Introduction
Oval cell-mediated liver regeneration has been a
focus of hepatic stem cell studies in recent years. The liver has a
notable regeneration capacity and under normal conditions damaged
liver tissue is repaired through the proliferation of mature
hepatocytes. When the ability of hepatocytes to divide and replace
the damaged tissue is compromised, oval cells become activated.
These cells then proliferate and differentiate into mature liver
cells, thereby aiding liver regeneration. As a result, hepatic stem
cell therapy is currently considered as a new approach to liver
disease treatment (1–3). However, the safe use of hepatic stem
cells must also be considered due to the possibility of malignant
transformation (4,5). Therefore, regulation of the
activation and expansion of liver progenitor cells has also been
analyzed. In addition, studies on pharmacologically effective
agents from natural products associated with low toxicity have also
been performed.
Matrine is one of the main alkaloid components
extracted from the Sophora root and has a molecular formula
of C15H24N2O (6). Studies have revealed that matrine
protects the stability of liver cell membranes, inhibiting
proliferation of mesenchymal cells and regulating satellite cells.
In addition, the compound exhibits anti-inflammatory (7), antiviral (8,9),
immunoinhibitory, antifibrotic (10) and antidiarrheal (11) effects. However, its role in
oval-cell mediated regeneration remains unclear. Therefore, the
aims of the present study were to determine the effects of matrine
on liver regeneration and elucidate its molecular mechanisms.
The signals mediating cellular specification are
produced by adjacent or distant cells and these signals are often
received by the cell simultaneously. Experimental evidence has
demonstrated that Notch receptors and ligands are required for
mammalian development and growth in a number of organ systems,
including the liver (12–14). In particular, the Notch signaling
pathway is key to regulating the differentiation of various stem
cells. However, only a limited number of studies have been
conducted on hepatic stem cells. Therefore, in this study, the
potential role of Notch signaling in the regulation of oval
cell-mediated liver regeneration was examined. An oval-cell
mediated liver regeneration model was constructed and treated with
matrine. In addition, the expression of recombination signal
sequence-binding protein Jκ (RBP-Jκ) and HES1, key molecular
components of the Notch signaling pathway in regenerated liver
tissue, was determined. The results of the current study are likely
to demonstrate the effects of matrine in the proper differentiation
and growth of oval cells regulated by Notch signaling.
Materials and methods
Animal models
Male Sprague Dawley (SD) rats (weight, 200±20 g)
were used. All procedues were performed in strict accordance with
the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health. The animal use protocol was reviewed
and approved by the Institutional Animal Care and Use Committee of
Beijing Ditan Hospital (Beijing, China).
Male SD rats were randomly divided into two groups,
model and matrine (both n=24). Rats were fed with pellet chow and
provided access to water ad libitum. Rats were maintained in
a temperature-controlled room with a 12-h light/dark illumination
cycle. All rats received daily oral gavage of
N-2-acetylaminofluorene (2-AAF; Sigma-Aldrich, St. Louis, MO, USA)
at a dosage of 15 mg/kg for 1 week prior to and 2 weeks following
partial hepatectomy (PH) to inhibit hepatocyte proliferation. The
2-AAF was dissolved in dimethyl sulfoxide (Sigma-Aldrich). The
matrine group also received daily oral gavage of matrine (batch no.
110780-201012, China Drugs and Biological Products Inspection
Institute, Beijing, China) at a dosage of 20 mg/kg. Matrine was
reconstituted in distilled water. Following 1 week of daily gavage,
all rats were anesthetized and two-thirds PH was performed by
surgically removing the left and median liver lobes. No dosing was
performed on the day of the surgery. Three rats from each group
were sacrificed on days 1, 3, 7 and 14 following PH. Formalin-fixed
and paraffin-embedded serial liver tissue sections (4 μm) were used
for immunohistochemistry and reverse-transcription polymerase chain
reaction (RT-PCR).
Survival rate test
The number of rats that survived in each group was
counted on days 1, 4, 7, 10, 14 and 21 of analysis and the survival
rate was calculated for each group.
Liver function test
Aminotransferase (ALT) and total bilirubin (TBil)
levels, which reflect liver function, were tested on days 1, 4, 7,
10, 14 and 21 of analysis. Blood from the rats tails was used to
test for ALT and TBil.
