Introduction
Psoriasis is a chronic inflammatory skin disease
that is characterized by erythema, epidermal hyperplasia,
inflammatory infiltrates, and enlarged, tortuous and hyperpermeable
blood vessels (1,2). Previous studies have shown that the
endothelial microvascular bed was increased four-fold in psoriatic
skin compared with normal skin (3). Dermal microvascular expansion with
abnormal orientation, and dilatation of capillaries in the biopsies
of the psoriatic skin revealed that the disease was angiogenesis
dependent (4,5) and signified the importance of
angiogenesis in psoriasis.
Numerous growth factors and cytokines are involved
in angiogenesis. Of all known angiogenic molecules, vascular
endothelial growth factor (VEGF) is the key mediator of
angiogenesis. Several studies indicate a vital role of VEGF in the
pathogenesis of psoriasis: epidermal-derived VEGF is highly
upregulated in psoriatic skin lesions (6–8);
VEGF serum levels are correlated with disease severity (9); VEGF expression in basal keratinocytes
showed psoriatic-like skin inflammation with increased tortuosity
and branching of dermal blood vessels in a mouse model (10,11).
VEGF receptors 1 and 2 were expressed on the surface of
keratinocytes, and the VEGF secreted by the keratinocytes was able
to bind to these receptors and activate the signaling pathway in an
autocrine manner (12–14). Thus, anti-vascular strategies to
treat psoriasis by blocking VEGF binding to VEGFR are anticipated,
although to date the majority of anti-angiogenic approaches have
primarily focused on the development of cancer therapeutics
(15–17).
In the present study, we used a therapeutic approach
in a transgenic mouse model (18)
of chronic, psoriasis-like skin inflammation, using the anti-VEGFR2
small molecule compound SKLB1002. Systemic treatment of transgenic
K14-VEGF mice with SKLB1002 strongly reduced skin inflammation in
contrast with control animals. The mice showed a marked improvement
in psoriatic phenotype, normalization of the epidermal
architecture, and a decrease in the number and size of blood
vessels. Furthermore, the immune infiltrate in the skin was reduced
in SKLB1002-treated mice.
Materials and methods
Animals
K14-VEGF transgenic homozygous mice (18) displaying symptoms of human
psoriasis were provided by the State Key Laboratory of Biotherapy
and Cancer Center, Sichuan University (Sichuan, China). Eight to
twelve-week-old mice with moderate to serious psoriasis were
selected for the experiment. The mice were housed under specific
pathogen-free (SPF) conditions. Animal experiments were approved by
the Institutional Animal Care and Use Committee of Southwest
University for Nationalities (Chengdu, Sichuan, China).
SKLB1002
The small molecule compound was donated by Dr
Sheng-Yong Yang from the State Key Laboratory of Biotherapy and
Cancer Center, West China Hospital, West China Medical School,
Sichuan University (Sichuan, China).
Antibodies
For immunohistochemistry, rabbit anti-mouse
antibodies against K6 (Abcam, Cambridge, MA, USA), CD4 (Abcam), CD8
(Abcam), CD31 (Abcam), VEGF (Boster, Fremont, CA, USA), CD54
(Boster) and E-selectin (Boster) were used.
In vivo treatment with the tyrosine
kinase inhibitor SKLB1002
K14-VEGF transgenic homozygous mice were divided
into three groups as follows: a treatment group receiving SKLB1002
and control groups receiving only vehicle or saline. Mice were
dosed with SKLB1002, vehicle or saline once daily by
intraperitoneal (i.p.) injection for four weeks. Mice in the
treatment group received 50 mg/kg SKLB1002 (dissolved in 5% DMSO +
35% PEG400 suspension, i.p.), which was a dose previously
determined to be effective in preparatory experiments (data not
shown). Animals in two control groups were treated with the same
volume of vehicle (5% DMSO + 35% PEG400) and saline, respectively.
Twenty-four hours after the final treatment, photographs of mouse
ears were obtained before all mice were sacrificed. Ear samples
were fixed in 4% neutral buffered paraformaldehyde for histological
analysis.
