Introduction
The lung is a radiosensitive organ, and the
radiotherapy of cancer in the thoracic region is able to cause
radiation-induced lung injury (RILI), sometimes resulting in
mortality (1). Protection of the
lungs against irradiation renders radiation therapy safer for
patients with risk factors for RILI and may enable the delivery of
more or greater radiation doses to tumors, to improve clinical
outcomes.
Neutrophil elastase (NE), a serine protease present
in the azurophil granules of neutrophils, is released in an
inflammatory state and disintegrates extracellular matrices due to
its low substrate specificity, resulting in tissue injury (2,3).
Sivelestat [sodium N-[2-[4-(2,2-dimethethyl-propionyloxy)
phenylsulfonylamino] benzoyl] aminoacetate tetrahydrate (ONO-5046
Na); Elaspol®; Ono Pharmaceutical, Co., Osaka, Japan] is
a synthetic human neutrophil elastase inhibitor. It has a
significantly small molecular weight of 528.5 Da and a short
half-life of ~2 h in the human body (4).
We previously reported that irradiation to bilateral
whole lungs of mice caused an elevation of the plasma NE activity
and that sivelestat decreased this activity (5), although a single administration of
sivelestat failed to maintain the low level of NE activity until 48
h after irradiation. Since sivelestat has a short half-life and
irradiation induces an immediate release of cytokines that activate
neutrophil migration and the release of NE, it was thought that
sivelestat should be administered twice or more as early as
possible after irradiation. In our histopathological examination,
hematoxylin and eosin (H&E) staining showed some mild
histopathological changes, while a large percentage of the lung
area seemed to be the same in the irradiated groups with or without
sivelestat compared to the non-irradiated group. Thus, it was
unclear whether sivelestat contributed to the mitigation of the
lung injury. The aim of the present study was to investigate the
efficacy of immediate and multiple administrations of sivelestat by
measuring NE activity and by evaluating the histopathological
changes in the irradiated lungs in the acute and scarred
phases.
Materials and methods
Animals
This experiment was conducted with the approval of
the Experimental Animal Ethics Committee at Osaka Medical College.
Forty-week-old female C57BL/6J mice were used. The animals were
kept in an air-conditioned room at 23±2°C and 55±10% humidity in a
12-h light/dark cycle with food and water ad libitum.
Irradiation and injection of drugs
For the irradiation, each mouse was fixed on an
exclusive jig under pentobarbital anesthesia. A dose of 20 Gy was
delivered with a 4-MV photon beam to the bilateral whole lungs in a
single fraction via a posterior field with a 5-mm bolus. Sivelestat
(provided by Ono Pharmaceutical, Co., Osaka, Japan) was dissolved
in physiological saline and adjusted to pH 7.8 with
Na2CO3. It was administered through an
intraperitoneal injection at a dose of 30 mg/kg as follows: group
RE2, immediately before and 1 h after the irradiation; group RE4,
immediately before and 1, 3 and 6 h after irradiation (Table I). The mice in group R were
administered irradiation only, without sivelestat injection. Group
C were the controls, mice that were administered neither sivelestat
injection nor irradiation.
 | Table IStratification of mice. |
Table I
Stratification of mice.
| Time before and after
irradiation | Group R | Group RE2 | Group RE4 |
|---|
| Just before | PS | S | S |
| 1 h | PS | S | S |
| 3 h | PS | PS | S |
| 6 h | PS | PS | S |
| 24 h | Measurement of NE
activity (blood plasma) and extirpation of the lungs |
| 48 h | Measurement of NE
activity (blood plasma) and extirpation of the lungs |
| 15 weeks | Extirpation of the
lungs |
Measurement of plasma NE activity
NE activity was measured 24 and 48 h after
irradiation. Blood samples were obtained via cardiopuncture from
the mice under pentobarbital anesthesia. After obtaining the
supernatant by centrifuging blood (1,700 × g, 10 min, 4°C), which
was immediately followed by freezing for preservation at −20°C, the
NE activity level was measured in the blood plasma via absorption
spectroscopy, using a specific synthetic substrate,
N-methoxysuccinyl-Ala-Pro-Val (pNA) for NE (6). Blood plasma was incubated in 0.1 M
Tris-HCl buffer solution (pH 8.0) containing 0.5 M NaCl and 1 mM
substrate for 24 h at 37°C. The absorbance of free pNA at 405 nm
was measured on a microplate reader.