Immunohistochemistry
Paraffin sections of the formalin-fixed liver
tissues were stained using a mouse monoclonal antibody against OV6
(MAB 2020; R&D Systems, Minneapolis, MN, USA), a marker of
hepatic oval cells in ductular reactions. Tissue sections were
rehydrated using descending concentrations of ethanol. Endogenous
peroxidase activity was blocked with 3% hydrogen peroxide in
methanol. Tissues used for OV6 immunohistochemistry were microwaved
to boiling for 15 min in 10 mmol/l Tris buffer and 1 mmol/l
ethylenediamine tetraacetic acid (EDTA) at pH 9.0 for antigen
retrieval. Following antigen retrieval, tissue sections were
blocked with 10% normal serum from the donor species of the
secondary antibodies for 15 min at room temperature and incubated
with primary antibodies overnight at 4°C. The ratio of anti-OV6
dilution was 1:10. Following rinsing with phosphate-buffered
solution (PBS), the primary antibody was detected by incubation for
30 min with biotinylated rabbit anti-mouse immunoglobulin, rinsed
further with PBS and incubated with horseradish
peroxidase-conjugated streptavidin/biotin complex (85-9843,
Histostain Plus kits; Zymed Laboratories Inc., Carlsbad, CA, USA).
Peroxidase activity was developed with 0.05% diaminobenzidine and
0.03% H2O2. Finally, sections were
counterstained for 5 min in hematoxylin, dehydrated using graded
alcohol and then mounted under glass coverslips.
RNA isolation and RT-PCR
Total RNA from fresh liver tissues of model and
matrine groups was extracted with TRIzol according to the
manufacturer’s instructions at the beginning of the experiment and
on days 7, 14 and 21. RNA (1 μg) was reverse transcribed to cDNA.
The number of cycles corresponded to the mid-logarithmic phase for
semi-quantitative PCR. Primers were then designed using GenBank
sequences (Table I). PCR
amplification was performed using PCR Master Mix (Taqman, Takara,
Dalian, China) according to the manufacturer’s instructions. PCR
