Introduction
Coronary heart disease is the leading cause of
mortality worldwide (1).
Regardless of rapid progress in the management of coronary heart
disease and acute myocardial infarction (MI), heart failure
following MI remains a great challenge in clinical practice
(2). Thus, novel therapies are
required to improve the long-term prognosis, and to reduce the
morbidity and mortality following MI.
During post-MI remodeling, compensatory eccentric
hypertrophy of the viable myocardium occurs and progressive
dilatation of the left ventricle (LV) results in heart failure,
which may lead to mortality (2). A
crucial mediator in the pathogenesis of cardiac remodeling and the
fibrogenic pathways in the healing infarct is interleukin 1 (IL-1)
signaling. Interventions targeting the IL-1 system may therefore
prevent remodeling following MI (3). Previous studies have demonstrated
that NLRP3-IL-1β signaling is involved in cardiac dysfunction. One
such study revealed that NLRP3 promoted myocardial inflammation and
systolic dysfunction through the production of proinflammatory
IL-1β (4). Another study
demonstrated that NLRP3 was upregulated in the heart in an
experimental mouse model of MI. Moreover, the inhibition of the
NLRP3 pathway prevented the formation of the inflammasome, and
limited the infarct size and cardiac enlargement following MI
(5).
Modulation of the inflammasome may therefore
represent a novel strategy to prevent cardiac dysfunction following
MI. It had remained unclear whether blocking cathepsin B, which is
proposed to be upstream of NLRP3 activation (6), results in beneficial effects on
post-MI remodeling. In the present study, we aimed to investigate
the effects of the novel cathepsin B inhibitor, CA-074Me, on
cardiac dysfunction, remodeling and fibrosis following MI in a rat
model, and to explore the underlying mechanisms of action.
Materials and methods
Induction of rat MI
Male Sprague-Dawley rats were purchased from Vital
River Laboratories Co., Ltd. (Beijing, China). Animals were housed
and maintained under standard conditions in the Experimental Animal
Center of Shandong University. All experiments conformed to the
Guide for the Care and Use of Laboratory Animals, provided by our
institute. The study was approved by the ethics committee of
Shandong University. MI was induced in male rats (age, 10 weeks;
weight, 180–320 g) by ligating the left anterior descending
coronary artery, as previously described (7). Briefly, rats were anesthetized with
isoflurane (5% induction, 2% maintenance) prior to intubation and
ventilation. The adequacy of the anesthesia was monitored by the
loss of reflexes and the degree of muscle relaxation. A left-sided
thoracotomy was performed between the fifth and sixth ribs, the
pericardium was opened and the heart was exteriorized. The coronary
artery was localized 1–2 mm below the junction of the pulmonary
conus and the left atrial appendage. A 5.0 silk suture
(Sigma-Aldrich, St. Louis, MO, USA) was used to permanently
constrict the artery from the left border of the pulmonary conus to
the right border of the left atrial appendage. The heart was
returned to the chest cavity, and the lungs were re-expanded prior
to closure of the chest wall with a 4.0 silk suture. Sham animals
underwent the aforementioned procedures, with the exception of the
coronary artery ligation.
Treatment
Stock solutions of the cathepsin B inhibitor,
CA-074Me, were prepared at a concentration of 10 mg/ml in dimethyl
sulfoxide (DMSO). This was diluted at a ratio of 1:10 in saline,
and administered at a dose of 10 mg/kg by intraperitoneal
injection, according to previously validated protocols (8–9). All
rats were randomly assigned to three groups: Group I, sham-operated
rats as the normal control (n=10); Group II, rats with MI treated
with vehicle (10% DMSO) for 4 weeks (n=15); Group III, rats with MI
treated with CA-074Me treatment at a dosage of 10 mg/kg/day for 4
weeks (n=15).
Cathepsin B activity assay
Cathepsin B activity was measured using the
synthetic fluorometric substrate, Z-Arg-Arg-NHMec, as previously
described (10). Briefly, 150 μM
Z-Arg-Arg-NHMec (pH 6.0) was added to the assay buffer and the
fluorescence was measured in triplicate, at 1-min intervals for 30
min, and at an excitation of 360 nm and an emission of 465 nm. Data
are represented as relative fluorescent units, and presented as the
mean ± standard deviation (SD) of three independent
experiments.