Histopathological examination of the
lungs
The mice were sacrificed at 24 h, 48 h and 15 weeks
after irradiation. The lungs were extirpated and fixed in
neutral-buffered formalin, and then embedded in paraffin. Paraffin
sections were stained with H&E, silver impregnation and the
Elastica van Gieson (EvG) method.
The number of cells were counted in five randomly
selected non-overlapping fields at a magnification of ×400 from
each H&E-stained section of the individual lungs of the mice
sacrificed at 24 h, 48 h and 15 weeks after irradiation.
To evaluate the degree of lung injury, 10
non-overlapping fields at a magnification of ×400 were randomly
selected from each section stained with silver impregnation of the
individual lungs of the mice sacrificed 15 weeks after irradiation.
One observer evaluated the thickening and tearing of basement
membrane, thickening of interstitium and the disordered structure
of pulmonary alveoli, and the lung injury was graded as mild (1
point), moderate (2 points) or severe (3 points).
Statistical analysis
The Wilcoxon rank sum test was used to analyze
differences between the groups, using JMP software, version 8.0.2
(SAS Institute, Cary, NC, USA). Probability values <0.05 were
considered to indicate a statistically significant difference.
Results
In this study, 20 mice for each group (R, RE2 and
RE4) were used. In each group, 5 mice were sacrificed at 24 h, 5 at
48 h, and 10 at 15 weeks after irradiation to measure NE activity
and observe histopathological changes. One mouse in group RE4 at 24
