1. Introduction
The retina originates in the neuroectodermal region
and is derived from the anterior neural tube; thus, it is
considered to be part of the central nervous system. The mature
mammalian retina is classically divided into ten layers, which
order from the inside to outside; the inner limiting membrane,
nerve fiber layer, ganglion cell layer, inner plexiform layer,
inner nuclear layer, outer plexiform layer, outer nuclear layer,
external limiting membrane, photoreceptor layer and retinal pigment
epithelium. The retina contains neuronal elements identified as
photoreceptors and horizontal, bipolar and amacrine cells and
retinal ganglion cells (RGCs) (1,2).
Comprising ~1% of all retinal cells, RGCs are the final output
neurons of the retina (3). RGCs
receive synaptic inputs from bipolar, amacrine and interplexiform
cells, as well as from gap junctions. Their axons cross the retina
and exit the eye via the optic disk, where they form the optic
nerve, relaying information to the visual centers of the brain.
Information received at the retinal level is conveyed to visual
centers via discharge patterns of RGCs. Thus, the intrinsic
membrane properties of RGCs are crucial in determining the methods
by which visual information is transmitted to the brain (4). Discharge patterns of RGCs are
primarily determined by the presence of ion channels, including
sodium (Na+) and potassium (K+) ion channels.
Of these ion channels, K+ channels are important in RGC
development, neurite outgrowth, axon guidance and action potential
and repetitive firing regulation (5,6).
A number of eye diseases, including glaucoma,
ischemic optic neuropathy, retinal degeneration and trauma, may
cause injury or the death of RGCs, and subsequently permanent
visual dysfunction. The study of ion channels, particularly
K+ channels in RGCs, may be beneficial in elucidating
the pathophysiology of RGCs and exploring novel RGC protection
strategies. In the past three decades, there has been considerable
progression in research on the function of K+ channels
in RGCs (7,8). The aim of the current review was to
summarize the roles of K+ channels in RGC development,
neurite outgrowth, axon guidance and the modulation of electrical
properties.
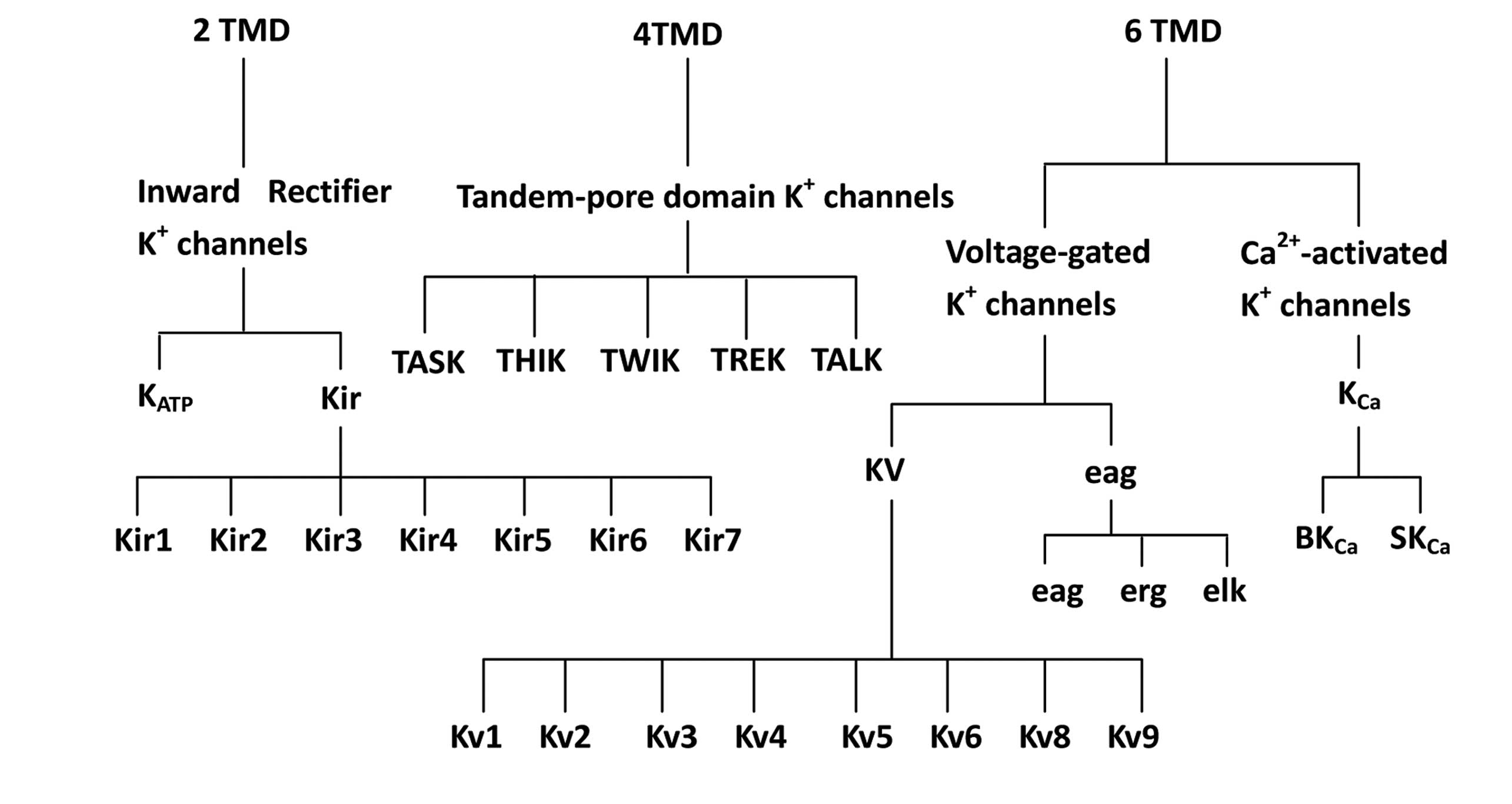
2. Classification of K+
channels
There are several types of K+ channels,
including voltage-gated K+ (Kv),
Ca2+-activated K+ (KCa),
inward-rectifier K+ (Kir), tandem-pore domain
K+ (TASK; also named ‘leak’ K+) and
ATP-sensitive K+ (KATP) channels (7,8).
K+ channels are classified into three groups based on
their predicted membrane topology; those with two, four and six
transmembrane domains (TMDs; Fig
1). The first group, consisting of two TMDs, comprises
Kir channels. The second group, consisting of four TMDs,
comprises ‘leak’ K+ channels (known as two-pore
channels) and the third group with six TMDs comprises Kv
and KCa channels. Each of these groups is divided into
families, which are further divided into subfamilies, the majority
of which have several closely related members (8).