products were analyzed via electrophoresis (Gel-Pro Analyzer
Version 3.0; Media Cybernetics, Inc., Bethesda, MD, USA) on a 1.5%
agarose gel. Results of the semi-quantitative PCR were expressed
using the optical density ratio of the value of RBP-Jκ and HES1 to
β-actin.
 | Table IPrimer sequences used for RT-PCR. |
Table I
Primer sequences used for RT-PCR.
| Gene | Primer sequence | Annealing temperature
(°C) | Cycles | PCR product length
(bp) |
|---|
| RBP-Jκ | 5′-CCA ATT TCA GGC
CAC TCC A-3′ | 54.2 | 35 | 253 |
| 5′-CTC TAC ATC CCC
AAA CCA CAC TC-3′ | | | |
| HES1 | 5′-CAA CAC GAC ACC
GGA CAA ACC-3′ | 51.8 | 35 | 349 |
| 5′-AGT GCG CAC CTC
GGT GTT AAC-3′ | | | |
| β-actin | 5′-GCC ATG TAC GTA
GCC ATC CA-3′ | | | 375 |
| 5′-GAA CCG CTC ATT
GCC GAT AG-3′ | | | |
Statistical analysis
Data from at least three independent experiments
were used for statistical analysis. All results are expressed as
mean ± SD. Measurement data were analyzed using one-way analysis of
variances and performed using SPSS v17.0 (SPSS Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
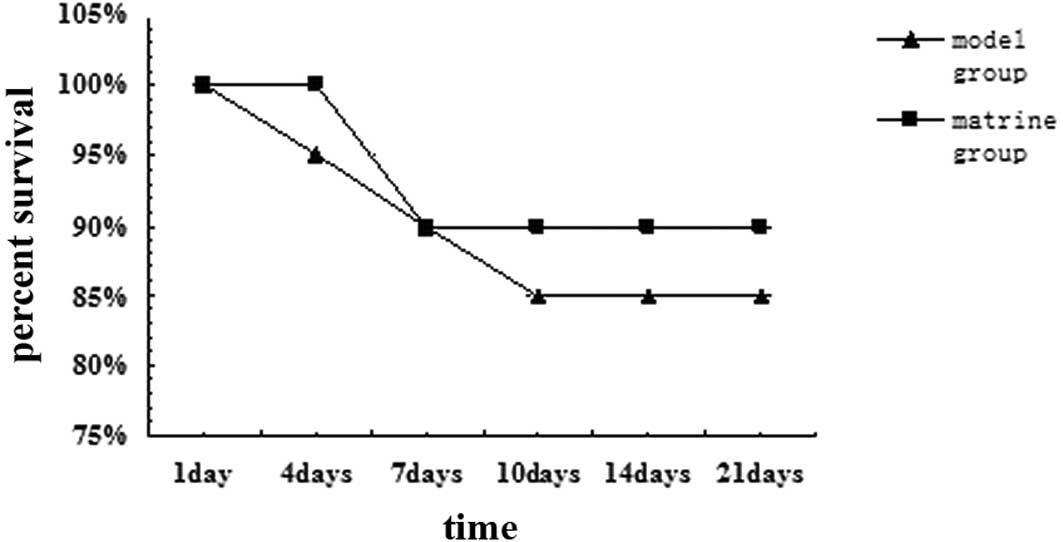
Survival rate
The majority of rats had poor appetites, drank and
exercised little and appeared dejected on days 1–3 following
surgery. One week following PH the characteristics/behavior of the
rats had almost returned to those observed prior to surgery. Wounds
were re-sutured to ensure improved healing in 3 and 2 rats from the
model and matrine groups, respectively. The same number of rats
from each group died 1 week following surgery. The survival rates
of the model group on days 1, 4, 7, 10, 14 and 21 of analysis were
100, 95, 90, 85, 85 and 85%, respectively, whereas those of the
matrine group were 100, 100, 90, 90, 90 and 90%, respectively. No
statistically significant difference was observed between the
survival rates of the two groups (Fig.
1).
Liver function recovery
Rats from the model and matrine groups were observed
