Introduction
Almost 3% of the world’s population is infected with
hepatitis C virus (HCV) (1).
Chronic infection with HCV may result in both hepatic and
extrahepatic disease, such as inflammation of the liver, which in
turn may progress to cirrhosis, liver failure or cancer (2). The mechanism whereby HCV damages the
liver is unclear, but there is much evidence to suggest that the
immunity disorder is one of the important reasons for this
(3). Active T cells in the liver
may target those hepatocytes infected with HCV and clear them to
prevent virus prevalence. Once infected with HCV, the HCV particles
are processed by antigen-presenting cells (APC) and then presented
to naive T cells by major histocompatibility complex (MHC)
molecules, which deliver a primary signal to initiate T-cell
activation by the engagement of the T-cell receptor/CD3 complex
with foreign antigens associated with MHC. In addition, the optimal
activation of T cells requires a co-stimulatory signal.
Co-stimulatory signals include CD28/B7 and TNFR/TNF superfamilies;
however, numerous novel molecules, such as programmed death 1
(PD-1), inducible T-cell co-stimulator (ICOS), B and T lymphocyte
attenuator (BTLA), B7-H1 (PD-L1), B7RP-1 (B7H,B7-H2), B7-DC
(PD-L2), B7-H3 and B7-H4, have recently been identified (4). These signals are also important in
physiological functions and pathologic progress (4). Cytotoxic T lymphocytes (CTL) are
hypothesized to control HCV replication, but these fail in numerous
persistently infected individuals (5–7).
Additionally, the CTL numbers are often low in chronic HCV
patients, as the HCV core protein is able to suppress the host
immune response (8–11). Monocyte-derived dendritic cells
(Mo-DCs) generated from hepatitis C patients have an impaired
ability to stimulate allogeneic CD4+ T cells that are
not able to release a significant amount of effective cytokines
(12). However, the exact
immunopathological mechanisms by which HCV escapes the host immune
response remain unknown.
Previous studies have demonstrated that
co-stimulators, PD-1 and ICOS, are involved in the HCV pathogenic
progress. PD-1 receptor expression is greater in dysfunctional
HCV-specific T cells compared with normal cells (13,14).
In acute HCV affections, PD-1 also upregulates CD4+ and
CD8+ T lymphocytes. Interaction with its ligand, PD-L1,
in the HCV-specific T cells inhibits effector functions and induces
T-cell apoptosis. In an HCV core(+) mouse model, CD8+
cells were accompanied with enhanced PD-1 expression. The core
antigen of HCV is an immunomodulatory protein that alters the
adaptive immune response (11).
The aforementioned findings demonstrate that PD-1 is randomly
expressed in HCV-specific immune cells, although its function has
not yet been fully elucidated.
Another co-stimulator, ICOS, also assists in
activating adaptive immunity, particularly by aiding antibody
secretion by B cells. However, there is limited number of studies
concerning ICOS in HCV patients. Ribavirin downmodulates ICOS in
CD4+ T cells and assists HCV clearance, indicating that
ICOS prevention is a novel choice for HCV treatment (15). In addition, ICOS has been found to
be upregulated in chronic HBV-infected cells.
Previous studies have demonstrated that there are
different isoforms of PD-1 and ICOS from alternatively spliced
messenger RNA (mRNA), and the soluble isoforms of these may
regulate immune homeostasis (16).
In addition, soluble ICOS (sICOS) and soluble PD-1 (sPD-1) have
been identified to be present in numerous diseases, particularly
autoimmune diseases, such as systemic lupus erythematosus (SLE) and
cancer. However, the clinical significance of these markers in
chronic HCV infection remains uncertain. The aim of this study was
to elucidate the correlation between these soluble molecules and
T-cell activation in chronic HCV patients, and to investigate their
possible association with the pathogenesis of HCV.
Materials and methods
Subjects
Sixty-three Chinese patients with chronic HCV and 30
volunteers (normal controls) were recruited in this study. The
individuals with chronic HCV were patients at The Affiliated
Hospital of North Sichuan Medical College and the Yongchuan
Hospital of Chongqing Medical University. All patients were
anti-HCV antibody- and HCV RNA-positive, but negative for HBV, HDV
and human immunodeficiency virus, and without any other
disease-related liver damage. Patients with concomitant illness and
autoimmune disease were excluded. No patients had accepted
administration of anti-HCV agents or steroids one year prior to
sampling. Each individual signed the written informed consent, and
the protocol was approved by the Clinical Research Ethics Committee
of the affiliated hospital of North Sichuan Medical College.