The first group has a predicted membrane topology of
two TMDs (M1–M2) and a pore (P) domain; this group includes
Kir and KATP channels. Currently, seven
subfamilies (Kir1–7) have been identified, the majority
of which form K+ channels with varying degrees of inward
rectification when expressed in heterologous expression systems
(8).
The second group contains four putative TMDs (M1–4)
and two P domains (P1 and 2) (9,10)
and are referred to as TASK channels. There are currently five
members in this family (mechano-gated TREK, alkaline-activated
TALK, calcium-activated TWIK, acid-inhibited TASK and
halothane-inhibited THIK channels; Fig
1) (11–13), however, there is a possibility of
new members being cloned in the future. The current responds to
changes in the extracellular K+ concentration, as
described by the Goldman-Hodgkin-Katz equation, thus these channels
are also referred to as ‘leak’ K+ channels (9). A number of these channels may be
extensively modulated by specific factors (e.g., arachidonic acid
or pH) (14).
The third group contains six TMDs (S1–S6) with a
conserved P (pore or H5) domain. When expressed in heterologous
expression systems, Kv and/or KCa channels
are formed. This group contains the Kv channel family
(with eight subfamilies, Kv1–6, 8 and 9), as well as
members of the ether-à-go-go (Eag) and KCa channel
families (8).
3. Expression of K+ channels in
RGCs
Biochemical, molecular and pharmacological studies
have identified numerous types of K+ channels in RGCs,
including Kir(15–17),
KATP(18), TASK
(19), Kv(6,15,20),
Eag (21) and KCa
channels (Table I) (22,23).
Various subtypes of Kir channels have been confirmed to
be expressed in RGCs in Xenopus laevis(15) and rats (16,17,22).
Specific subunits of Kv channels have been observed to
be expressed in RGCs in Xenopus laevis(6,15,24,25),
goldfish (26), trout (27), mice (28–30,31),
rats (20,32,33)
and cats (34). Eag1 and Eag2 have
been reported to be expressed in RGCs in rats (21) and cattle (35). Large-conductance KCa
channels (BKCa) and small-conductance KCa
channels (SKCa) have been identified to express RGCs in
ferrets (36), trout (27), mice (37) and rats (22,23).
Various subunits of K+ channels are expressed in RGCs
with distinct subcellular localization. Chen et al(16) previously reported that
Kir1.1 was mainly expressed in the axons of RGCs, and
Kir2.1 and Kir2.3 were present in the somata
of RGCs. Staining methods demonstrated that Kir3.1 was
primarily present in an endoplasmic reticulum-like structure and
Kir3.2 was expressed in the cytoplasm and the
cytomembrane of somata, dendrites and axons of RGCs. Faint, sparse
labeling for Kir3.3 was observed in the cytomembrane.
Tian et al(17) reported
marked staining of Kir2.1 in the cytoplasm and staining
for Kir1.1, 2.3, 3.1, 3.2, 3.3 and 4.2 was predominantly
observed on the cell membrane. Concentrated Kv1.1 and
Kv1.3 staining was present in RGC somata, while
Kv1.2 distribution was restricted, with intense staining
in RGC axon fascicles (20,29).
Jow and Jeng (21) observed the
expression of Eag1 and Eag2 in the somata of rat RGCs.
 | Table IPotassium ion channels in retinal
ganglion cells. |
Table I
Potassium ion channels in retinal
ganglion cells.
| Classes of
K+ channel | K+
channel | Species | Reference |
|---|
| Inward-rectifier K
+ channels |
Kir1.1 | Rat | 16, 17 |
|
Kir2.1 | Xenopus
laevis | 15 |
| | Rat | 16, 17 |
|
Kir2.3 | Rat | 16,17 |
| Kir3
(GIRK) | Rat | 22 |
|
Kir3.1 | Rat | 16, 17 |
|
Kir3.2 | Rat | 16, 17 |
|
Kir3.3 | Rat | 16, 17 |
|
Kir4.2 | Rat | 17 |
|
KATP | Rat | 18 |
| Tandem-pore domain
K+ channels | TASK-2 | Rat | 19 |
| Voltage-gated
K+ channels | Kv | Mouse | 28 |
| | Cat | 33 |
|
Kv1.1 | Xenopus
laevis | 15 |
| | Rat | 20, 31 |
|
Kv1.2 | Mouse | 29 |
| | Rat | 20 |
|
Kv1.3 | Xenopus
laevis | 6 |
| | Mouse | 29 |
| | Rat | 20, 31 |
|
Kv1.4 | Mouse | 29 |
|
Kv1.5 | Xenopus
laevis | 6 |
|
Kv2.1 | Mouse | 29 |
|
Kv3.1 | Trout | 27 |
| | Rat | 32 |
|
Kv3.2 | Rat | 32 |
|
Kv3.4 | Xenopus
laevis | 6 |
|
Kv4.2 | Goldfish | 26 |
| | Xenopus
laevis | 6 |
| | Mouse | 29, 30,
37 |
|
Kv4.3 | Xenopus
laevis | 24, 25 |
| Eag1 | Rat | 21 |
| | Bovine | 34 |
| Eag2 | Rat | 21 |
| | Bovine | 34 |
|
Ca2+-activated K+
channels |
SKCa | Ferret | 35 |
| | Rat | 22, 23 |
|
BKCa | Ferret | 35 |
| | Trout | 27 |
| | Mouse | 36 |
4. Effects of K+ channels on
RGCs
RGCs function as output neurons in the retina,
encoding visual signals by producing spikes with characteristic
spatial and temporal patterns. The encoded signals are relayed to
visual centers via RGC axons. As the most diverse group of ion
channels, K+ channels play a key role in modulating the
electrical properties of neurons (5,6).
5. Kir channels
Kir channels are characterized by inward
rectification, which allows more current to flow inward than
outward via the channels (38).
These channels are important for regulating neuronal signaling and
membrane excitability (16,39–41).
Kir channels are important in the maintenance of resting
membrane potentials, thereby controlling the excitability of
neurons (42). Kir
channels are characterized by an increasing conductance under
hyperpolarization and a decreasing conductance under depolarization
(43). There are seven
Kir subfamilies (Kir1–7) (44), including ATP-regulated
(Kir1), classical (Kir2) and
G-protein-coupled (Kir3; GIRK). These subfamilies have
varying properties and kinetics, mediating distinct physiological
functions (45). It has previously
been reported that GIRK channels containing Kir3.2 and
3.3 subunits mediate the hyperpolarization and decrease in the
firing rate of rat locus coeruleus neurons caused by acute opioid
administration (46). Activation
of Kir channels serves as an underlying mechanism for
improved RGC survival (43).