Histology
Paraffin-embedded skin sections (~5 μm) from
treated-K14/VEGF mice were stained by HE and immunohistochemistry.
Quantitative assessments of the pathological score, based on
Baker’s method (19), were
performed in five randomly selected high-power fields (x400) in
each HE-stained section to assess the severity of psoriasis. The
mean epidermal thickness of the ear skin was also measured as an
indication of epidermal proliferation. Immunohistochemistry
staining was performed according to the antibody protocol, and
images were acquired using an Olympus BX60 microscope (Olympus,
Japan). Paraffin sections were deparaffinized and rehydrated, then
heat-induced antigen retrieval was required for 5 min prior to
immunohistochemical staining. The tissue sections were incubated
with the primary antibodies overnight at 4°C. Cells were counted in
five randomly chosen fields (x400) in each immunohistochemically
stained section (n=5 for each group). The evaluations were
performed by two blinded observers.
Statistical analysis
SPSS 16.0 was used for statistical analysis (SPSS,
Inc., Chicago, IL, USA). Data are expressed as the means ± SD. The
statistical analysis in all experiments was performed using one-way
analysis of variance (ANOVA) or t-test. P<0.05 was considered to
indicate a statistically significant result.
Results
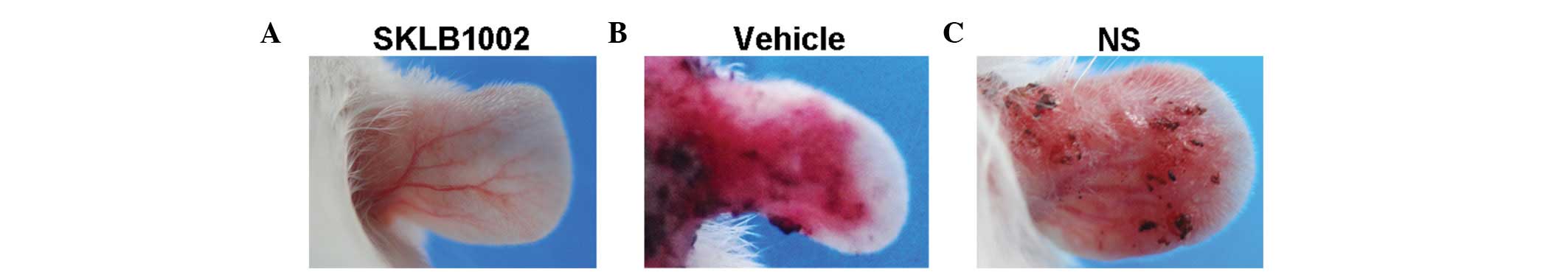
Systemic treatment with SKLB1002 reduces
symptoms of ear inflammation in K14/VEGF transgenic mice
The effects of a newly identified VEGF receptor
tyrosine-kinase inhibitor, SKLB1002, were tested in K14/VEGF
transgenic mice exhibiting numerous characteristic features of
psoriasis. Fifteen 8–12-week-old K14-VEGF transgenic mice received
consecutive i.p. injections of SKLB1002, vehicle or saline for 4
weeks. The mice treated with the vehicle or saline exhibited focal
skin lesions on their ears, which were highly similar to human
psoriasis. By comparison, the mice treated with SKLB1002 showed
only hyperaemia and very slight incrassation of the skin on their
ears (Fig. 1). SKLB1002 treatment
was well-tolerated; the mice showed no signs of sickness and did
not lose weight (data not shown).