Virological detection and biochemistry
assays
Anti-HCV antibody was determined by a kit (i2000;
Abbort, Wiesbaden, Germany). The HCV RNA level was determined by
real-time RT-PCR using the LightCycler® 480 System
(Roche Diagnostics GmbH, Mannheim, Germany) with a detection
sensitivity of 1×103 IU/ml. Serum alanine
aminotransferase (ALT), aspartate aminotransferase (AST), total
bilirubin (TB) and direct bilirubin (DB) were measured by the
Synchron LX20 auto-analyzer (Beckman Coulter Inc., Fullerton, CA,
USA).
ELISA assay of sICOS and sPD-1
Serum concentrations of sICOS and sPD-1 of all
patients and the normal control group were detected with ELISA
(R&D Systems, Inc., Minneapolis, MN, USA). The assay was
conducted according to the manufacturer’s instructions.
Real-time RT-PCR
Peripheral blood mononuclear cells (PBMC) were
obtained according to the specification of the kit (Tianjin Hao
Yang Biological Manufacture Co., Ltd., Tianjin, China). RNA
extraction and cDNA synthesis were performed according to the
experimental guidelines of Promega Corporation (Madison, WI, USA).
The PCR primer sequences used were: Sense 5′-GTTCCCTGAGTTGTTTG-3′
and antisense: 5′-TCATCTTGAGGTGTCCC-3′ for ICOS; sense
5′-CCGCCTTCTGTAATGGTTTGA-3′ and antisense:
5′-GGGCAGCTGTATGATCTGGAA-3′ for PD-1 (17). GAPDH was used as an internal
control. Relative quantification was calculated using the
2−ΔΔCt formula.
Statistical analysis
The Mann-Whitney rank sum test was used to analyze
the difference in sPD-1 and sICOS levels between normal controls
and patients. The Spearman’s rank correlation test was used to
assess the correlation between sPD-1, sICOS and ALT or anti-HCV
antibody levels. Results are expressed as the median (interquartile
range). The data were analyzed with OriginPro 8.5 statistical
software. P<0.05 was considered to indicate a statistically
significant difference.
Results
Numerous biochemical marker levels were
increased in chronic HCV patients compared with the normal
controls
Sixty-three patients with chronic HCV infection and
30 normal controls were studied. The detection results of ALT, AST,
DB and TB are summarized in Table
I. Serum AST, ALT, TB and DB were significantly elevated in the
HCV group compared with the normal control group, indicating that
HCV chronic infection causes liver damage.
 | Table IComparison of multiple biochemical and
immunological markers between normal controls and chronic HCV
patients. |
Table I
Comparison of multiple biochemical and
immunological markers between normal controls and chronic HCV
patients.