Adenosine-induced hyperpolarization of RGCs is produced via the
activation of A1 receptors, initiating a signaling
cascade that activates GIRK and SKCa channels. This
represents a novel mechanism of adenosine-mediated neuromodulation
that may contribute to the regulation of RGC activity (22). Flupirtine, a drug approved for
patients suffering from chronic pain, may protect RGCs from
degeneration in a non-inflammatory animal model of optic nerve
transection. It has been verified through patch-clamp studies that
the activation of Kir channels is involved in
flupirtine-mediated neuroprotection (47).
6. KATP channels
KATP channels belong to the family of
Kir channels, and are composed of pore-forming units and
a sulfonylurea binding site (48).
These channels are located in the plasma membrane, as well as in
the mitochondrial inner membranes (43,49,50).
Mitochondria supply energy to the cell via the synthesis of ATP.
Respiring mitochondria transport H+ into the cytoplasm,
forming a transmembrane potential and pH gradient at the
mitochondrial membrane. An influx of K+, due to the
opening of mitochondrial KATP channels, decreases the
electrical gradient, but not the pH gradient, at the mitochondrial
membrane. Consequently, mitochondria no longer have to maintain two
gradients and therefore become more resistant to stress by
conserving energy (48,51). This indicates that KATP
channels may be involved in RGC survival and neuroprotection. A
number of studies have shown that KATP channels are
important in enhancing retinal resistance against ischemic insult
(18,52,53).
Retinal ischemic injury induces retinal neuron cell death by
excitotoxicity. In excitotoxic injury, increased glutamate causes
the continuous opening of NMDA or kainate channels, damaging the
retinal ion balance. The disturbed ionic environment is deleterious
to retinal neurons and leads to cell death. ATP depletion leads to
an opening of plasmalemmal channels, which hyperpolarizes the cell
membrane in states of energy deficiency or ischemia (54). Yamauchi et al(52) previously reported that the opening
of mitochondrial KATP channels may inhibit glutamate.
The opening of K+ channels induces the reduction of
K+ ion levels in the cytoplasm, which is accompanied by
an increase in ischemic tolerance, mimicking ischemic
preconditioning (55). This has
been hypothesized to function in a similar manner to the way that
low levels of K+ ions inhibit or delay neuronal cell
toxicity by Ca2+ influx in excitotoxicity (56). A number of potent
KATP-channel openers, including KR-31378 and
gabapentin-lactam, exhibit RGC neuroprotection by regulating ion
balance during excitotoxicity (56,57).
KATP channel agonists may prevent ischemia-induced
expression of the immediate early genes, c-fos and c-jun (58). In addition, KATP
channels are essential for cerebral ischemic preconditioning
(59–61). Early ischemic preconditioning has
been demonstrated in the rat retina, and KATP channel
openers mimic the effect of ischemic preconditioning (59). The activation of KATP
channels following a sublethal ischemic stimulus may involve
adenosine/adenosine receptors, as KATP channels are
localized in close proximity to adenosine A1 receptors
(62). A release of endogenous
adenosine from the retina following K+ depolarization or
ischemic insult has been reported (63,64).
It is therefore conceivable that adenosine formed from the
breakdown of ATP may be released during the initial ischemia and
indirectly activates KATP channels via the G-protein
pathway following binding to the adenosine A1 receptor
(65). Sakamoto et
al(53) observed that the
stimulation of adenosine receptors, opening of KATP
channels and activation of protein kinase C may be involved in the
underlying protective mechanisms of early ischemic
preconditioning.
7. TASK channels
TASK channels belong to the four putative TMD and
two pore domain channels (13).
These channels are divided into three subtypes, TASK-1, −2 and −3.
TASK channels are distinguished from other family members of
K+ channels due to their sensitivities to changes in
extracellular pH. Although they have varying ranges of pH
sensitivity, these channels are activated by extracellular alkaline
pH and inhibited by acidic pH (19). TASK channels are not all
voltage-gated and are resistant to conventional K+
channel blockers. In addition, these channels have been
hypothesized to contribute to the regulation of resting membrane
potentials and firing patterns of neurons (11,13).
TASK-2 is expressed in RGCs, indicating that TASK-2 may be involved
in the regulation of resting membrane potentials and firing
patterns of RGCs.
8. Kv channels
Kv channels contain eight potassium
channel subfamilies (Kv1–6, 8 and 9). All the
electrically active Kv channel subfamilies are
represented in the adult rodent retina in spatially restricted
patterns (29,66,67).
The coordinated expression of Kv channels is of key
importance for the maturation of membrane excitability and
electrical signaling behavior in the retina. These channels
determine the resting membrane potential of retinal neurons,
particularly RGCs, modulating their intrinsic firing properties
(68) and regulating neuronal
differentiation processes (24).
There are three aspects of the effect of Kv channels on
RGCs.
RGC electrical activity
Kv currents are important regulators of
cellular excitability, functioning to modulate the amplitude,
duration and frequency of action potentials and subthreshold
depolarizations. Altering Kv channel function is useful
for identifying the cellular processes that are regulated by
excitability (69). Kv
channels carry outward currents that repolarize the membrane in
response to action potentials or spontaneous depolarizations.
Therefore, Kv channels are critical for determining the
shape of the action potential, the time course and extent of the
hyperpolarization following a spike, return to resting potential,
the delay to spike onset and in regulating repetitive firing
(5,44,70,71).
In RGCs, Kv channels exhibit a distinct subcellular
localization pattern in the axon, thereby shaping the firing
patterns of action potentials (72). Kv1.3 channels produce a
slowly inactivating current, whereas Kv1.1 and 1.2
produce currents with fast or slow inactivation, depending on
accessory molecules (73).
Kuznetsov et al(33)
observed that Kv3.1/3.2 channels underlie the fast
firing of rat RGCs and provide, at a given firing frequency, a
1.8-fold restriction of Ca2+ influx, which protects the
cells from its cytotoxic action.
RGC development
A number of studies have indicated that
Kv channels have important and varied roles in the
development of neuronal cell types, and they have been implicated
in numerous processes, including cell proliferation or
differentiation, neurite outgrowth and axon guidance. Pollock et
al(6) revealed that the
retinal expression patterns of different Kv channels
have various roles in retinal development. Kv1.3, 1.5,
3.4 and 4.2 channels became restricted to postmitotic retinal cells
and/or synaptic layers, indicating additional roles for these
channels in cell differentiation and synaptogenesis. The restricted
and transient nature of Kv4.2 protein expression in RGCs
is particularly intriguing, as it implicates the Kv
channel in the differentiation of a specific RGC subtype. The
presence of Kv1.3 channels in axons indicates that they
may be involved in the myelination of the optic nerve.