to exhibit the most serious liver impairments at day 1 and 3
following surgery. Liver slowly recovered at day 7 and appeared
almost normal by day 14. Compared with the model group, lower ALT
and TBil values on days 7, 10, 14 and 21 were identified in matrine
rats, indicating that matrine may aid repair of the impaired liver
and protect liver function during liver regeneration (Table II).
 | Table IILiver function of model and matrine
groups. |
Table II
Liver function of model and matrine
groups.
| Day 1 | Day 7 | Day 10 | Day 14 | Day 21 |
|---|
|
|
|
|
|
|
|---|
| Group | ALT | TBil | ALT | TBil | ALT | TBil | ALT | TBil | ALT | TBil |
|---|
| Model | 52.1±7.7 | 14.3±7.3 | 933.8±246.2 | 97.2±44.6 | 1465.2±572.7 | 120.8±53.4 | 716.6±253.8 | 60.1±27.4 | 267.8±120.5 | 19.9±8.7 |
| Matrine | 58.8±6.0 | 16.3±5.9 |
672.5±241.7a | 87.7±39.6 |
1038.8±455.2b |
96.1±40.3a |
493.4±196.7b |
38.68±16.0b |
62.5±26.4b | 18.3±9.5 |
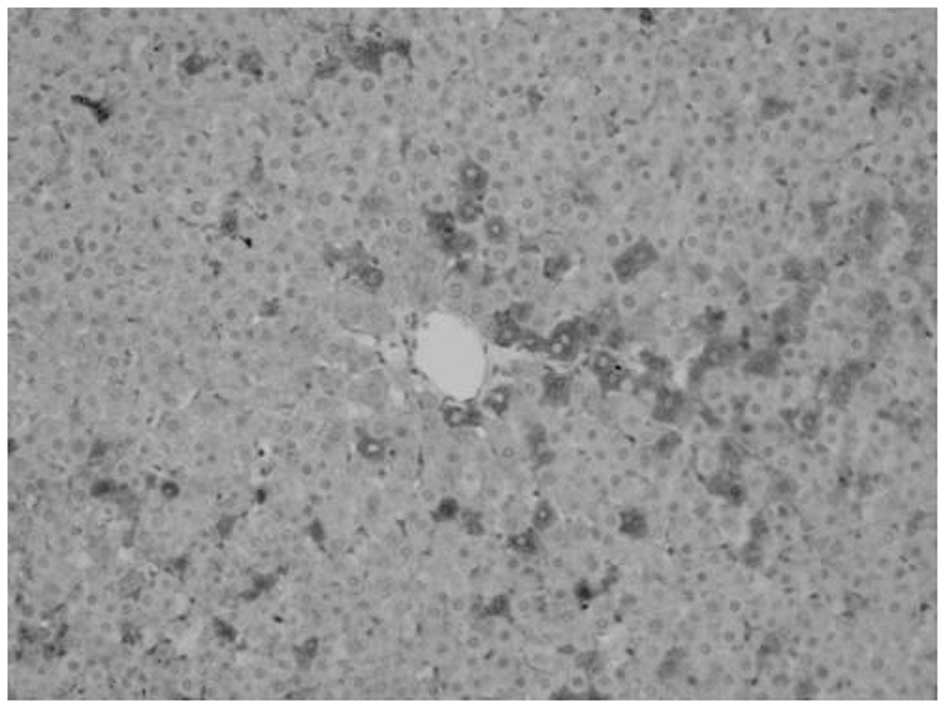
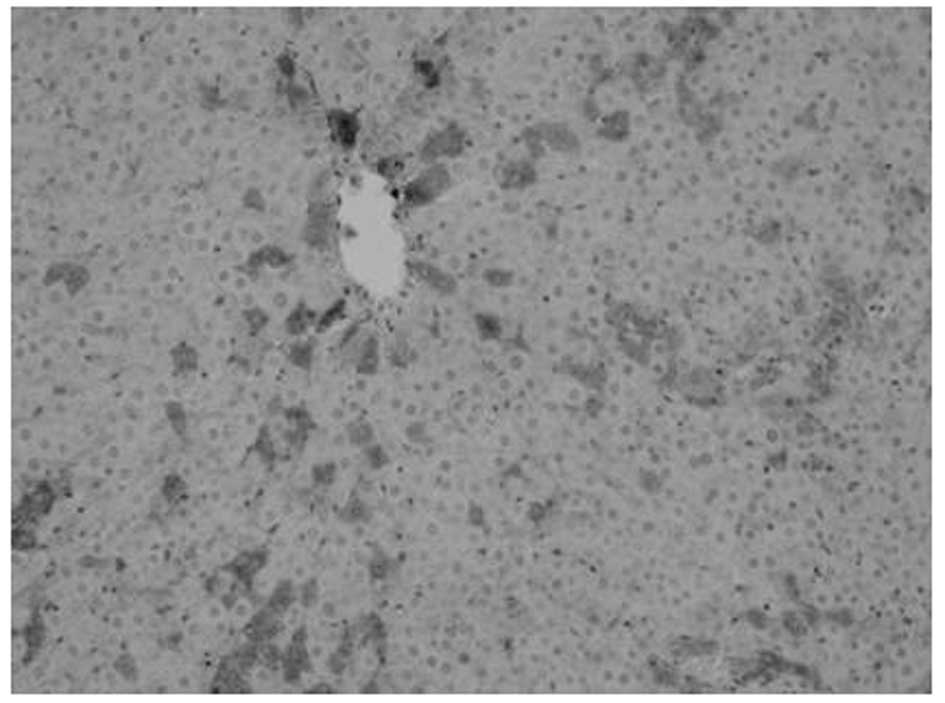
Proliferation of oval cells
Oval cells were found in the ductular area on day 1
following PH. Compared with mature hepatocytes, oval cells were
observed to exhibit reduced volume, higher ratios of nucleus to
cytoplasm, round- or oval-shaped nuclei and antibody OV6
expression. The number of oval cells increased with time.
OV6-expressing cells were found to be distributed along the
ductular to the parenchymal regions of the liver on days 3–7. Cell
numbers peaked at day 7 and then decreased. A marked decrease was
noted at day 14 following surgery, however, OV6-expressing cells
were noted only in the ductular region. Compared with model, fewer
OV6-expressing cells were identified in the matrine group (Figs. 2–5
and Table III).
 | Table IIINumber of OV6-expressing cells in the
liver tissue of model and matrine groups at various days. |
Table III
Number of OV6-expressing cells in the
liver tissue of model and matrine groups at various days.