Western blot analysis
Total protein extracts were prepared by homogenizing
the heart tissues in lysis buffer (Cell Signaling Technology, Inc.,
Danvers, MA, USA), before storage at −20°C. The protein content was
determined using the bicinchoninic assay method (Pierce
Biotechnology, Inc., Rockford, IL, USA). Equal amounts of protein
were boiled with sodium dodecyl sulfate (SDS) buffer, loaded onto
8–12% denaturing polyacrylamide gels and blotted onto a
polyvinylidene fluoride (PVDF) membrane. Subsequent to blocking
with 5% skimmed milk, the following specific primary antibodies
were added: Anti-NLRP3 (dilution, 1:2,000; BD Biosciences, Franklin
Lakes, NJ, USA); anti-caspase-1p20, anti-pro-IL-1β/-IL-1β and
anti-pro-IL-18/-IL-18 (all with dilution, 1:2,000; all from Cell
Signaling Technology, Inc.). The immunoblots were visualized by
enhanced chemiluminescence, and quantified for the specific protein
content by densitometry with normalization for the housekeeping
gene, β-actin (dilution, 1:2,000; Cell Signaling Technology,
Inc.).
Enzyme-linked immunosorbent assay
(ELISA)
The serum IL-1β and IL-18 levels were determined by
ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the
manufacturer’s instructions.
Cardiac function assessed by
echocardiography
The cardiac function of all rats was evaluated by
noninvasive echocardiography, as described previously (11). Briefly, images were recorded using
a 10- to 12-MHz phased-array transducer (model 21380A with HP SONOS
5500 imaging system; Agilent Technologies, Inc., Santa Clara, CA,
USA). Diastolic and systolic left ventricle internal dimensions
(LVIDD and LVIDS, respectively) and LV fractional shortening (LVFS)
were measured with M-mode tracing from the short-axis view of the
LV at the papillary muscle level. All measurements were performed
in a blinded manner according to the guidelines of the American
Society for Echocardiology, and were averaged over three
consecutive cardiac cycles. All data were acquired and analyzed by
a single blinded observer, using EchoPac (GE Vingmed, USA) off-line
processing.
Infarct size
The Masson’s trichrome-stained slides were examined
by light microscopy, digitized and analyzed using image analysis
software (Analytical Imaging Station, AIS, Version 6.0; Imaging
Research, Inc., St. Catherines, ON, Canada). The infarct size was
assessed morphologically and calculated as the ratio of the average
scar circumferences of the endocardium and the epicardium to the
average LV circumferences of the endocardium and the epicardium, as
previously described (12).
Extracellular matrix (ECM)
deposition
Sections were stained with Masson’s trichrome stain
to examine the ECM deposition, as previously described (13). All tissues were assessed with the
examiner blinded to the experimental groups. The accumulation of
matrix within the non-infarct zone (NIZ) was then quantified as
described previously (14).
Briefly, stained sections from the mid-left ventricle were
digitally captured in their entirety with a standard polarizing
filter, and loaded onto a Pentium III computer (IBM Corporation,
Armonk, NY, USA). To isolate the NIZ from the infarct and the
peri-infarct zone, the infarct and a 2-mm zone on either side of it
were excluded from the analysis. The remaining myocardium comprised
the NIZ and was analyzed using computer-assisted image analysis
(15–16) with the AIS software. The whole NIZ
was used for quantification of the ECM in order to prevent possible
bias from using selected fields. An area of blue on a
trichrome-stained section, representing the ECM, was selected for
its color range. For sham animals, the ECM content of the entire LV
was quantitated by the same method.
Histological and immunofluorescence
analysis
The rat hearts were collected 4 weeks following MI
and fixed with buffered 10% PFA for 1 h, followed by a 1-h
incubation in 0.1 M glycine and overnight incubation at 4°C in 0.6
M sucrose. Samples were embedded in optimal cutting temperature
(OCT) media, and sections (4 μm) were cut and stored at −20°C.
Sections for Masson’s trichrome staining were processed according
to the manufacturer’s instructions (Sigma-Aldrich). In a separate
set of immunohistochemistry (IHC) experiments, rat heart sections
were permeabilized and blocked for 1 h in 1% bovine serum albumin
(BSA) and probed with primary polyclonal antibodies to α-sarcomeric
actin (dilution, 1:50; Sigma-Aldrich), wheat germ hemagglutinin
(WGA), and anti-His (both with a dilution of 1:50; both from
Invitrogen Life Technologies, Carlsbad, CA, USA). The secondary
antibodies (1:100; Invitrogen Life Technologies) were used at the
recommended dilution and incubated with sections for 1 h at room
temperature. The nuclei were counterstained with DAPI. Images were
acquired on an inverted epifluorescent microscope (Zeiss; Leica,
Germany).
Statistical analysis
Data are presented as the mean values ± standard
deviation. Statistical analyses included Student’s t-test and
one-way analysis of variance (ANOVA) followed by the Tukey and
Bonferroni multiple comparison tests. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using SPSS 16.0 (SPSS, Inc., Chicago, IL,
USA).