Kv1.3, 1.5 and 3.4 subunits continue to be expressed by
RGCs beyond the time the visual system first becomes functional,
indicating that they are eventually involved in the regulation of
electrical activity (6). It is
proposed that these Kv channels, through their control
of membrane potential, regulate the downstream signaling of axon
growth and guidance cues (74).
Membrane excitability regulates the earliest differentiation of RGC
dendritic arbors (15), while key
regulators of membrane excitability are Kv channels
(44,71). This indicates that Kv
may regulate the differentiation of RGC dendritic arbors. McFarlane
and Pollock (24) observed that
RGCs and their growth cones express Kv channels during
progression to the midbrain target, the optic tectum. It was also
observed that the blockade of Kv channels using
4-aminopyridine, a Kv channel blocker, inhibited RGC
axon extension and caused the aberrant routing of numerous RGC
fibers. Inhibiting Kv channels affects the ability of
RGC axons to extend in culture and causes extension and pathfinding
defects of the axons in vivo. These observations indicate
that Kv channel activity regulates the guidance of
growing axons of RGCs. Pollock et al(75) reported that the chemorepellent
fibroblast growth factor-2 repulsed RGC growth cones in the
presence of 4-aminopyridine, but not tetraethylammonium, indicating
that tetraethylammonium- and 4-aminopyridine-sensitive
Kv channels differ in the manner by which they regulate
the response of RGC axons to extension and guidance cues. Qu et
al(30) noted that
Kv4.2-mediated currents were important for development
in a subset of RGCs, particularly around postnatal day 10 as the
bipolar cells mature. In addition, the majority of mouse and cat
RGCs express Kv currents soon after birth, before the
cells have the ability to generate spontaneous action potentials
(28,33).
The regulation of intracellular calcium
([Ca2+]i) appears to be a particularly
attractive function for Kv channels, since resting
[Ca2+]i and dynamic changes in
[Ca2+]i are important for regulating the
response of the growth cone to extrinsic cues (76–78).
In growth cones, Kv channel activity may function to
modulate [Ca2+]i by regulating the membrane
potential and opening voltage-gated Ca2+ channels.
Maruoka et al(79) and
Petrecca et al(80)
observed that Kv1.5 and 4.2 channels
coimmunoprecipitated with the cytoskeletal components of
α-actinin-2 and filamin, respectively, indicating the possibility
of specific Kv channels functioning directly or
indirectly in the reorganization of the cytoskeleton in response to
extrinsic cues.
RGC protection
Apoptosis in several cell types is accompanied by
increased K+ currents, the depletion of cytoplasmic
K+ and cell shrinkage (81–84).
This ‘apoptotic volume decrease’ precedes mitochondrial
depolarization, apoptosome formation and cell fragmentation, and is
considered to be a triggering event (81–84).
The volume decrease of apoptotic cells occurs through the channel-
and transporter-mediated efflux of osmolytes, particularly
K+ and chloride ions. This ion efflux creates an osmotic
gradient that draws water out of the cells (85–87).
The decrease in cytoplasmic K+ concentration may
activate molecules in the apoptotic cascade. Consequently, one
experimental strategy has been to target K+ channels. An
increasing number of studies support the hypothesis that
Kv channels are involved in the protection of RGCs.
Kv1.1 and Kv1.3 channels contribute to
cell-autonomous death of RGCs through various components of the
apoptotic machinery (20).
Kv1.1 depletion increases the anti-apoptotic gene,
Bcl-xL. By contrast Kv1.3 depletion reduces the
pro-apoptotic genes, caspase-3, caspase-9 and Bad (20). It has been reported that
Kv contributions depend on their location.
Kv1.1 and 1.3 are highly expressed in RGC somata and
have the greatest effect on cell survival, whereas the contribution
of the predominantly axonal Kv1.2 channel is limited
(20). By contrast, Kv
channels are functionally linked to RGC degeneration indirectly via
non-neuronal cells, most likely by blocking Kv1.3
channels in microglia (31,88–90).
The Kv1.3 channel is highly expressed in microglia and
contributes to microglial activation and neurotoxicity (91). Utilizing the optic nerve
transection model, Koeberle et al(32) reported the following observations:
(i) Following optic nerve transection, intraocular injection of
agitoxin-2, a potent blocker of Kv1.3 channels, reduced
microglial activation and the expression of several inflammatory
genes in the damaged retina, which indicated that Kv
channels contribute to inflammation in the adult retina in
vivo; (ii) the retinal expression of several growth factors was
upregulated following axotomy. Intraocular injection of margatoxin,
a blocker of Kv1.3 channels, increased retinal b-FGF. By
contrast, RGC-specific depletion of Kv1.3 from RGCs
decreased GDNF and b-FGF levels. Agitoxin-2 injection did not
affect growth factor levels; (iii) injecting agitoxin-2 increased
c-Fos, TGFβ, IL-1β, IL-1ra and TNFα beyond any effects of
Kv1.1 or Kv1.3 channel knockdown in RGCs; and
(iv) combining siRNA-mediated knockdown of Kv1.1 or 1.3
with intraocular injection of agitoxin-2 or margatoxin provided
increased RGC rescue, with up to 55% of RGCs surviving at day 14
following optic nerve transection (31). In addition, as the Kv1.3
channel is important for the activation of T lymphocytes, it is
also possible that this channel is involved in immune-mediated
damage in the retina (92).
9. Eag channels
The Eag channel was the first reported member of
the Eag family of voltage-gated K+ channels (93). In mammals, two Eag channel subunit
isoforms have been identified, Eag1 and Eag2, sharing ~70% identity
in amino acid sequence (94–96).
Jow and Jeng (21) observed that
Eag1 channels are localized at the dendrites and somata of RGCs,
and Eag2 channels are localized at the somata of RGCs. Eag1 and
Eag2 channels have also been identified to express RGCs in cattle
(34). The widespread expression
of Eag1 channels in the somatodendritic compartment indicates that
these channels may contribute to dendritic repolarization during
excitatory postsynaptic potentials and to the attenuation of the
back propagation of action potentials. Thus, Eag1 channels are
critical in regulating electrical coupling between dendrites and
cell bodies in RGCs (21). In
addition, Eag channels are involved in the formation of
IKx channels and may contribute to the dark current in
the rod inner segment (34).