h, 2 mice in group RE2 at 48 h and 2 mice in group RE4 at 48 h were
lost for reasons not related to the radiation injury. One mouse in
group R was lost probably due to radiation injury at 12 weeks after
irradiation.
Plasma NE activity
Lung irradiation induced elevation of NE activity at
24 and 48 h after irradiation. Compared to group R, the NE
activities in groups RE2 and RE4 were significantly suppressed at
24 and 48 h after the irradiation. However, there was no
significant difference between group RE2 and group RE4 in the
suppression of NE activity (Fig.
1).
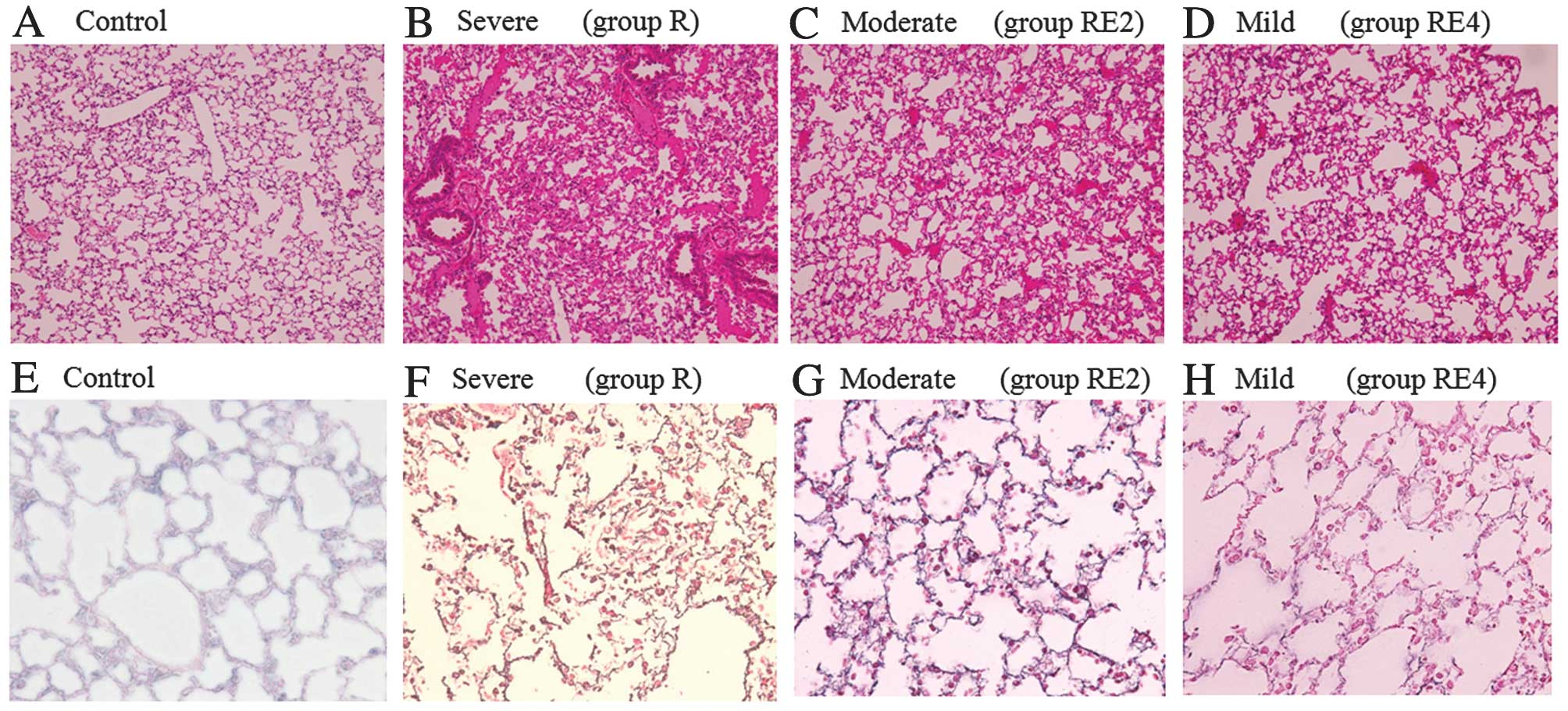
Histopathological examination of the
lungs
In the irradiated lungs (groups R, RE2 and RE4),
there were some small atelectatic foci and thrombi in the vessels.
However, a large percentage of the lungs appeared to be the same as
that of the control mice at 24 and 48 h. At 15 weeks, thickening of
the interstitium, a disordered structure of pulmonary alveoli and
thickening and tearing of basement membrane in the irradiated lungs
were observed by H&E staining or silver impregnation; these
findings were more severe in group R compared to groups RE2 and RE4
(Fig. 2). There were no apparent
fibrotic or elastofibrotic foci when EvG staining was used at 15
weeks in all the groups.
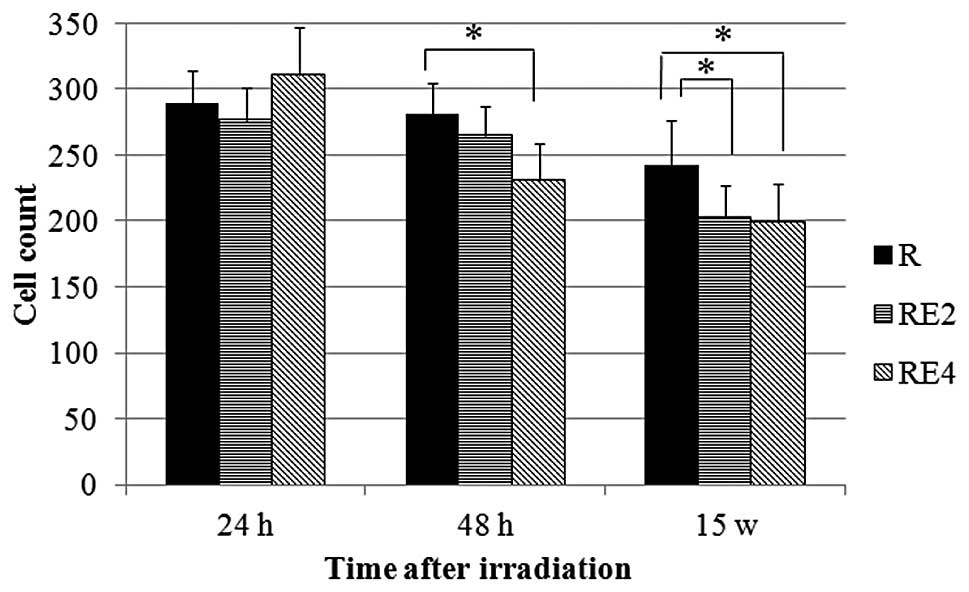
Fig. 3 shows the
cell count results. The cell counts in groups R, RE2 and RE4 showed
a tendency to decrease with time. Compared to group R, there were
significant decreases in the cell counts of group RE2 at 15 weeks
and group RE4 at 48 h and 15 weeks.