| Characteristic | Normal controls
(n=30) | Chronic hepatitis C
(n=63) |
|---|
| Age (mean ± SD,
years) | 44.6±14.4 | 48±15.7 |
| Female/male | 17/13 | 24/39 |
| ALT [median (IQ
range), U/ml] | 13.4 (3.2–29.5) | 37
(6.9–285.1)a |
| AST [median (IQ
range), U/ml] | 11.2 (2.7–27.4) | 36.5
(11.7–187)a |
| TB [median (IQ
range), μmol/l] | 5.6 (2.1–16.5) | 12.5 (2.8–80.6) |
| DB [median (IQ
range), μmol/l] | 2.1 (0.4–6.6) | 3.7 (1.1–46.8) |
| Anti-HCV [median (IQ
range)] | 0.46 (0.05–0.91) | 3.7
(3.1–19.62)a |
| HCV RNA log10 (mean ±
SD, U/ml) | <3 | 6.01±1.62a |
| Autoimmune
diseases | None | None |
Serum levels of sPD-1 and sICOS were
elevated in chronic HCV patients compared with the normal
controls
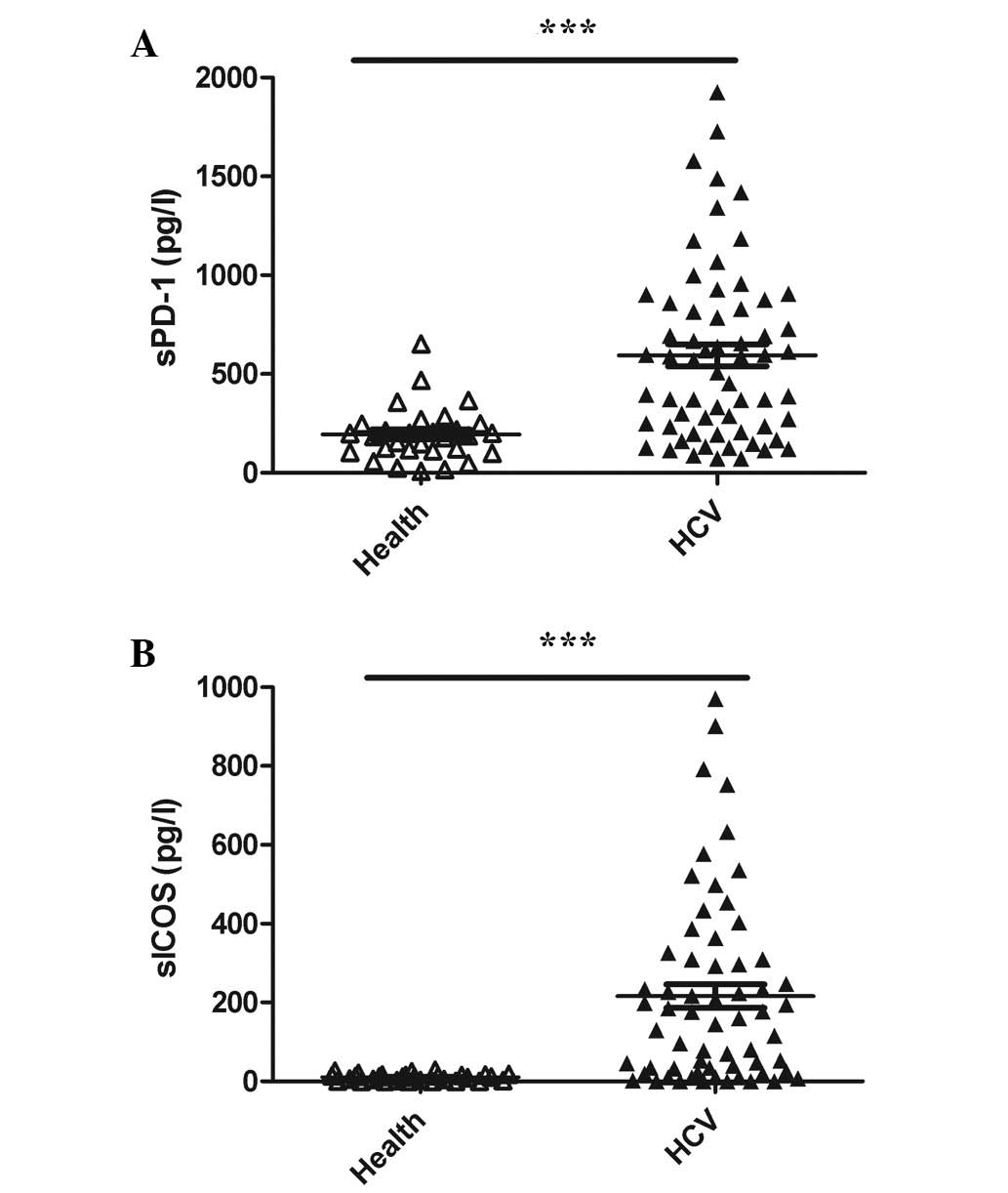
The sPD-1 and sICOS serum levels were extremely low
in the normal controls, a large number of which were below the
detection limit of the two kits (Fig.