10. KCa channels
Single channel studies have revealed several types
of calcium-activated potassium channels, which may be divided into
two distinct groups based on their pharmacological and biophysical
properties, BKCa and SKCa. BKCa
channels, which may be blocked by charybdotoxin (CTX), have a high
unitary conductance and exhibit sensitivity to voltage and
submicromolar concentrations of CTX (97). The current passing through these
channels has been implicated in action potential repolarization and
fast hyperpolarization following the spike (98). By contrast, SKCa
channels have a markedly lower unitary conductance, are voltage-
and CTX-insensitive and are activated by nanomolar concentrations
of calcium (97). The current
flowing through these channels is sensitive to apamin, a
SKCa channel blocker, and has been shown to underlie the
slow after-hyperpolarization that is responsible for action
potential frequency adaptation in a number of cells (99,100). KCa currents have been
reported to be important in the regulation of neuronal activity. In
particular, these currents have been shown to contribute towards
the repolarizing phase of the action potential (98), control the repetitive discharge of
spikes (101–103) and are involved in various forms
of oscillatory membrane behavior (104).
BKCa and SKCa channels are
located in RGCs (3,35,105). KCa channels contribute
to repetitive firing in RGCs (105), and blocking KCa
channels has been shown to increase the current-evoked firing rate
of RGCs in ferret retinas (35).
Whole-cell recordings from isolated and intact RGCs revealed that
conductances regulate the frequency of spike discharges in response
to maintained depolarizations. Activation of these channels leads
to an increase in the time to spike threshold and in the
hyperpolarization following the spike, decreasing the rate of
sustained discharges (35). Wang
et al(106) revealed that
apamin induced low-frequency bursts of a relatively long duration,
a pattern similar to that observed in developing RGCs. By contrast,
CTX induced high-frequency bursts of a short duration that were
periodic; these observations indicate that the modulation of
KCa conductances provides an effective means for
affecting the spontaneous discharge patterns of RGCs. In addition,
firing patterns were evident following blockade of the small
conductance and resembled the spontaneous discharges noted during
development, indicating a possible link between the functional
state of KCa conductances and the spontaneous discharges
manifested by immature RGCs (106). Utilizing patch-clamp recordings
in mouse RGCs, Nemargut et al(37) observed that during dark adaptation,
the blockage of BKCa channels increased the spontaneous
excitatory postsynaptic currents (EPSCs) and light-evoked on-EPSCs,
while it decreased the light-evoked off-inhibitory postsynaptic
currents (IPSCs). However, under light adaptation, it decreased the
light-evoked on-EPSCs, the spontaneous IPSCs and the light-evoked
on- and off-IPSCs. The blockage of BKCa channels
significantly altered the outputs of RGCs by changing their
light-evoked responses into a bursting pattern and increasing the
light-evoked depolarization of the membrane potentials, while it
did not significantly change the peak firing rates of light-evoked
responses (36). These
observations indicate that BKCa channels play various
roles in mediating visual signals in the retina under different
ambient light conditions.
11. Conclusions
The discharge patterns of RGCs are primarily
determined by the presence of ion channels. As the most diverse
group of ion channels, K+ channels are key in modulating
the electrical properties of RGCs. Biochemical, molecular and
pharmacological studies have identified numerous types of
K+ channels in RGCs, including Kir,
KATP, TASK, Kv, Eag and KCa.
Kir channels are important in maintaining the resting
membrane potential and modulating RGC excitability. KATP
channels are involved in RGC survival and neuroprotection. TASK
channels are considered to contribute to the regulation of resting
membrane potentials and the firing patterns of RGCs. Kv
channels are important regulators of cellular excitability,
functioning to modulate the amplitude, duration and frequency of
action potentials and subthreshold depolarizations. Kv
channels are important in RGC development and protection. Eag
channels may contribute to dendritic repolarization during
excitatory postsynaptic potentials and to the attenuation of the
back propagation of action potentials. KCa channels have
been observed to contribute to repetitive firing in RGCs.
Considering these important roles of K+ channels on
RGCs, the study of K+ channels may be conducive to
elucidating the pathophysiology of RGCs and to explore new RGC
protection strategies.
Acknowledgements
This study was funded by the Shanghai Leading
Academic Discipline Project (no. S30205) and the Shanghai ‘Science
and Technology Innovation Action Plan’ Basic Research Key Project
(nos. 11JC1407700 and 11JC1407701).
References
|
1
|
Pycock CJ: Retinal neurotransmission. Surv
Ophthalmol. 29:355–365. 1985. View Article : Google Scholar
|
|
2
|
Marquardt T and Gruss P: Generating
neuronal diversity in the retina: one for nearly all. Trends
Neurosci. 25:32–38. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lipton SA and Tauck DL: Voltage-dependent
conductances of solitary ganglion cells dissociated from the rat
retina. J Physiol. 385:361–391. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robinson DW and Chalupa LM: The intrinsic
temporal properties of alpha and beta retinal ganglion cells are
equivalent. Curr Biol. 7:366–374. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Augustine GJ: Regulation of transmitter
release at the squid giant synapse by presynaptic delayed rectifier
potassium current. J Physiol. 431:343–364. 1990. View Article : Google Scholar
|
|
6
|
Pollock NS, Ferguson SC and McFarlane S:
Expression of voltage-dependent potassium channels in the
developing visual system of Xenopus laevis. J Comp Neurol.
452:381–391. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rudy B: Diversity and ubiquity of K
channels. Neuroscience. 25:729–749. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coetzee WA, Amarillo Y, Chiu J, Chow A,
Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D,
Saganich M, Vega-Saenz de Miera E and Rudy B: Molecular diversity
of K+ channels. Ann NY Acad Sci. 868:233–285. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldstein SA, Wang KW, Ilan N and Pausch
MH: Sequence and function of the two P domain potassium channels:
implications of an emerging superfamily. J Mol Med (Berl).
76:13–20. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lesage F, Guillemare E, Fink M, Duprat F,
Lazdunski M, Romey G and Barhanin J: TWIK-1, a ubiquitous human
weakly inward rectifying K+ channel with a novel
structure. EMBO J. 15:1004–1011. 1996.PubMed/NCBI
|
|
11
|
Honoré E: The neuronal background K2P
channels: focus on TREK1. Nat Rev Neurosci. 8:251–261.
2007.PubMed/NCBI
|
|
12
|
Mathie A: Neuronal two-pore-domain
potassium channels and their regulation by G protein-coupled
receptors. J Physiol. 578:377–385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Connell AD, Morton MJ and Hunter M:
Two-pore domain K+ channels-molecular sensors. Biochim
Biophys Acta. 1566:152–161. 2002. View Article : Google Scholar
|
|
14
|
Fink M, Lesage F, Duprat F, Heurteaux C,
Reyes R, Fosset M and Lazdunski M: A neuronal two P domain
K+ channel stimulated by arachidonic acid and
polyunsaturated fatty acids. EMBO J. 17:3297–3308. 1998.PubMed/NCBI
|
|
15
|
Hocking JC, Pollock NS, Johnston J, Wilson
RJ, Shankar A and McFarlane S: Neural activity and branching of
embryonic retinal ganglion cell dendrites. Mech Dev. 129:125–135.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen L, Yu YC, Zhao JW and Yang XL:
Inwardly rectifying potassium channels in rat retinal ganglion
cells. Eur J Neurosci. 20:956–964. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tian M, Chen L, Xie JX, Yang XL and Zhao
JW: Expression patterns of inwardly rectifying potassium channel
subunits in rat retina. Neurosci Lett. 345:9–12. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ettaiche M, Heurteaux C, Blondeau N,
Borsotto M, Tinal N and Lazdunski M: ATP-sensitive potassium
channels (K(ATP)) in retina: a key role for delayed ischemic
tolerance. Brain Res. 890:118–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang XM, Zhong YM and Yang XL: TASK-2 is
expressed in proximal neurons in the rat retina. Neuroreport.