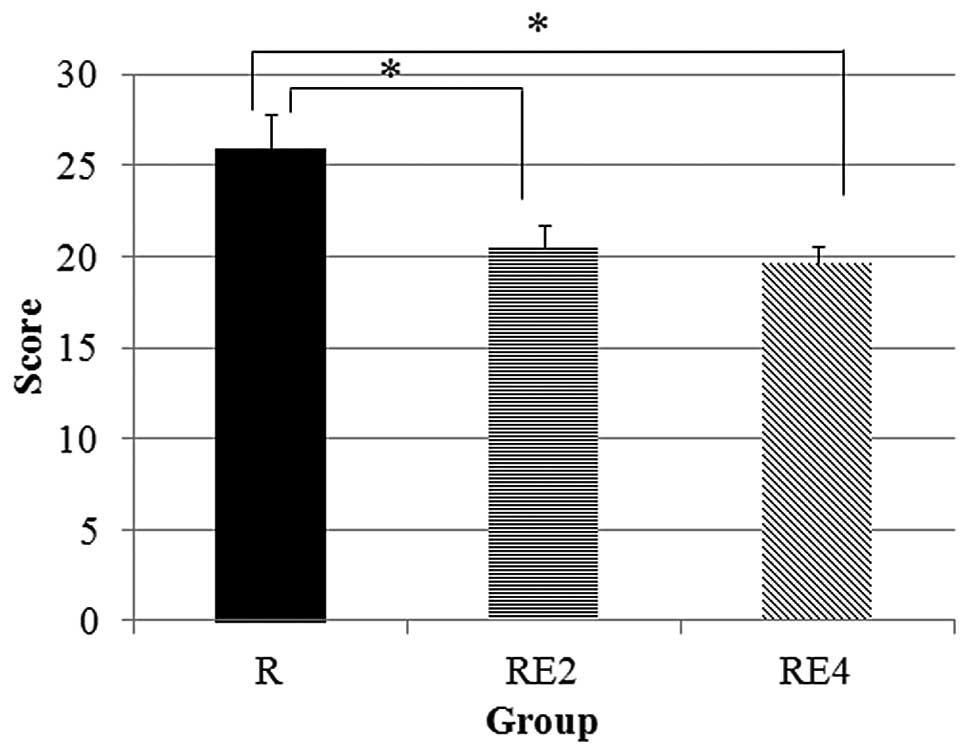
The lung injury scores are shown in Fig. 4. In groups RE2 and RE4, the lung
injury scores were significantly lower compared to those in group
R.
Discussion
Early histopathologic findings after radiation
therapy are characterized by damaged endothelial cells and
pneumocytes (7,8). Endothelial cell injury increases
vascular permeability and induces perivascular edema, congestion
and an infiltration of inflammatory cells such as neutrophils and
macrophages. Vessel thrombosis and intra-alveolar hemorrhage may
also occur. Shedding of type I pneumocytes and depletion of
surfactant secretions from type II pneumocytes are observed
immediately after irradiation, which induces the loss of pulmonary
functions. In the scarred phase, the proliferation of type II
pneumocytes is accelerated to repopulate the alveolar epithelium,
and alveolar septae are more hypercellular with fibroblasts and
macrophages (7).
The present study has shown that the cell counts in
the sivelestat-treated groups (RE2 and RE4) at 15 weeks were lower
compared to those in the no-sivelestat group R (Fig. 3). When sivelestat suppressed acute
inflammation in the irradiated lungs by blocking NE, the degree of
lung injury and subsequent repair of lung structures by various
cells such as type II pneumocytes were more mild compared to the
irradiated lungs without sivelestat. Therefore, it is thought that
the lower cell counts in groups RE2 and RE4 compared to group R at
15 weeks reflect less damage to the lungs by sivelestat.
Previous studies have demonstrated early and
persistent alterations in pro-inflammatory cytokines such as tumor
necrosis factor-α (TNF-α), interleukin (IL)-1 and IL-6 mRNA levels
by irradiation to the lungs (9–11).
TNF-α and IL-1 enhance the expression of IL-8, which is a strong
chemoattractant and activator of neutrophils (12). These cytokines promote the release
and activation of proteolytic enzymes such as NE and matrix
metalloproteases. These proteolytic enzymes disintegrate
extracellular matrix components such as collagens, proteoglycans
and fibronectins, thereby causing lung tissue injury (13,14).