1A and B, respectively). However, the sPD-1 and sICOS levels
were significantly higher in patients with chronic HCV infection
compared with the normal controls (P<0.01 for both; Fig. 1A and B, respectively).
sPD-1 and sICOS are correlated with
anti-HCV antibody levels in chronic HCV patients
A potential correlation was identified between serum
ALT and sPD-1/sICOS. A significant correlation between ALT and
sPD-1 levels was observed (r=0.268, P=0.033; Fig. 2F), although sICOS levels did not
correlate with ALT levels (Fig.
2E). sPD-1 levels did not correlate with the DB and TB levels
(Fig. 2A and B, respectively),
whereas the sICOS levels correlated with DB and TB levels (Fig. 2C and D, respectively), this
correlation may have been due to the detection bias. However, the
upregulation of DB may have been as a result of other factors.
sPD-1 and sICOS serum levels correlated with anti-HCV antibody
levels (sPD-1, r=0.344, P=0.005; sICOS, r=0.322, P=0.009; Fig. 3A and B, respectively), indicating
that their infection may be an immune reaction in chronic HCV
patients. However, no difference in sICOS or sPD-1 levels between
HCV patients with normal and abnormal ALT results was detected (F
sig. 4A and B, respectively).
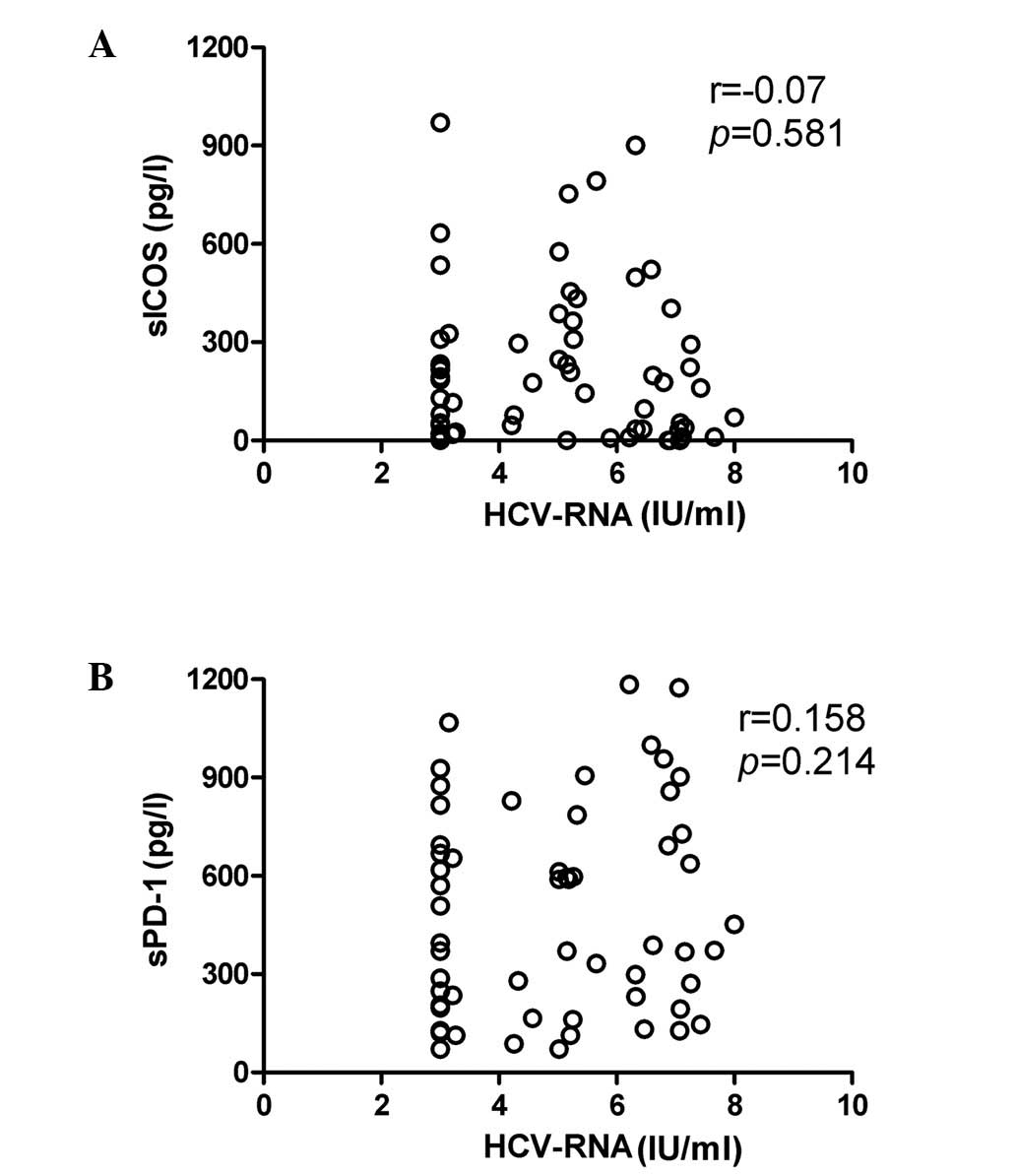
No correlation was evident between HCV
RNA levels and sPD-1/sICOS concentration
HCV RNA levels were measured using real-time RT-PCR
and the statistical analysis revealed that there was no significant
correlation between HCV RNA levels and sICOS (P>0.05) or sPD-1
(P>0.05) levels in patients with chronic HCV infection (Fig. 4A and B, respectively). In addition,
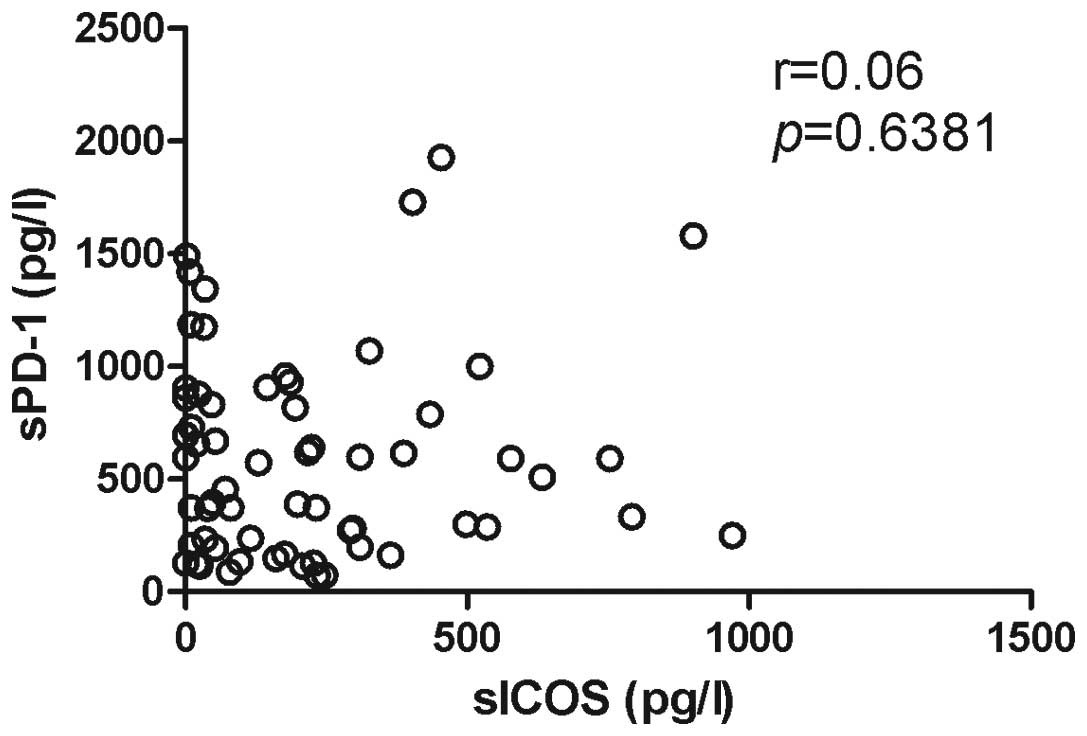
the correlation between sPD-1 and sICOS levels was not significant
(Fig. 5).
PD-1 and ICOS mRNA levels were elevated
in PBMC of HCV patients
Quantitative RT-PCR demonstrated that the mRNA
levels of PD-1 and ICOS in PBMC were elevated in chronic HCV
patients compared with the normal controls (Fig. 6A and B, respectively). Considering
that sPD-1 and sICOS may be shed from the membrane of these cells,
the upregulation of these molecules may be one of the reasons to
explain the high levels of PD-1 and ICOS in HCV patients.