Results
CA-074Me inhibits the activation of the
cathepsin B-NLRP3-IL-1β pathway
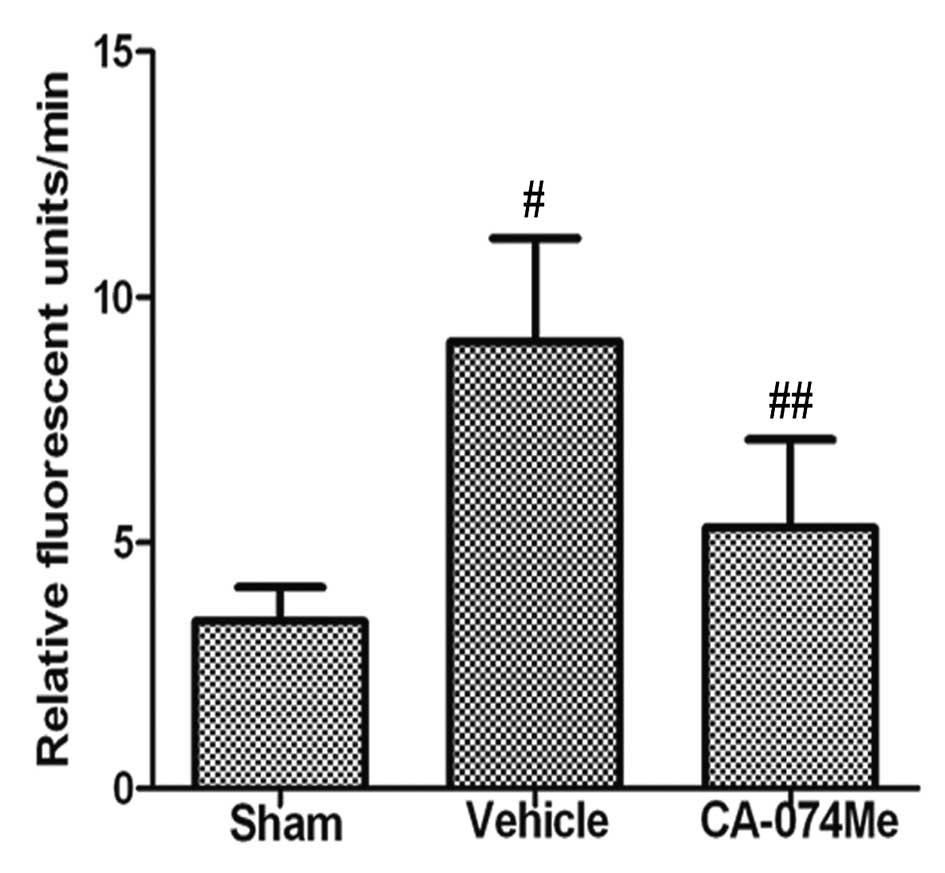
We initially confirmed that cathepsin B activity was
inhibited by CA-074Me treatment (Fig.
1). Furthermore, we identified, by ELISA, whether CA-074Me
treatment affected the serum levels of the proinflammatory
cytokines, IL-1β and IL-18. As demonstrated in Fig. 2A and B, a significant increase in
the serum levels of these cytokines was observed in the
vehicle-treated rats compared with the low baseline levels observed
in the sham control rats (P<0.01 and P<0.001 for IL-1β and
IL-18, respectively). In addition, the serum levels in the
CA-074Me-treated rats were significantly lower than those in the
vehicle-treated animals (P<0.05 and P<0.01, for IL-1β and
IL-18, respectively).
 | Figure 2CA-074Me inhibits NLRP3 inflammasome
activation. The serum levels of (A) IL-1β and (B) IL-18 4 weeks
following MI, as measured by ELISA. (C) Representative western blot
analysis of NLRP3, IL-1β, IL-18 and Casp1 in the ventricular tissue
4 weeks following MI. The appearance of mature 17-kDa IL-1β, 18-kDa
IL-18 and the Casp1 p20 subunit indicates activation. β-actin was
used as an internal control. (D) Quantification of the
NLRP3/β-actin, mature IL-1β/β-actin, mature IL-18/β-actin and
Casp1/β-actin ratios. In the histograms, data are presented as the
mean ± SEM for 10 mice per group. Sham (white bars), vehicle (10%
DMSO)-treated (black bars) and CA-074Me-treated (hatched bars)
mice. *P<0.05, **P<0.01 and
***P<0.001. MI, myocardial infarction; ELISA,
enzyme-linked immunosorbent assay; Casp1, caspase-1; IL,
interleukin. |
As demonstrated by western blot analysis, increasing
levels of mature IL-1β (Fig. 2C and
D) were observed in the lysates of vehicle-treated hearts, but
not in those of the CA-074Me-treated or sham control hearts. We
then measured whether inflammasome activation was suppressed by
CA-074Me administration. The NLRP3 protein levels in the heart
tissues were observed using western blot analysis. In the
vehicle-treated rats, the levels of NLRP3 protein (Fig. 2C and D) were significantly
increased compared with that of the sham controls, and these
effects were inhibited by CA-074Me treatment. Consistent with
cytokine maturation, caspase-1 activation (Fig. 2C and D) was also observed in the
vehicle-treated hearts, as demonstrated by the appearance of the
p20 subunit. This effect was significantly inhibited by CA-074Me
treatment.