| Day 1 | Day 7 | Day 14 | Day 21 |
|---|
|
|
|
|
|
|---|
| Group | n | Cell number | n | Cell number | n | Cell number | n | Cell number |
|---|
| Model | 6 | 8.3±3.8 | 6 | 26.3±9.1 | 6 | 38.7±16.8 | 3 | 20.2±8.3 |
| Matrine | 6 | 7.9±3.9 | 6 | 18.2±8.3a | 6 | 21.1±9.4a | 4 | 11.0±4.7a |
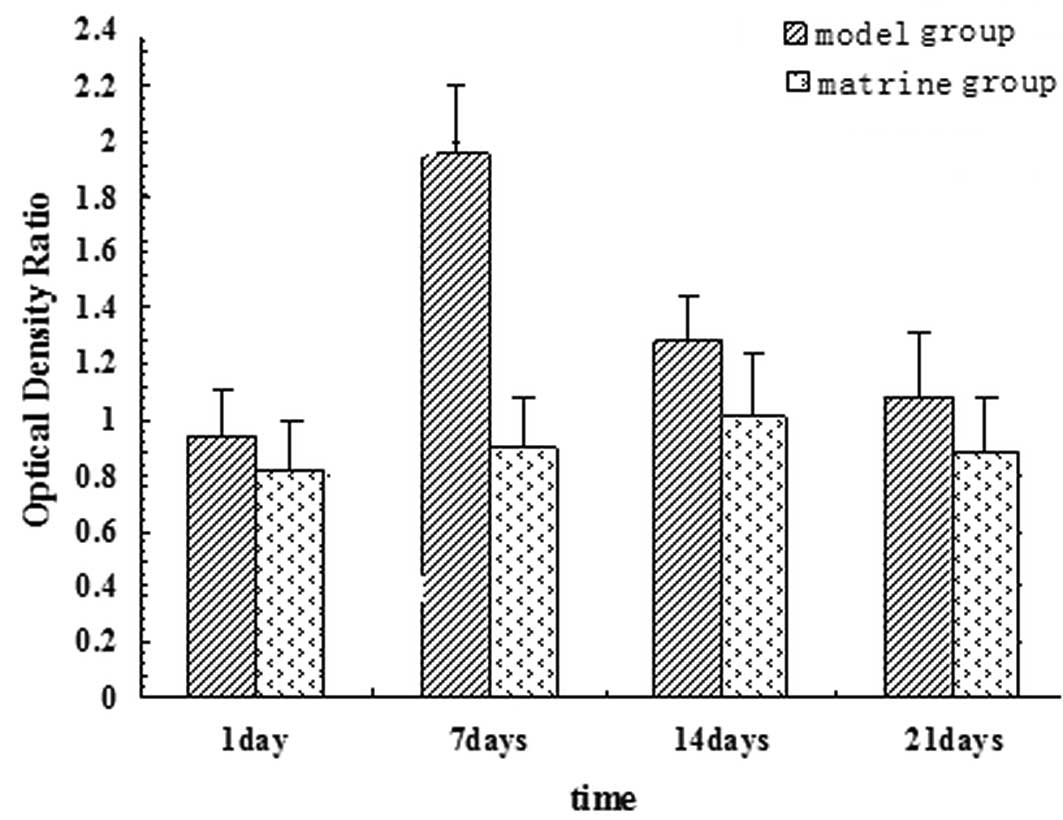
RBP-Jκ mRNA and HES1 mRNA expression
RBP-Jκ and HES1 mRNA expression was analyzed on days
1, 7, 14 and 21 of analysis. Expression peaked at day 7 of the
experiment (one day following PH) and decreased over time in the
model group. On day 1 of the experiment, no significant difference
was observed between RBP-Jκ and HES1 mRNA expression of the model
and matrine groups. By contrast, on days 7, 14 and 21 of the
experiment, a significant difference was found in RBP-Jκ and HES1
mRNA expression between the groups (P<0.05). The matrine trial
group experienced a larger decrease in expression levels (Figs. 6 and 7).
Discussion
Damaged liver tissue is mainly rebuilt through the
proliferation of mature hepatocytes and extracellular matrix.
However, the ability of the liver to regenerate becomes compromised
if it suffers excess damage or if the proliferation process of
hepatocytes is blocked. Oval cell activation and proliferation
serve as sources of tissue repair and cell replenishment, aiding
liver regeneration. The proliferation and differentiation of oval
cells to mature hepatocytes and cholangiocytes occurs in a specific
micro-environment of extracellular matrix and defined signaling
pathways. Therefore, it is not only difficult to determine the
mechanisms of proliferation and differentiation of oval cells, but
also which drugs induce differentiation. These are the two main
issues in the study of liver stem cells (14).