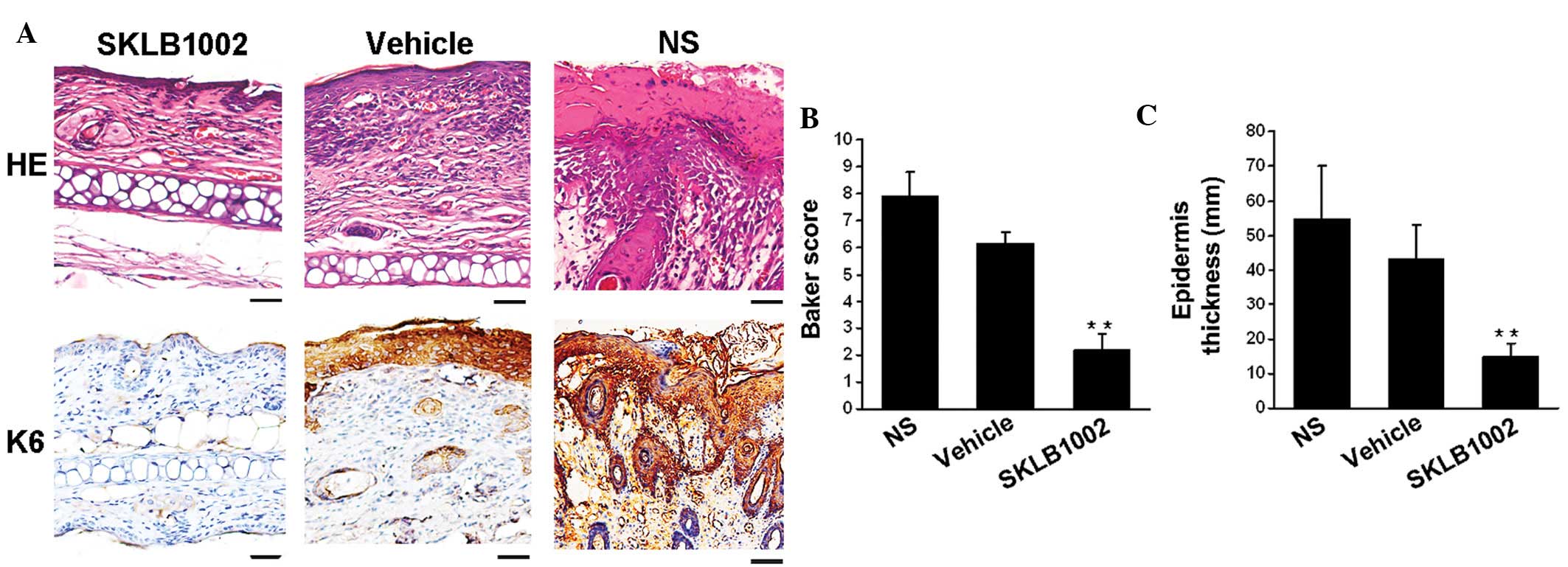
SKLB1002 treatment normalizes the
epidermal architecture in inflamed skin
To better characterize the efficacy of systemic VEGF
blockade in reducing psoriasis-like skin inflammation, histological
analyses were performed on ear sections obtained from K14/VEGF
littermates treated with SKLB1002, vehicle and saline. After 4
weeks of treatment, HE-stained sections revealed the typical
histopathological signs of the psoriasis-like phenotype in the
control-treated mice, including acanthosis (thickened epidermis),
epidermal rete elongation, focal parakeratosis (retention of nuclei
in the stratum corneum), hyperkeratosis (thickening of the stratum
corneum), hemangiectasis, abundant infiltrated lymphocytes and
microabscesses. By contrast, systemic inhibition of VEGF led to a
notable reduction in psoriasis-like histological features (Fig. 2).
As shown in Fig. 2,
SKLB1002 treatment led to a significant improvement in skin
inflammation, which was confirmed by the pathological score for
HE-stained preparations based on the Baker score system. The
pathological scores of the mice treated with SKLB1002 were
significantly different (n=5 in each group; P<0.01, one-way
ANOVA) from controls. In addition, the average epidermal thickness
was reduced by 80% in the group treated with SKLB1002 when compared
with the control groups (n=5 in each group; P<0.01, one-way
ANOVA), whereas the difference between the vehicle-treated group
and saline-treated group was not significant. During psoriatic
hyperproliferation of keratinocytes, the epidermal
hyperproliferation marker keratin 6 displayed a much broader
staining pattern. Treatment with SKLB1002 significantly inhibited
the expression of keratin 6 in the epidermis. By contrast, keratin
6 expression was marked in the epidermis of vehicle-treated mice.