Treatment with CA-074Me improves cardiac
function
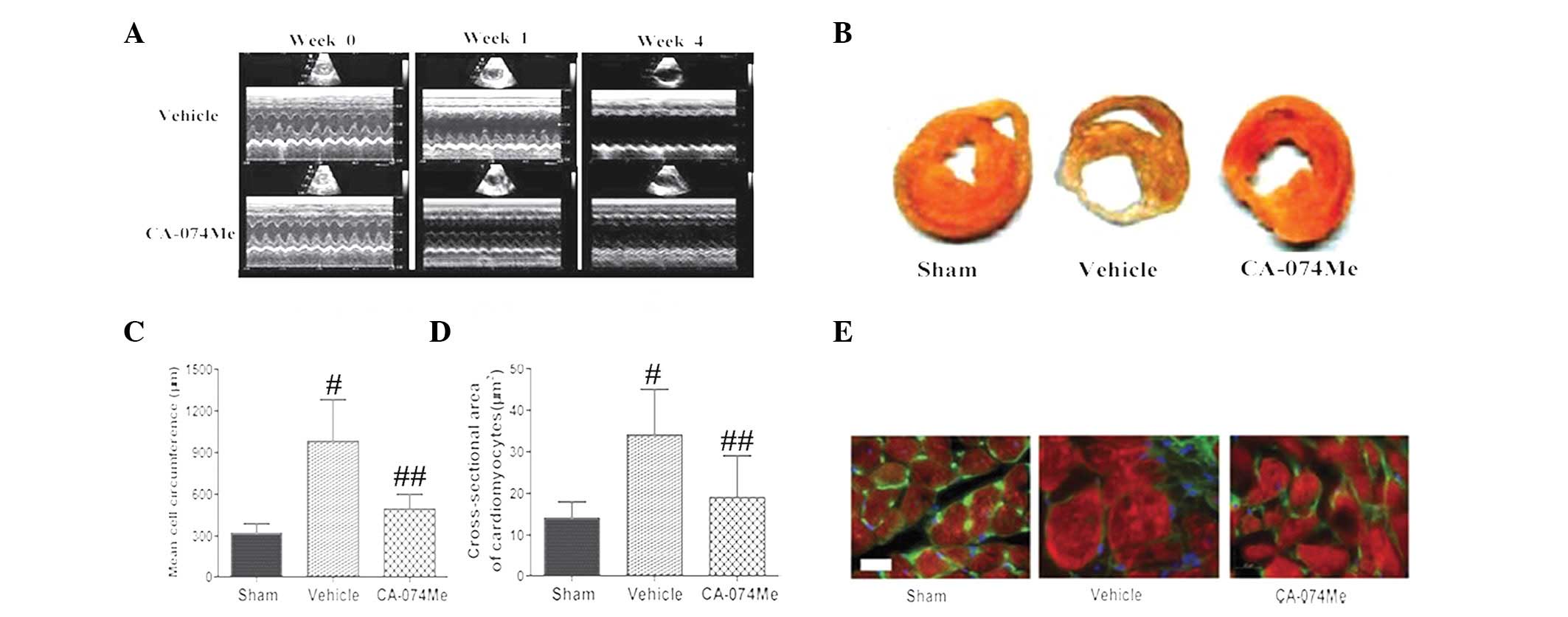
Echocardiography was used to examine the cardiac
structure and function of the rats. At the baseline, no differences
were observed between the three groups (Fig. 3A). Treatment with CA-074Me for 4
weeks contributed to significant improvements in the LVIDD, LVIDS,
LVFS and ejection fraction (EF) (Fig.
3A and Table I).
 | Table IEchocardiography of each group. |
Table I
Echocardiography of each group.
| Sham | MI + vehicle | MI + CA-074Me |
|---|
| HR (beats/min) | 254±39 | 243±15 | 251±18 |
| LVIDD (cm) | 0.86±0.02 | 0.98±0.03a | 0.84±0.05b |
| LVIDS (cm) | 0.54±0.04 | 0.79±0.04a | 0.67±0.06b |
| LVFS (%) | 38.24±2.10 | 21.15±1.10a | 24.82±2.90b |
| EF (%) | 73.25±2.80 | 46.08±3.40a | 53.81±2.90b |
Infarct size and LV cardiomyocyte size
were reduced by CA-074Me
Treatment with CA-074Me significantly reduced the
infarct size compared with that of the vehicle-treated MI groups
(22±2.8 vs. 38±5.4%, respectively; P<0.01; Fig. 3B). The cardiomyocyte size was
assessed using high magnification microscopy to quantify the
circumference (Fig. 3C) and the
cross-sectional area (Fig. 3D and
E). These data demonstrated that treatment with CA-074Me
prevented cardiac remodeling when compared with the vehicle-treated
and sham groups (P<0.01 for all comparisons, n=15).