20:946–950. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koeberle PD, Wang Y and Schlichter LC:
Kv1.1 and Kv1.3 channels contribute to the degeneration of retinal
ganglion cells after optic nerve transection in vivo. Cell Death
Differ. 17:134–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jow GM and Jeng CJ: Differential
localization of rat Eag1 and Eag2 potassium channels in the retina.
Neurosci Lett. 431:12–16. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clark BD, Kurth-Nelson ZL and Newman EA:
Adenosine-evoked hyperpolarization of retinal ganglion cells is
mediated by G-protein-coupled inwardly rectifying K+ and
small conductance Ca2+-activated K+ channel
activation. J Neurosci. 29:11237–11245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klöcker N, Oliver D, Ruppersberg JP, Knaus
HG and Fakler B: Developmental expression of the small-conductance
Ca(2+)-activated potassium channel SK2 in the rat retina. Mol Cell
Neurosci. 17:514–520. 2001.
|
|
24
|
McFarlane S and Pollock NS: A role for
voltage-gated potassium channels in the outgrowth of retinal axons
in the developing visual system. J Neurosci. 20:1020–1029.
2000.PubMed/NCBI
|
|
25
|
Lautermilch NJ and Spitzer NC: The KV4.3
Shal gene is developmentally upregulated in Xenopus embryos
and encodes a potassium current modulated by arachidonic acid. Soc
Neurosci Abstr. 23:17381997.
|
|
26
|
Yazulla S and Studholme KM:
Co-localization of Shaker A-type K+ channel (Kv1.4) and
AMPA-glutamate receptor (GluR4) immunoreactivities to dendrites of
OFF-bipolar cells of goldfish retina. J Neurocytol. 28:63–73. 1999.
View Article : Google Scholar
|
|
27
|
Henne J and Jeserich G: Maturation of
spiking activity in trout retinal ganglion cells coincides with
upregulation of Kv3.1- and BK-related potassium channels. J
Neurosci Res. 75:44–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rörig B and Grantyn R: Ligand- and
voltage-gated ion channels are expressed by embryonic mouse retinal
neurones. Neuroreport. 5:1197–1200. 1994.PubMed/NCBI
|
|
29
|
Pinto LH and Klumpp DJ: Localization of
potassium channels in the retina. Prog Retin Eye Res. 17:207–230.
1998.PubMed/NCBI
|
|
30
|
Qu J, Mulo I and Myhr KL: The development
of Kv4.2 expression in the retina. Neurosci Lett. 464:209–213.
2009. View Article : Google Scholar
|
|
31
|
Klumpp DJ, Song EJ and Pinto LH:
Identification and localization of K+ channels in the
mouse retina. Vis Neurosci. 12:1177–1190. 1995. View Article : Google Scholar
|
|
32
|
Koeberle PD and Schlichter LC: Targeting
K(V) channels rescues retinal ganglion cells in vivo directly and
by reducing inflammation. Channels (Austin). 4:337–346. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuznetsov KI, Grygorov OO, Maslov VY,
Veselovsky NS and Fedulova SA: Kv3 channels modulate calcium
signals induced by fast firing patterns in the rat retinal ganglion
cells. Cell Calcium. 52:405–411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Skaliora I, Robinson DW, Scobey RP and
Chalupa LM: Properties of K+ conductances in cat retinal
ganglion cells during the period of activity-mediated refinements
in retinofugal pathways. Eur J Neurosci. 7:1558–1568. 1995.
|
|
35
|
Frings S, Brüll N, Dzeja C, Angele A,
Hagen V, Kaupp UB and Baumann A: Characterization of ether-à-go-go
channels present in photoreceptors reveals similarity to IKx, a
K+ current in rod inner segments. J Gen Physiol.
111:583–599. 1998.
|
|
36
|
Wang GY, Robinson DW and Chalupa LM:
Calcium-activated potassium conductances in retinal ganglion cells
of the ferret. J Neurophysiol. 79:151–158. 1998.PubMed/NCBI
|
|
37
|
Nemargut JP, Zhu J, Savoie BT and Wang GY:
Differential effects of charybdotoxin on the activity of retinal
ganglion cells in the dark- and light-adapted mouse retina. Vision
Res. 49:388–397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Isomoto S, Kondo C and Kurachi Y: Inwardly
rectifying potassium channels: their molecular heterogeneity and
function. Jpn J Physiol. 47:11–39. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nichols CG and Lopatin AN: Inward
rectifier potassium channels. Annu Rev Physiol. 59:171–191. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Neusch C, Weishaupt JH and Bähr M: Kir
channels in the CNS: emerging new roles and implications for
neurological diseases. Cell Tissue Res. 311:131–138.
2003.PubMed/NCBI
|
|
41
|
Tanaka S, Wu N, Hsaio CF, Turman J Jr and
Chandler SH: Development of inward rectification and control of
membrane excitability in mesencephalic v neurons. J Neurophysiol.
89:1288–1298. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pongs O: Molecular biology of
voltage-dependent potassium channels. Physiol Rev. 72(4 Suppl):
S69–S88. 1992.PubMed/NCBI
|
|
43
|
Kubo Y, Baldwin TJ, Jan YN and Jan LY:
Primary structure and functional expression of a mouse inward
rectifier potassium channel. Nature. 362:127–133. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jan LY and Jan YN: Voltage-gated and
inwardly rectifying potassium channels. J Physiol. 505:267.b1–282.
1997.PubMed/NCBI
|
|
45
|
Reimann F and Ashcroft FM: Inwardly
rectifying potassium channels. Curr Opin Cell Biol. 11:503–508.
1999. View Article : Google Scholar
|
|
46
|
Torrecilla M, Marker CL, Cintora SC,
Stoffel M, Williams JT and Wickman K: G-protein-gated potassium
channels containing Kir3.2 and Kir3.3 subunits mediate the acute
inhibitory effects of opioids on locus ceruleus neurons. J
Neurosci. 22:4328–4334. 2002.
|
|
47
|
Sättler MB, Williams SK, Neusch C, Otto M,
Pehlke JR, Bähr M and Diem R: Flupirtine as neuroprotective add-on
therapy in autoimmune optic neuritis. Am J Pathol. 173:1496–1507.