This induces a further release of cytokines and a subsequent
vicious inflammatory spiral. Transforming growth factor-β (TGF-β)
gene expression is also increased by irradiation in acute and
fibrotic phases (15). As a key
cytokine in the remodeling and fibrotic process, TGF-β stimulates
the secretion of extracellular matrix components (16,17).
The acute and fibrotic changes of the lung comprise
a complex process involving proinflammatory and profibrotic
cytokines as well as proteolytic enzymes produced by various
damaged and activated cells. However, these genetic, molecular, and
histopathological changes are not specific for RILI, which have
also been observed after drug infusions (18).
Experiments have shown the efficacy of sivelestat in
animal models of lung injuries caused by endotoxin (6), bleomycin (19) and hydrochloric acid (20). In bleomycin-induced pulmonary
fibrosis of mice, sivelestat has been shown to suppress the
infiltration of fibroblasts and inflammatory cells (e.g.,
neutrophils and macrophages) as well as the expression of cytokines
(e.g., IL-1 and PDGF) in bronchoalveolar lavage fluid, and it
mitigated histopathological changes such as thickened alveolar wall
with inflammatory cells and fibrotic changes (21). In addition, other experimental
models of lung injury have shown the suppression of plasma NE
activity and other cytokines such as TNF-α, IL-6 and TGF-β by
sivelestat (6,19,20,22,23).
Since inflammation and subsequent tissue injury are
very complex interactions involving various proteins and cells, NE
constitutes only a part of inflammatory processes. The important
role of sivelestat, a specific inhibitor of NE, is in the control
of excessive inflammation and prevention of the vicious
inflammatory spiral. Since acute reactions to irradiation were
observed immediately and sivelestat has a short half-life, it was
determined that sivelestat should be administered immediately
before the initiation of the inflammatory cascade, and repeatedly
or continuously. In the present study, multiple administrations of
sivelestat successfully suppressed NE activity until 48 h after
irradiation, and it is thought that this resulted in mitigation of
the lung injury at 15 weeks. However, more precise mechanisms of
sivelestat-induced suppression of inflammatory reactions and tissue
damage are necessary.
In Japan, sivelestat is widely used in clinics to
improve the acute lung injury that accompanies systemic
inflammatory response syndrome, and its safety has been confirmed
in clinical studies (24,25). For future clinical practice,
sivelestat is a promising agent which improves the therapeutic
ratio by decreasing the risk of RILI or escalation of the delivered
dose when patients need curative irradiation for thoracic
lesions.
In conclusion, in irradiated mouse lung, multiple
administrations of sivelestat continuously suppressed NE activity
and mitigated lung injury at the scarred phase.
References
|
1
|
Sekine I, Sumi M, Ito Y, et al:
Retrospective analysis of steroid therapy for radiation-induced
lung injury in lung cancer patients. Radiother Oncol. 80:93–97.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Havemman K and Gramse M: Physiology and
pathophysiology of neutral proteinases of human granulocytes. Adv
Exp Med Biol. 84:1–20. 1984. View Article : Google Scholar
|
|
3
|
Kawabata K, Hagio T and Matsuoka S: The
role of neutrophil elastase in acute lung injury. Eur J Pharmacol.
451:1–10. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakashima H, Akimoto A, Kitagawa T, et al:
General pharmacological studies of sodium
N-[2-[4-(2,2-dimethethyl-propionyloxy) phenylsulfonylamino]
benzoyl] aminoacetate tetrahydrate (ONO-5046 Na). Pharmacometrics.
54:267–277. 1997.(In Japanese).
|
|
5
|
Shimbo T, Inomata T, Takahashi M, Tatsumi
T, Uesugi Y, Narabayashi I and Sonobe H: Effects of sivelestat
sodium hydrate on the reduction of radiation pneumonitis. Int J Mol
Med. 20:817–822. 2007.PubMed/NCBI
|
|
6
|
Kawabata K, Hagio T, Matsumoto S, Nakao S,
Orita Y, Aze Y and Ohno H: Delayed neutrophil elastase inhibition
prevents subsequent progression of acute lung injury induced by
endotoxin inhalation in hamster. Am J Respir Crit Care Med.
161:2013–2018. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Movsas B, Raffin TA, Epstein AH and Link
CJ Jr: Pulmonary radiation injury. Chest. 111:1061–1076. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsoutsou PG and Koukourakis MI: Radiation
pneumonitis and fibrosis: mechanisms underlying its pathogenesis
and implications for future research. Int J Radiat Oncol Biol Phys.