CA-074Me treatment reduced cardiac
fibrosis
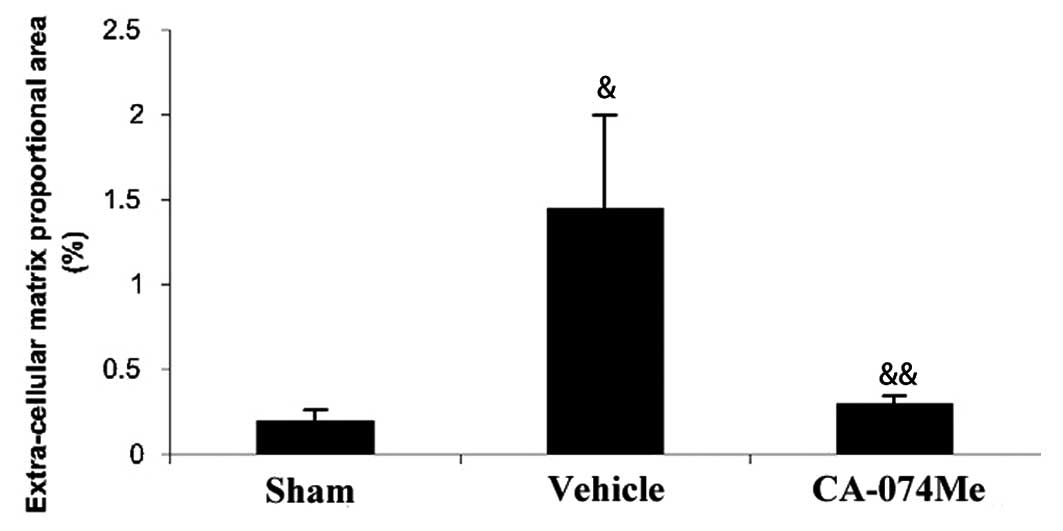
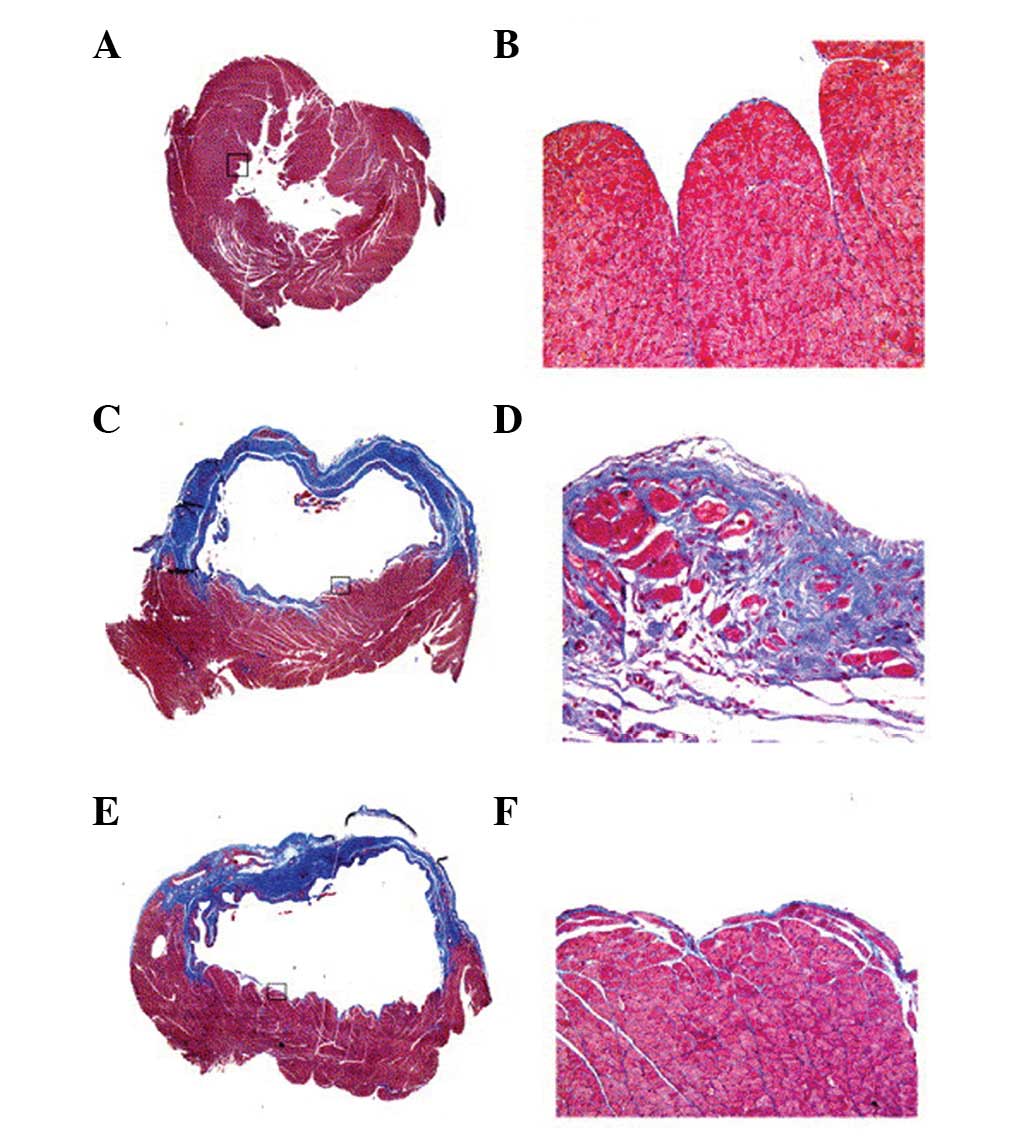
The extent of the cardiac fibrosis was evaluated by
Masson’s trichrome staining 4 weeks following MI. In the
trichrome-stained sections, ECM deposition in the NIZ was
>7-fold higher in MI animals compared with sham animals. The ECM
deposition was particularly pronounced in the subendocardial region
of the NIZ (Figs. 4 and 5). Treatment with CA-074Me resulted in a
reduction in the ECM throughout the NIZ, to levels similar to those
observed in the sham animals (Figs.