2008.PubMed/NCBI
|
|
48
|
Szewczyk A and Marbán E: Mitochondria: a
new target for K channel openers? Trends Pharmacol Sci. 20:157–161.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Inagaki N, Gonoi T, Clement JP 4th, Namba
N, Inaza J, Gonzalez G, Aguilar-Bryan L, Seino S and Bryan J:
Reconstitution of IKATP: an inward rectifier subunit plus the
sulfonylurea receptor. Science. 270:1166–1170. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Inagaki N, Inazawa J and Seino S: cDNA
sequence, gene structure and chromosomal localization of the human
ATP-sensitive potassium channel, uKATP-1, gene (KCNJ8). Genomics.
30:102–104. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kicińska A, D bska G, Kunz W and Szewczyk
A: Mitochondrial potassium and chloride channels. Acta Biochim Pol.
47:541–551. 2000.
|
|
52
|
Yamauchi T, Kashii S, Yasuyoshi H, Zhang
S, Honda Y and Akaike A: Mitochondrial ATP-sensitive potassium
channel: a novel site for neuroprotection. Invest Ophthalmol Vis
Sci. 44:2750–2756. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sakamoto K, Yonoki Y, Kuwagata M, Saito M,
Nakahara T and Ishii K: Histological protection against
ischemia-reperfusion injury by early ischemic preconditioning in
rat retina. Brain Res. 1015:154–160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kersten JR, Gross GJ, Pagel PS and
Warltier DC: Activation of adenosine triphosphate-regulated
potassium channels: mediation of cellular and organ protection.
Anesthesiology. 88:495–513. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rodrigo GC and Standen NB: ATP-sensitive
potassium channels. Curr Pharm Des. 11:1915–1940. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Choi A, Choi JS, Yoon YJ, Kim KA and Joo
CK: KR-31378, a potassium-channel opener, induces the protection of
retinal ganglion cells in rat retinal ischemic models. J Pharmacol
Sci. 109:511–517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pielen A, Kirsch M, Hofmann HD, Feuerstein
TJ and Lagrèze WA: Retinal ganglion cell survival is enhanced by
gabapentin-lactam in vitro: evidence for involvement of
mitochondrial KATP channels. Graefes Arch Clin Exp Ophthalmol.
242:240–244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Heurteaux C, Bertaina V, Widmann C and
Lazdunski M: K+ channel openers prevent global
ischemia-induced expression of c-fos, c-jun, heat shock protein and
amyloid β-protein precursor genes and neuronal death in rat
hippocampus. Proc Natl Acad Sci USA. 90:9431–9435. 1993.
|
|
59
|
Heurteaux C, Lauritzen I, Widmann C and
Lazdunski M: Essential role of adenosine, adenosine A1 receptors
and ATP-sensitive K+ channels in cerebral ischemic
preconditioning. Proc Natl Acad Sci USA. 92:4666–4670. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu Y, Sato T, Seharaseyon J, Szewczyk A,
O'Rourke B and Marbán E: Mitochondrial ATP-dependent potassium
channels. Viable candidate effectors of ischemic preconditioning.
Ann NY Acad Sci. 874:27–37. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tanno M, Miura T, Tsuchida A, Miki T,
Nishino Y, Ohnuma Y and Shimamoto K: Contribution of both the
sarcolemmal K(ATP) and mitochondrial K(ATP) channels to infarct
size limitation by K(ATP) channel openers: differences from
preconditioning in the role of sarcolemmal K(ATP) channels. Naunyn
Schmiedebergs Arch Pharmacol. 364:226–232. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kvanta A, Seregard S, Sejersen S, Kull B
and Fredholm BB: Localization of adenosine receptor messenger RNAs
in the rat eye. Exp Eye Res. 65:595–602. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Roth S, Park SS, Sikorski CW, Osinski J,
Chan R and Loomis K: Concentrations of adenosine and its
metabolites in the rat retina/choroid during reperfusion after
ischemia. Curr Eye Res. 16:875–885. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Roth S, Rosenbaum PS, Osinski J, Park SS,
Toledano AY, Li B and Moshfeghi AA: Ischemia induces significant
changes in purine nucleoside concentration in the retina-choroid in
rats. Exp Eye Res. 65:771–779. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kurachi Y: G protein regulation of cardiac
muscarinic potassium channel. Am J Physiol. 269:C821–C830.
1995.PubMed/NCBI
|
|
66
|
Serôdio P and Rudy B: Differential
expression of Kv4 K+ channel subunits mediating
subthreshold transient K+ (A-type) currents in rat
brain. J Neurophysiol. 79:1081–1091. 1998.
|
|
67
|
Yazulla S and Studholme KM: Differential
distribution of Shaker-like and Shab-like K+-channel
subunits in goldfish retina and retinal bipolar cells. J Comp
Neurol. 396:131–140. 1998.PubMed/NCBI
|
|
68
|
Dantzker JL and Callaway EM: The
development of local, layer-specific visual cortical axons in the
absence of extrinsic influences and intrinsic activity. J Neurosci.
18:4145–4154. 1998.PubMed/NCBI
|
|
69
|
Ribera AB and Spitzer NC: Developmental
regulation of potassium channels and the impact on neuronal
differentiation. Ion Channels. 3:1–38. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jan LY and Jan YN: Cloned potassium
channels from eukaryotes and prokaryotes. Annu Rev Neurosci.
20:91–123. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wei A, Jegla T and Salkoff L: Eight
potassium channel families revealed by the C. elegans genome
project. Neuropharmacology. 35:805–829. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Van Wart A, Trimmer JS and Matthews G:
Polarized distribution of ion channels within microdomains of the
axon initial segment. J Comp Neurol. 500:339–352. 2007.PubMed/NCBI
|
|
73
|
Grissmer S, Nguyen AN, Aiyar J, Hanson DC,
Mather RJ, Gutman GA, Karmilowicz MJ, Auperin DD and Chandy KG:
Pharmacological characterization of five cloned voltage-gated
K+ channels, types Kv1.1, 1.2, 1.3, 1.5 and 3.1, stably
expressed in mammalian cell lines. Mol Pharmacol. 45:1227–1234.