In the present study, proliferation and
differentiation of oval cells in oval cell-mediated liver
regeneration in 2-AAF/PH rat models was analyzed. The proliferation
of mature hepatocytes was inhibited by 2-AAF. Activation,
proliferation and differentiation of oval cells occurred shortly
following removal of two-thirds of the liver. An oval cell-mediated
liver regeneration model was successfully constructed and the
survival rate of the rats was >85% in the model and matrine
groups. No statistically significant difference in survival rate
was identified between the groups. Bleeding and serious infections
were minimized during the surgical procedure, leading to improved
recovery rates in the majority of the rats, therefore verifying the
high regenerative ability of the liver. Changes in liver function
were also analyzed and ALT and TBil were observed to alter over
time. The results demonstrate that ALT and TBil levels in the model
group were increased at day 1 following surgery, peaked at day 3
and then decreased at day 7. Compared with the model group, the
trial group exhibited improved liver function on days 1, 3, 7 and
14, particularly on days 3 and 7.
Results indicate that matrine may aid liver damage
recovery, consistent with previous studies. The mechanism by which
matrine mediates this function may be associated with its ability
to clear free radicals and induce the chondriosome drug metabolism
enzyme of hepatocytes (10,11).
The current study indictates that matrine regulates oval
cell-mediated liver regeneration through its effects on specific
signaling pathways that implement this mechanism.
In this study, proliferation of oval cells was
revealed to occur in accordance with the recovery of liver function
during regeneration of liver. Results indicate that liver function
was most impaired at days 3 and 7 following surgery, whereas oval
cell proliferation was the highest at day 7. The proliferation of
oval cells decreased following the recovery of liver function.
Compared with the model, the matrine trial group exhibited a
reduced number of proliferating oval cells on days 3, 7 and 14,
indicating that matrine inhibits the excessive proliferation of
oval cells. When proliferation of mature hepatocytes was blocked,
oval cells were induced to proliferate and differentiate in
response to the inhibition. During the process of oval
cell-mediated liver regeneration, matrine inhibited excessive
proliferation of oval cells, enabling regeneration of liver tissue
and the recovery of liver function to proceed normally. The
mechanism of the effects of matrine on oval cell-mediated liver
regeneration remains to be studied. In addition, it remains unclear
whether matrine directly induces oval cells to differentiate into
mature hepatocytes to repair the damaged liver tissue or whether it
affects differentiation, migration and proliferation indirectly by
regulating the extracellular matrix of the microenvironment
(15,16).
The Notch-RBP-Jκ signaling pathway is important for
maintenance of the characteristics and cell fate specification of
various types of stem cells. Alteration of signaling pathway
activity or unregulated expression of its ligands and receptors is
associated with specific diseases. RBP-Jκ is a key protein of the
Notch signaling pathway, and as a kind of DNA combination protein,
it is combined with specific DNA sequence to induce gene priming
(17,18). A number of studies have
demonstrated that upregulation of Notch signaling inhibits embryo
stem cells from differentiating into a number of specialized cell
types, including neural and insulin B cells (19,20).
With respect to liver stem cell regulation, previous studies have
reported high expression of Notch-1 protein in bone mesenchymal
stem cells and that this expression decreases following bone
mesenchymal stem cell differentiation into mature hepatocytes
(21,22).
Results of the present study are in agreement with
those obtained in previous studies. Serious damage to the liver led
to proliferation of a large number of oval cells, accompanied by
upregulation of RBP-Jκ and HES1 mRNA expression in the liver
tissue. As the liver was repaired, proliferation of oval cells
decreased and expression of RBP-Jκ and HES1 mRNA was downregulated,
indicating that upregulation of RBP-Jκ and HES1 mRNA is a pivotal
process during stem cell maintenance of oval cells. Compared with
model, the matrine group was identified to express low levels of
RBP-Jκ and HES1 mRNA on days 1, 7 and 14 following surgery,
indicating that downregulation of the Notch-RBP-Jκ signaling
pathway is closely associated with oval cell-mediated liver
regeneration.
The current study demonstrates that matrine inhibits
excessive proliferation of oval cells, protects liver function and
promotes liver regeneration through downregulation of the
Notch-RBP-Jκ signaling pathway. These results are consistent with
the hypothesis that Chinese medicine may contribute to advancement
of liver regeneration. However, there may be more signaling
pathways involved in this procdure. Therefore, additional studies
should be performed.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 30873423), the Youth Science
Research Program of Traditional Chinese Medicine Association of
Beijing (QN2010-7) and the Traditional Chinese Medicine Personnel
Training Plan.