4 and 5). Non-infarcted
animals treated with CA-074Me exhibited similar levels of ECM
compared with the untreated sham-operated animals (0.26±0.07 vs.
0.19±0.07%, respectively).
Discussion
The present study indicated that CA-074Me attenuated
cardiac dysfunction, cardiomyocyte hypertrophy and fibrosis
following MI. This resulted in improved systolic function in a rat
model of MI, which was associated with inhibition of the
NLRP3-IL-1β signaling pathway.
The significance of the proinflammatory cytokine,
IL-1β, in the pathogenesis of heart disease has been demonstrated
in animal experiments, and exogenous administration in vivo
and in vitro has led to structural remodeling with reduced
cardiac function (17–18). Additional studies in animals have
validated these results (19).
Previous studies have suggested that IL-1β may induce systolic
dysfunction in patients with heart failure, further supporting a
negative role for this cytokine in heart disease (20). In post-MI clinical trials,
administration of the IL-1-receptor antagonist (anakinra) reduced
adverse remodeling and improved cardiac function (21). In addition, previous studies have
indicated that upstream of IL-1β processing, the NLRP3 inflammasome
promotes adverse cardiac remodeling following MI in mice (5). Cathepsin B release is proposed to be
upstream of NLRP3 activation (22). However, it remains unknown as to
whether the inhibition of cathepsin B improves systolic dysfunction
in post-MI heart failure, by affecting NLRP3 activation. In the
present study, we identified a critical role for cathepsin B-NLRP3
in proinflammatory cytokine production, and in the progression of
systolic dysfunction.
The inflammatory response during heart failure has
severe consequences on cardiac contractility. Dying cells release
danger signals within the injured tissue microenvironment, which
are subsequently recognized by danger-sensing systems. NLRP3
oligomerization with pro-caspase-1 and apoptosis-associated
speck-like protein containing a CARD domain (ASC) leads to the
activation of caspase-1, which processes pro-IL-1β for secretion,
inducing sterile inflammation (23–24).
A previous study, in which cardiac contractility was improved via
NLRP3 siRNA in mice following MI (5), demonstrated that inflammasome/IL-1β
antagonism is involved in acutely evolving injuries, including
ischemia/reperfusion and infarction, as well as in the progression
of heart disease, where ongoing myocardial stress yields chronic
tissue damage.
Cathepsin B is a prominent lysosomal protease and is
highly abundant in the left ventricular myocardium of patients with
hypertensive heart failure. Thus, it has been implicated in cardiac
remodeling in this disease (25).
It has been demonstrated that cathepsin B is
involved in apoptosis, as well as in the degradation of
myofibrillar proteins in MI (26).
The activity of cathepsin B has been demonstrated to significantly
increase in the serum and the hearts of isoproterenol (ISO)-induced
myocardial infarcted rats (27).
Increased myocardial expression of cathepsin B in failing human
hearts suggests that cathepsin B may be involved in the development
of heart failure (26).
In addition, although there has been substantial
investigation into the roles of hypertrophy and dilatation, certain
studies have demonstrated the importance of fibrosis, remote from
the site of infarction, in the pathogenesis of post-MI cardiac
dysfunction (28). The
predilection for fibrosis in the subendocardium of the NIZ is
viewed as a significant contributory factor to mechanical
dysfunction (29) and propensity
for dysrhythmia (30) following
MI. In the present study, in contrast to its neutral effect on
fibrosis in the infarct zone, CA-074Me treatment significantly
reduced ECM deposition in the areas remote from the site of
infarction, particularly the subendocardial region, which was
associated with a reduction in the level of in LV function.