Discussion
Communication between T cells and APC initiates the
immune response to HCV infection. Dysfunction of T cells and
abnormal immunomodulation molecules of APC in HCV patients have
been demonstrated (18,19). However, the soluble co-stimulatory
molecules in chronic HCV patients has not previously been
investigated.
In the present study, we demonstrated that the serum
concentrations of sPD-1 and sICOS were markedly elevated in chronic
HCV patients compared with the normal controls. The levels of sPD-1
and sICOS were significantly correlated with the anti-HCV antibody
serum levels in HCV patients. The anti-HCV antibody levels are
recognized as HCV infection markers, and high anti-HCV antibody
levels indicate active replication of HCV. However, in the present
study, the sPD-1 and sICOS levels were not significantly correlated
with the HCV RNA level. This may be due to the fact that the immune
regulation ability of PD-1 and ICOS is not very effective at
influencing the virus replication; thus, they are not sensitively
reactive to the changes in HCV RNA levels. There are numerous
co-stimulatory molecules, such as CD28, CD80, CD86 and CTLA, all of
which have two forms (the membrane and soluble forms) (20). sPD-1 and sICOS are formed from
alternative mRNA splicing. Since sPD-1, and not sICOS, has been
demonstrated to be highly expressed in the active lymphocytes of
autoimmune diseases (21), sPD-1
may not be a specific molecule in chronic HCV infection.
Additionally, sICOS has not been investigated previously.
Therefore, these two markers are not necessarily disease-specific
molecules. However, due to their correlation with the anti-HCV
antibody, sPD-1 and sICOS may be partially associated with virus
replication and HCV pathogenesis, which may lead to the
dysregulation of T-cell co-stimulation.
In the present study, we found that the sPD-1 and
sICOS levels were significantly higher in the HCV group compared
with the normal control group. However, the biological significance
of these results remains unknown. Both of these molecules have an
immunomodulatory function. Increased production of sPD-1 and sICOS
may interfere with the patient’s adaptive immune function. sPD-1
and sICOS may compete and interfere with the PD-1 and ICOS
interactions with their respective ligands, leading to immune
dysregulation and a defective immune response. PD-1/PD-L has been
demonstrated to negatively regulate the T-cell response, both in
the primary and secondary immune responses (22). Blocking the PD-1/PD-L pathway could
enhance the anti-virus immunity. Thus, the abnormal expression of
sPD-1 may interact with PD-L and inhibit PD-1/PD-L axis-induced
T-cell apoptosis, leading to continuation of T-cell activation and
causing inflammation. ICOS is usually expressed in activated T
cells and amplifies the first stimulator signal. Abnormal sICOS is
likely to compete with ICOS-L and reduce T-cell reaction, which may
cause T-cell exhausion. Both PD-1 and ICOS activation have been
demonstrated to induce Th2 cytokine release and in turn suppress
Th1 inflammation, leading to liver damage (23). Humoral immune function may also be
enhanced by ICOS. ICOS knockout may prevent T cell-specific
germinal center formation, as B cells have been observed to be
inactive and incapable of migrating to form the germinal center.
The T cells specifically induced immunoglobulin dyspoiesis,
indicating that high antibody production is ICOS-dependent
(24). In the present study, the
high levels of sICOS were concordant with the high levels of
anti-HCV antibody, which is explained by the fact that sICOS is
predominantly shed from the active T-cell membranes. Thus, the
aberrant production of sPD-1 and sICOS may be important in HCV
infection. The detailed immunopathological roles of those soluble
co-stimulatory molecules in HCV infection require further
study.
PD-1 expression levels in HCV-specific
CD8+ and CD4+ T cells do not correlate with
clinical outcomes (13). In
addition, sPD-1 is upregulated in certain autoimmune diseases.
Notably, HCV has recently been classified as an autoimmune disease.