References
|
1
|
Kuhlmann WD and Peschke P: Hepatic
progenitor cells, stem cells and AFP expression in models of liver
injury. Int J Exp Pathol. 87:343–359. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Libbrecht L and Roskams T: Hepatic
progenitor cells in human liver diseases. Semin Cell Dev Biol.
13:389–396. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Santoni-Rugiu E, Jelnes P, Thorgeirsson SS
and Bisgaard HC: Progenitor cells in liver regeneration: molecular
responses controlling their activation and expansion. APMIS.
113:876–902. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JS, Heo J, Libbrecht L, et al: A novel
prognostic subtype of human hepatocellular carcinoma derived from
hepatic progenitor cells. Nat Med. 12:410–416. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shupe T and Petersen BE: Evidence
regarding a stem cell origin of hepatocellular carcinoma. Stem Cell
Rev. 1:261–264. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Wang B, Zhou C and Bi Y: Matrine
induces apoptosis in angiotensin II-stimulated hyperplasia of
cardiac fibroblasts: effects on Bcl-2/Bax expression and caspase-3
activation. Basic Clin Pharmacol Toxicol. 101:1–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng H, Xia B, Zhang L, et al: Matrine
improves 2,4,6-trinitrobenzene sulfonic acid-induced colitis in
mice. Pharmacol Res. 53:202–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Zhu M, Shi R and Yang M: Radix
Sophorae flavescentis for chronic hepatitis B: a systematic
review of randomized trials. Am J Chin Med. 31:337–354. 2003.
View Article : Google Scholar
|
|
9
|
Long Y, Lin XT, Zeng KL and Zhang L:
Efficacy of intramuscular matrine in the treatment of chronic
hepatitis B. Hepatobiliary Pancreat Dis Int. 3:69–72.
2004.PubMed/NCBI
|
|
10
|
Zhang JP, Zhang M, Zhou JP, et al:
Antifibrotic effects of matrine on in vitro and in vivo models of
liver fibrosis in rats. Acta Pharmacol Sin. 22:183–186.
2001.PubMed/NCBI
|
|
11
|
Ai J, Gao HH, He SZ, Wang L, Luo DL and
Yang BF: Effects of matrine, artemisinin, tetrandrine on cytosolic
[Ca2+]i in guinea pig ventricular myocytes.
Acta Pharmacol Sin. 22:512–515. 2001.PubMed/NCBI
|
|
12
|
Baldi A, de Falco M, de Luca L, et al:
Characterization of tissue-specific expression of Notch-1 in human
tissues. Biol Cell. 96:303–311. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fausto N and Campbell JS: The role of
hepatocytes and oval cells in liver regeneration and repopulation.
Mech Dev. 120:117–130. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sautin YY, Jorgensen M, Petersen BE,
Saulnier-Blache JS, Crawford JM and Svetlov SI: Hepatic oval (stem)
cell expression of endothelial differentiation gene receptors for
lysophosphatidic acid in mouse chronic liver injury. J Hematother
Stem Cell Res. 11:643–649. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fiegel HC, Havers J, Kneser U, et al:
Influence of flow conditions and matrix coatings on growth and
differentiation of three-dimensionally cultured rat hepatocytes.
Tissue Eng. 10:165–174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leite AR, Correa-Giannella ML, Dagli ML,
et al: Fibronectin and laminin induce expession of islet cell
markers in hepatic oval cells in culture. Cell Tissue Res.
327:529–537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hansson EM, Lendahl U and Chapman G: Notch
signaling in development and disease. Semin Cancer Biol.
14:320–328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stoekhausen MT, Kristofersen K and Poulsen
HS: The functional role of Notch signaling in human gliomas. Neuro
Oncol. 12:199–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kobayashi T and Kageyama R: Hesl regulates
embryonic stem cell diferentiation by suppressing Notch signaling.
Genes Cells. 15:689–698. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murtaugh LC, Stanger BZ, Kwan KM and
Melton DA: Notch sin signaling controls multiple steps of
pancreatic differentiation. Proc Natl Acad Sci USA.
100:14920–14925. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okumoto K, Saito T, Hattori E, et al:
Differentiation of bone marrow cells into cells that express
liver-specific genes in vitro implication of the Notch signals in
differentiation. Biochem Biophys Res Commun. 304:691–695. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schwartz RE, Reyes M, Koodie L, et al:
Multipotent adult progen itor cells from bone marrow differentiate
into functional hepatocyte-like cells. J Clin Invest.
109:1291–1302. 2002. View Article : Google Scholar : PubMed/NCBI
|