Notably, studies have revealed that NLRP3 is closely associated
with organ fibrosis in the lung, liver and kidney (31–33).
We speculated that the reduced cardiac fibrosis due to CA-074Me
treatment was associated with inhibited activation of the NLRP3
pathway.
Therefore, our study has provided support for the
hypothesis that the inhibition of cathepsin B induces a significant
decrease in NLRP3-IL-1β activation, resulting in improved cardiac
function and reduced levels of fibrosis. In addition, complex
signaling pathways are involved in myocyte hypertrophy, and our
results demonstrated that the increase in cardiomyocyte size was
also attenuated by such inhibition. This supports the hypothesis
that the cathepsin B-NLRP3-IL-1β pathway has an adverse effect on
cardiac contractility and function.
References
|
1
|
Weber C and Noels H: Atherosclerosis:
current pathogenesis and therapeutic options. Nat Med.
17:1410–1422. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yousef Z, Redwood S and Marber M:
Postinfarction left ventricular remodelling: where are the theories
and trials leading us? Heart. 83:76–80. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bujak M, Dobaczewski M, Chatila K, Mendoza
LH, Li N, Reddy A and Frangogiannis NG: Interleukin-1 receptor type
I signaling critically regulates infarct healing and cardiac
remodeling. Am J Pathol. 173:57–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bracey NA, Beck PL, Muruve DA, Hirota SA,
Guo J, Jabagi H, Wright JR Jr, Macdonald JA, Lees-Miller JP, Roach
D, et al: The Nlrp3 inflammasome promotes myocardial dysfunction in
structural cardiomyopathy through IL-1β. Exp Physiol. 98:462–472.
2013.PubMed/NCBI
|
|
5
|
Mezzaroma E, Toldo S, Farkas D, Seropian
IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF and
Abbate A: The inflammasome promotes adverse cardiac remodeling
following acute myocardial infarction in the mouse. Proc Natl Acad
Sci USA. 108:19725–19730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Niemi K, Teirilä L, Lappalainen J,
Rajamäki K, Baumann MH, Öörni K, Wolff H, Kovanen PT, Matikainen S
and Eklund KK: Serum amyloid A activates the NLRP3 inflammasome via
P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol.
186:6119–6128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boyle AJ, Kelly DJ, Zhang Y, Cox AJ, Gow
RM, Way K, Itescu S, Krum H and Gilbert RE: Inhibition of protein
kinase C reduces left ventricular fibrosis and dysfunction
following myocardial infarction. J Mol Cell Cardiol. 39:213–221.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van Acker GJ, Saluja AK, Bhagat L, Singh
VP, Song AM and Steer ML: Cathepsin B inhibition prevents
trypsinogen activation and reduces pancreatitis severity. Am J
Physiol Gastrointest Liver Physiol. 283:G794–G800. 2002.PubMed/NCBI
|
|
9
|
Matarrese P, Ascione B, Ciarlo L, Vona R,
Leonetti C, Scarsella M, Mileo AM, Catricalà C, Paggi MG and
Malorni W: Cathepsin B inhibition interferes with metastatic
potential of human melanoma: an in vitro and in vivo study. Mol
Cancer. 9:2072010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cavallo-Medved D, Dosescu J, Linebaugh BE,
Sameni M, Rudy D and Sloane BF: Mutant K-ras regulates cathepsin B
localization on the surface of human colorectal carcinoma cells.
Neoplasia. 5:507–519. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takaya T, Wada H, Morimoto T, Sunagawa Y,
Kawamura T, Takanabe-Mori R, Shimatsu A, Fujita Y, Sato Y, Fujita
M, et al: Left ventricular expression of lectin-like oxidized
low-density lipoprotein receptor-1 in failing rat hearts. Circ J.
74:723–729. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saito T, Rodger IW, Hu F, Robinson R,
Huynh T and Giaid A: Some histological methods: Inhibition of COX
pathway in experimental myocardial infarction. J Mol Cell Cardiol.
37:71–77. 2004. View Article : Google Scholar
|
|
13
|
Masson P: Trichrome stainings and their
preliminary technique. J Tech Methods. 2:75–90. 1929.
|
|
14
|
Lal A, Veinot JP and Leenen FH: Critical
role of CNS effects of aldosterone in cardiac remodeling
post-myocardial infarction in rats. Cardiovasc Res. 64:437–447.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lehr HA, Mankoff DA, Corwin D, Santeusanio
G and Gown AM: Application of photoshop-based image analysis to
quantification of hormone receptor expression in breast cancer. J
Histochem Cytochem. 45:1559–1565. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lehr HA, Van der Loos CM, Teeling P and
Gown AM: Complete chromogen separation and analysis in double
immunohistochemical stains using Photoshop-based image analysis. J
Histochem Cytochem. 47:119–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duncan DJ, Yang Z, Hopkins PM, Steele DS
and Harrison SM: TNF-alpha and IL-1beta increase Ca2+
leak from the sarcoplasmic reticulum and susceptibility to
arrhythmia in rat ventricular myocytes. Cell Calcium. 47:378–386.