1994.PubMed/NCBI
|
|
74
|
McFarlane S: Attraction vs. repulsion: the
growth cone decides. Biochem Cell Biol. 78:563–568. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pollock NS, Atkinson-Leadbeater K,
Johnston J, Larouche M, Wildering WC and McFarlane S: Voltage-gated
potassium channels regulate the response of retinal growth cones to
axon extension and guidance cues. Eur J Neurosci. 22:569–578. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Goldberg DJ and Grabham PW: Braking news:
calcium in the growth cone. Neuron. 22:423–425. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gomez TM and Spitzer NC: In vivo
regulation of axon extension and pathfinding by growth-cone calcium
transients. Nature. 397:350–355. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
78
|
Petersen OH and Cancela JM: Attraction or
repulsion by local Ca(2+) signals. Curr Biol. 10:R311–R314.
2000.
|
|
79
|
Maruoka ND, Steele DF, Au BP, Dan P, Zhang
X, Moore ED and Fedida D: alpha-actinin-2 couples to cardiac Kv1.5
channels, regulating current density and channel localization in
HEK cells. FEBS Lett. 473:188–194. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Petrecca K, Miller DM and Shrier A:
Localization and enhanced current density of the Kv4.2 potassium
channel by interaction with the actin-binding protein filamin. J
Neurosci. 20:8736–8744. 2000.PubMed/NCBI
|
|
81
|
Lang F, Föller M, Lang KS, Lang PA, Ritter
M, Gulbins E, Vereninov A and Huber SM: Ion channels in cell
proliferation and apoptotic cell death. J Membr Biol. 205:147–157.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Bortner CD and Cidlowski JA: Cell
shrinkage and monovalent cation fluxes: role in apoptosis. Arch
Biochem Biophys. 462:176–188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Burg ED, Remillard CV and Yuan JX:
Potassium channels in the regulation of pulmonary artery smooth
muscle cell proliferation and apoptosis: pharmacotherapeutic
implications. Br J Pharmacol. 153(Suppl 1): S99–S111. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yu SP: Regulation and critical role of
potassium homeostasis in apoptosis. Prog Neurobiol. 70:363–386.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yu SP, Yeh CH, Sensi SL, Gwag BJ,
Canzoniero LM, Farhangrazi ZS, Ying HS, Tian M, Dugan LL and Choi
DW: Mediation of neuronal apoptosis by enhancement of outward
potassium current. Science. 278:114–117. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bortner CD, Hughes FM Jr and Cidlowski JA:
A primary role for K+ and Na+ efflux in the
activation of apoptosis. J Biol Chem. 272:32436–32442.
1997.PubMed/NCBI
|
|
87
|
Szabò I, Lepple-Wienhues A, Kaba KN,
Zoratti M, Gulbins E and Lang F: Tyrosine kinase-dependent
activation of a chloride channel in CD95-induced apoptosis in T
lymphocytes. Proc Natl Acad Sci USA. 95:6169–6174. 1998.PubMed/NCBI
|
|
88
|
Chen L, Yang P and Kijlstra A:
Distribution, markers and functions of retinal microglia. Ocul
Immunol Inflamm. 10:27–39. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Langmann T: Microglia activation in
retinal degeneration. J Leukoc Biol. 81:1345–1351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Schuetz E and Thanos S: Microglia-targeted
pharmacotherapy in retinal neurodegenerative diseases. Curr Drug
Targets. 5:619–627. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Fordyce CB, Jagasia R, Zhu X and
Schlichter LC: Microglia KV1.3 channels contribute to their ability
to kill neurons. J Neurosci. 25:7139–7149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chandy KG, Wulff H, Beeton C, Pennington
M, Gutman GA and Cahalan MD: K+ channels as targets for
specific immunomodulation. Trends Pharmacol Sci. 25:280–289.
2004.
|
|
93
|
Warmke JW and Ganetzky B: A family of
potassium channel genes related to eag in Drosophila and
mammals. Proc Nat Acad Sci USA. 91:3438–3442. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ludwig J, Weseloh R, Karschin C, Liu Q,
Netzer R, Engeland B, Stansfeld C and Pongs O: Cloning and
functional expression of rat eag2, a new member of the
ether-à-go-go family of potassium channels and comparison of its
distribution with that of eag1. Mol Cell Neurosci. 16:59–70.
2000.PubMed/NCBI
|
|
95
|
Saganich MJ, Vega-Saenz de Miera E, Nadal
MS, Baker H, Coetzee WA and Rudy B: Cloning of components of a
novel subthreshold-activating K(+) channel with a unique pattern of
expression in the cerebral cortex. J Neurosci. 19:10789–10802.
1999.PubMed/NCBI
|
|
96
|
Schönherr R, Gessner G, Löber K and
Heinemann SH: Functional distinction of human EAG1 and EAG2
potassium channels. FEBS Lett. 514:204–208. 2002.
|
|
97
|
Blatz AL and Magleby KL: Calcium-activated
potassium channels. Trends in Neurosci. 10:463–467. 1987.
View Article : Google Scholar
|
|
98
|
Adams PR, Constanti A, Brown DA and Clark
RB: Intracellular Ca2+ activates a fast
voltage-sensitive K+ current in vertebrate sympathetic
neurons. Nature. 296:746–749. 1982.PubMed/NCBI
|
|
99
|
Lancaster B, Nicoll R and Perkel D:
Calcium activates two types of potassium channels in rat
hippocampal neurons in culture. J Neurosci. 11:23–30.
1991.PubMed/NCBI
|
|
100
|
Madison D and Nicoll R: Control of the
repetitive discharge of rat CA 1 pyramidal neurones in vitro. J
Physiol. 354:319–331. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Constanti A and Sim JA: Calcium-dependent
potassium conductance in guinea-pig olfactory cortex neurones in
vitro. J Physiol. 387:173–194. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lancaster B and Pennefather P: Potassium
currents evoked by brief depolarizations in bull-frog sympathetic
ganglion cells. J Physiol. 387:519–548. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Schwindt PC, Spain WJ, Foehring RC,
Stafstrom CE, Chubb MC and Crill WE: Multiple potassium
conductances and their functions in neurons from cat sensorimotor
cortex in vitro. J Neurophysiol. 59:424–449. 1988.PubMed/NCBI
|
|
104
|
Bourque CW: Transient calcium-dependent
potassium current in magnocellular neurosecretory cells of the rat
supraoptic nucleus. J Physiol. 397:331–347. 1988. View Article : Google Scholar
|
|
105
|
Rothe T, Jüttner R, Bähring R and Grantyn
R: Ion conductances related to development of repetitive firing in
mouse retinal ganglion neurons in situ. J Neurobiol. 38:191–206.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wang GY, Olshausen BA and Chalupa LM:
Differential effects of apamin- and charybdotoxin-sensitive
K+ conductances on spontaneous discharge patterns of
developing retinal ganglion cells. J Neurosci. 19:2609–2618.
1999.PubMed/NCBI
|