66:1281–1293. 2006. View Article : Google Scholar
|
|
9
|
Rubin P, Johnston CJ, Williams JP,
McDonald S and Finkelstein JN: A perpetual cascade of cytokines
postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol
Biol Phys. 33:99–109. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnston CJ, Piedboeuf B, Rubin P,
Williams JP, Baggs R and Finkelstein JN: Early and persistant
alterations in the expression of interleukin-1α, interleukin-1β and
tumor necrosis factor α mRNA levels in fibrosis-resistant and
sensitive mice after thoracic irradiation. Radiat Res. 145:762–767.
1996.
|
|
11
|
Rübe CE, Uthe D, Wilfert F, et al: The
bronchiolar epithelium as a prominent source of pro-inflammatory
cytokines after lung irradiation. Int J Radiat Oncol Biol Phys.
61:1482–1492. 2005.PubMed/NCBI
|
|
12
|
Mukaida N: Pathophysiological roles of
interleukin-8/CXCL8 in pulmonary diseases. Am J Physiol Lung Cell
Mol Physiol. 284:L566–L577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Araya J, Maruyama M, Sassa K, et al:
Ionizing radiation enhances matrix metalloproteinase-2 production
in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol.
280:L30–L38. 2001.PubMed/NCBI
|
|
14
|
Yang K, Palm J, König J, et al:
Matrix-metallo-proteinases and their tissue inhibitors in
radiation-induced lung injury. Int J Radiat Biol. 83:665–676. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rube CE, Uthe D, Schmid KW, et al:
Dose-dependent induction of transforming growth factor β (TGF-β) in
the lung tissue of fibrosis-prone mice after thoracic irradiation.
Int J Radiat Oncol Biol Phys. 47:1033–1042. 2000.
|
|
16
|
Martin M, Lefaix J and Delanian S:
TGF-beta1 and radiation fibrosis: a master switch and a specific
therapeutic target? Int J Radiat Oncol Biol Phys. 47:277–290. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishioka A, Ogawa Y, Mima T, et al:
Histopathologic amelioration of fibroproliferative change in rat
irradiated lung using soluble transforming growth factor-beta
(TGF-beta) receptor mediated by adenoviral vector. Int J Radiat
Oncol Biol Phys. 58:1235–1241. 2004. View Article : Google Scholar
|
|
18
|
Trott KR, Herrmann T and Kasper M: Target
cells in radiation pneumopathy. Int J Radiat Oncol Biol Phys.
58:463–469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takemasa A, Ishii Y and Fukuda T: A
neutrophil elastase inhibitor prevents bleomycin-induced pulmonary
fibrosis in mice. Eur Respir J. 40:1475–1482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hagio T, Matsumoto S, Nakao S, Abiru T,
Ohno H and Kawabata K: Elastase inhibition reduced death associated
with acid aspiration-induced lung injury in hamsters. Eur J
Pharmacol. 488:173–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taooka Y, Maeda A, Hiyama K, Ishioka S and
Yamakido M: Effect of neutrophil elastase inhibitor on
bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care
Med. 156:260–265. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hagio T, Nakao S, Matsuoka H, Matsumoto S,
Kawabata K and Ohno H: Inhibition of neutrophil elastase activity
attenuates complement-mediated lung injury in the hamster. Eur J
Pharmacol. 426:131–138. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang T, Zhang J, Sun L, et al: Combined
effects of a neutrophil elastase inhibitor (sivelestat sodium) and
a free radical scavenger (edaravone) on lipopolysaccharide-induced
acute lung injury in rats. Inflamm Res. 61:563–569. 2012.
View Article : Google Scholar
|
|
24
|
Tamakuma S, Ogawa M, Aikawa N, et al:
Relationship between neutrophil elastase and acute lung injury in
humans. Pulm Pharmacol Ther. 17:271–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hayakawa M, Katabami K, Wada T, Sugano M,
Hoshino H, Sawamura A and Gando S: Sivelestat (selective neutrophil
elastase inhibitor) improves the mortality rate of sepsis
associated with both acute distress syndrome and disseminated
intravascular coagulation patients. Shock. 33:14–18. 2010.
View Article : Google Scholar
|