In the present study, TB and DB were weakly correlated with sPD-1,
but not with sICOS. Similarly, ALT was correlated with sPD-1 and
not with sICOS. We suggest that sPD-1 and sICOS are not
disease-specific indicators, due to their weak immunomodulatory
ability and the fact that their levels do not change synchronously
with other biochemical markers. sPD-1 and sICOS are usually
aberrant in immune disorder disease; however, they may have little
correlation with the changes in liver function (Fig. 4).
To elucidate the mechanism of soluble sPD-1 and
sICOS generation, we detected the transcription level changes of
the two molecules. As expected, PD-1 and ICOS levels are both
higher in the HCV patient group compared with the normal control
group. As the sPD-1 and sICOS mRNA levels corresponded with their
membrane protein forms, the high ICOS and PD-1 mRNA levels may
indicate that their soluble forms predominantly shed from the
membrane, and may have a negative regulation of their function.
Although the exact mechanism of the soluble forms in immune
regulation is not yet clear, the different concentrations of
soluble forms may have different functions in HCV-infected
patients. Blocking PD-1/PD-L has been demonstrated to be a useful
way of recovering T-cell function. T cells proliferate
significantly following blocking of PD-1 (25). However, in our study, upregulation
of sPD-1 did not induce T-cell proliferation and clearing of the
virus. Thus, T-cell function and subtype in the HCV patients should
be identified, and the different concentrations and functions of
sPD-1 in the T cells from HCV patients should be investigated. No
studies are available regarding the manner in which sICOS affects
T-cell immunity and whether it functions as a competitor of ICOS,
both of which require further study.
In summary, this is the first study to identify
soluble co-stimulatory molecules in chronic HCV patients. Our
results provide a new direction and experimental basis to elucidate
the pathological mechanism of chronic HCV infection. The soluble
co-stimulatory molecules may be a new therapeutic target in HCV
therapy. The biological function of sPD-1 and sICOS in HCV
infection requires further study in order for their therapeutic
function to be investigated.
References
|
1
|
Sorrell MF, Belongia EA, Costa J, Gareen
IF, Grem JL, Inadomi JM, Kern ER, McHugh JA, Petersen GM, Rein MF,
et al: National Institutes of Health consensus development
conference statement: management of hepatitis B. Hepatology.
49:S4–S12. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodriguez-Luna H and Vargas HE: Management
of hepatitis C virus infection in the setting of liver
transplantation. Liver Transpl. 11:479–489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mengshol JA, Golden-Mason L and Rosen HR:
Mechanisms of Disease: HCV-induced liver injury. Nat Clin Pract
Gastroenterol Hepatol. 4:622–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharpe AH and Freeman GJ: The B7-CD28
superfamily. Nat Rev Immunol. 2:116–126. 2002. View Article : Google Scholar
|
|
5
|
Cooper S, Erickson AL, Adams EJ, Kansopon
J, Weiner AJ, Chien DY, Houghton M, Parham P and Walker CM:
Analysis of a successful immune response against hepatitis C virus.
Immunity. 10:439–449. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thimme R, Oldach D, Chang KM, Steiger C,
Ray SC and Chisari FV: Determinants of viral clearance and
persistence during acute hepatitis C virus infection. J Exp Med.
194:1395–1406. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Puig M, Mihalik K, Tilton JC, Williams O,
Merchlinsky M, Connors M, Feinstone SM and Major ME:
CD4+ immune escape and subsequent T-cell failure
following chimpanzee immunization against hepatitis C virus.
Hepatology. 44:736–745. 2006.
|
|
8
|
Rehermann B, Chang KM, McHutchinson J,
Kokka R, Houghton M, Rice CM and Chisari FV: Differential cytotoxic
T-lymphocyte responsiveness to the hepatitis B and C viruses in
chronically infected patients. J Virol. 70:7092–7102.
1996.PubMed/NCBI
|
|
9
|
Nelson DR, Marousis CG, Davis GL, Rice CM,
Wong J, Houghton M and Lau JY: The role of hepatitis C
virus-specific cytotoxic T lymphocytes in chronic hepatitis C. J
Immunol. 158:1473–1481. 1997.PubMed/NCBI
|
|
10
|
Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR,
Liu C and Nelson DR: An immunomodulatory role for CD4(+)CD25(+)
regulatory T lymphocytes in hepatitis C virus infection.