2010.PubMed/NCBI
|
|
18
|
Bujak M and Frangogiannis NG: The role of
IL-1 in the pathogenesis of heart disease. Arch Immunol Ther Exp
(Warsz). 57:165–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abbate A, Salloum FN, Vecile E, Das A,
Hoke NN, Straino S, Biondi-Zoccai GG, Houser JE, Qureshi IZ, Ownby
ED, et al: Anakinra, a recombinant human interleukin-1 receptor
antagonist, inhibits apoptosis in experimental acute myocardial
infarction. Circulation. 117:2670–2683. 2008. View Article : Google Scholar
|
|
20
|
Van Tassell BW, Arena RA, Toldo S,
Mezzaroma E, Azam T, Seropian IM, Shah K, Canada J, Voelkel NF,
Dinarello CA and Abbate A: Enhanced interleukin-1 activity
contributes to exercise intolerance in patients with systolic heart
failure. PLoS One. 7:e334382012.PubMed/NCBI
|
|
21
|
Abbate A, Kontos MC, Grizzard JD,
Biondi-Zoccai GG, Van Tassell BW, Robati R, Roach LM, Arena RA,
Roberts CS, Varma A, et al: Interleukin-1 blockade with anakinra to
prevent adverse cardiac remodeling after acute myocardial
infarction [Virginia Commonwealth University Anakinra Remodeling
Trial (VCU-ART) Pilot study]. Am J Cardiol. 105:1371–1377.
2010.PubMed/NCBI
|
|
22
|
Hoegen T, Tremel N, Klein M, Angele B,
Wagner H, Kirschning C, Pfister HW, Fontana A, Hammerschmidt S and
Koedel U: The NLRP3 inflammasome contributes to brain injury in
pneumococcal meningitis and is activated through ATP-dependent
lysosomal cathepsin B release. J Immunol. 187:5440–5451. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hirota SA, Ng J, Lueng A, Khajah M, Parhar
K, Li Y, Lam V, Potentier MS, Ng K, Bawa M, et al: NLRP3
inflammasome plays a key role in the regulation of intestinal
homeostasis. Inflamm Bowel Dis. 17:1359–1372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McDonald B, Pittman K, Menezes GB, Hirota
SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA and Kubes P:
Intravascular danger signals guide neutrophils to sites of sterile
inflammation. Science. 330:362–366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng XW, Obata K, Kuzuya M, Izawa H,
Nakamura K, Asai E, Nagasaka T, Saka M, Kimata T, Noda A, et al:
Elastolytic cathepsin induction/activation system exists in
myocardium and is upregulated in hypertensive heart failure.
Hypertension. 48:979–987. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ge J, Zhao G, Chen R, Li S, Wang S, Zhang
X, Zhuang Y, Du J, Yu X, Li G and Yang Y: Enhanced myocardial
cathepsin B expression in patients with dilated cardiomyopathy. Eur
J Heart Fail. 8:284–289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumaran KS and Prince PS: Preventive
effect of caffeic acid on lysosomal dysfunction in
isoproterenol-induced myocardial infarcted rats. J Biochem Mol
Toxicol. 24:115–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jugdutt BI: Ventricular remodeling after
infarction and the extracellular collagen matrix: when is enough
enough? Circulation. 108:1395–1403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Borg TK, Ranson WF, Moslehy FA and
Caulfield JB: Structural basis of ventricular stiffness. Lab
Invest. 44:49–54. 1981.PubMed/NCBI
|
|
30
|
Strain JE, Grose RM, Factor SM and Fisher
JD: Results of endomyocardial biopsy in patients with spontaneous
ventricular tachycardia but without apparent structural heart
disease. Circulation. 68:1171–1181. 1983. View Article : Google Scholar
|
|
31
|
Artlett CM: The role of the NLRP3
inflammasome in fibrosis. Open Rheumatol J. 6:80–86. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu JF, Washko GR, Nakahira K, Hatabu H,
Patel AS, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross C,
et al: Statins and pulmonary fibrosis: the potential role of NLRP3
inflammasome activation. Am J Respir Crit Care Med. 185:547–556.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Watanabe A, Sohail MA, Gomes DA, Hashmi A,
Nagata J, Sutterwala FS, Mahmood S, Jhandier MN, Shi Y, Flavell RA
and Mehal WZ: Inflammasome-mediated regulation of hepatic stellate
cells. Am J Physiol Gastrointest Liver Physiol. 296:G1248–G1257.
2009. View Article : Google Scholar : PubMed/NCBI
|