YK: Blocking endogenous glypican-3 expression releases
Hep 3B cells from G1 arrest. Mol Cells. 15:356–360. 2003.PubMed/NCBI
|
|
18
|
Sung YK, Hwang SY, Farooq M, Kim JC and
Kim MK: Growth promotion of HepG2 hepatoma cells by
antisense-mediated knockdown of glypican-3 is independent of
insulin-like growth factor 2 signaling. Exp Mol Med. 35:257–262.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oliver F, Christians JK, Liu X, et al:
Regulatory variation at glypican-3 underlies a major growth QTL in
mice. PLoS Biol. 3:e1352005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu B, Paranjpe S, Bowen WC, et al:
Investigation of the role of glypican 3 in liver regeneration and
hepatocyte proliferation. Am J Pathol. 175:717–724. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu B, Bell AW, Paranjpe S, et al:
Suppression of liver regeneration and hepatocyte proliferation in
hepatocyte-targeted glypican 3 transgenic mice. Hepatology.
52:1060–1067. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin CW, Mars WM, Paranjpe S, et al:
Hepatocyte proliferation and hepatomegaly induced by phenobarbital
and 1,4-bis [2-(3,5-dichloropyridyloxy)] benzene is suppressed in
hepatocyte-targeted glypican 3 transgenic mice. Hepatology.
54:620–630. 2011.PubMed/NCBI
|
|
23
|
Pilia G, Hughes-Benzie RM, MacKenzie A, et
al: Mutations in GPC3, a glypican gene, cause the
Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet. 12:241–247.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lapunzina P: Risk of tumorigenesis in
overgrowth syndromes: a comprehensive review. Am J Med Genet C
Semin Med Genet. 137C:53–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zittermann SI, Capurro MI, Shi W and
Filmus J: Soluble glypican 3 inhibits the growth of hepatocellular
carcinoma in vitro and in vivo. Int J Cancer. 126:1291–1301.
2010.PubMed/NCBI
|
|
26
|
Feng M, Kim H, Phung Y and Ho M:
Recombinant soluble glypican 3 protein inhibits the growth of
hepatocellular carcinoma in vitro. Int J Cancer. 128:2246–2247.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bansal R: Fibroblast growth factors and
their receptors in oligodendrocyte development: implications for
demyelination and remyelination. Dev Neurosci. 24:35–46. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Acevedo VD, Ittmann M and Spencer DM:
Paths of FGFR-driven tumorigenesis. Cell Cycle. 8:580–588. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pollak M: Insulin and insulin-like growth
factor signalling in neoplasia. Nat Rev Cancer. 8:915–928. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peters MG, Farías E, Colombo L, Filmus J,
Puricelli L and Bal de Kier Joffé E: Inhibition of invasion and
metastasis by glypican-3 in a syngeneic breast cancer model. Breast
Cancer Res Treat. 80:221–232. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin H, Huber R, Schlessinger D and Morin
PJ: Frequent silencing of the GPC3 gene in ovarian cancer cell
lines. Cancer Res. 59:807–810. 1999.PubMed/NCBI
|
|
32
|
Kim H, Xu GL, Borczuk AC, et al: The
heparan sulfate proteoglycan GPC3 is a potential lung tumor
suppressor. Am J Respir Cell Mol Biol. 29:694–701. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Murthy SS, Shen T, De Rienzo A, et al:
Expression of GPC3, an X-linked recessive overgrowth gene, is
silenced in malignant mesothelioma. Oncogene. 19:410–416. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiang YY, Ladeda V and Filmus J:
Glypican-3 expression is silenced in human breast cancer. Oncogene.
20:7408–7412. 2001. View Article : Google Scholar : PubMed/NCBI
|