Hepatology. 40:1062–1071. 2004.
|
|
11
|
Yao ZQ, King E, Prayther D, Yin D and
Moorman J: T cell dysfunction by hepatitis C virus core protein
involves PD-1/PDL-1 signaling. Viral Immunol. 20:276–287. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li S, Roberts S, Plebanski M, Gouillou M,
Spelman T, Latour P, Jackson D, Brown L, Sparrow RL, Prince HM, et
al: Induction of multi-functional T cells in a phase I clinical
trial of dendritic cell immunotherapy in hepatitis C virus infected
individuals. PLoS One. 7:e393682012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kasprowicz V, Schulze Zur Wiesch J,
Kuntzen T, Nolan BE, Longworth S, Berical A, Blum J, McMahon C,
Reyor LL, Elias N, et al: High level of PD-1 expression on
hepatitis C virus (HCV)-specific CD8+ and
CD4+ T cells during acute HCV infection, irrespective of
clinical outcome. J Virol. 82:3154–3160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rutebemberwa A, Ray SC, Astemborski J,
Levine J, Liu L, Dowd KA, Clute S, Wang C, Korman A, Sette A, et
al: High-programmed death-1 levels on hepatitis C virus-specific T
cells during acute infection are associated with viral persistence
and require preservation of cognate antigen during chronic
infection. J Immunol. 181:8215–8225. 2008. View Article : Google Scholar
|
|
15
|
Atsukawa M, Nakatsuka K, Kobayashi T,
Shimizu M, Tamura H, Harimoto H, Takahashi H and Sakamoto C:
Ribavirin downmodulates inducible costimulator on CD4+ T
cells and their interleukin-10 secretion to assist in hepatitis C
virus clearance. J Gastroenterol Hepatol. 27:823–831.
2012.PubMed/NCBI
|
|
16
|
Nielsen C, Ohm-Laursen L, Barington T,
Husby S and Lillevang ST: Alternative splice variants of the human
PD-1 gene. Cell Immunol. 235:109–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meng Q, Yang P, Li B, Zhou H, Huang X, Zhu
L, Ren Y and Kijlstra A: CD4+PD-1+ T cells
acting as regulatory cells during the induction of anterior
chamber-associated immune deviation. Invest Ophthalmol Vis Sci.
47:4444–4452. 2006.PubMed/NCBI
|
|
18
|
Klenerman P and Semmo N: Cellular immune
responses against persistent hepatitis C virus: gone but not
forgotten. Gut. 55:914–916. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koziel MJ: Cellular immune responses
against hepatitis C virus. Clin Infect Dis. 41(Suppl 1): S25–S31.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Magistrelli G, Jeannin P, Elson G, Gauchat
JF, Nguyen TN, Bonnefoy JY and Delneste Y: Identification of three
alternatively spliced variants of human CD28 mRNA. Biochem Biophys
Res Commun. 259:34–37. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishimura H, Nose M, Hiai H, Minato N and
Honjo T: Development of lupus-like autoimmune diseases by
disruption of the PD-1 gene encoding an ITIM motif-carrying
immunoreceptor. Immunity. 11:141–151. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Greenwald RJ, Freeman GJ and Sharpe AH:
The B7 family revisited. Annu Rev Immunol. 23:515–548. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Witsch EJ, Peiser M, Hutloff A, Buchner K,
Dorner BG, Jonuleit H, Mages HW and Kroczek RA: ICOS and CD28
reversely regulate IL-10 on re-activation of human effector T cells
with mature dendritic cells. Eur J Immunol. 32:2680–2686. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Warnatz K, Bossaller L, Salzer U,
Skrabl-Baumgartner A, Schwinger W, van der Burg M, van Dongen JJ,
Orlowska-Volk M, Knoth R, Durandy A, et al: Human ICOS deficiency
abrogates the germinal center reaction and provides a monogenic
model for common variable immunodeficiency. Blood. 107:3045–3052.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakamoto N, Cho H, Shaked A, Olthoff K,
Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ
and Chang KM: Synergistic reversal of intrahepatic HCV-specific CD8
T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog.
5:e10003132009. View Article : Google Scholar : PubMed/NCBI
|