Thus, inhibition of VEGF normalized the epidermal skin architecture
in this mouse model of psoriasis.
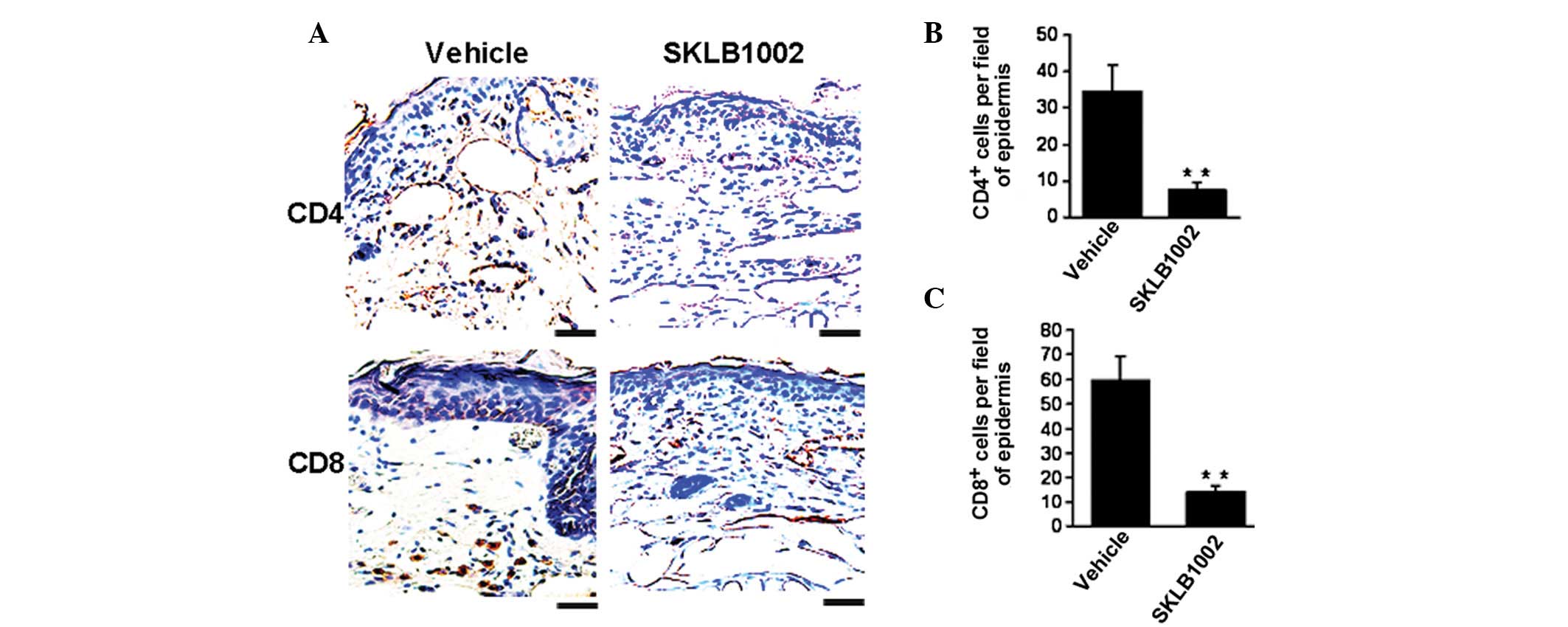
Decrease in T-cell infiltration mediated
by SKLB1002 in vivo
The defining histological features of psoriasis
include marked infiltration of T cells. We thus performed
immunohistochemical assays to examine the variations in
CD4+ and CD8+ cells in skin sections. As
shown in Fig. 3, when compared
with the control, SKLB1002 treatment significantly decreased the
number of CD4+ and CD8+ cells by 71.2% and
78.5%, respectively, which demonstrated inhibition of the
infiltration of inflammatory T lymphocytes.
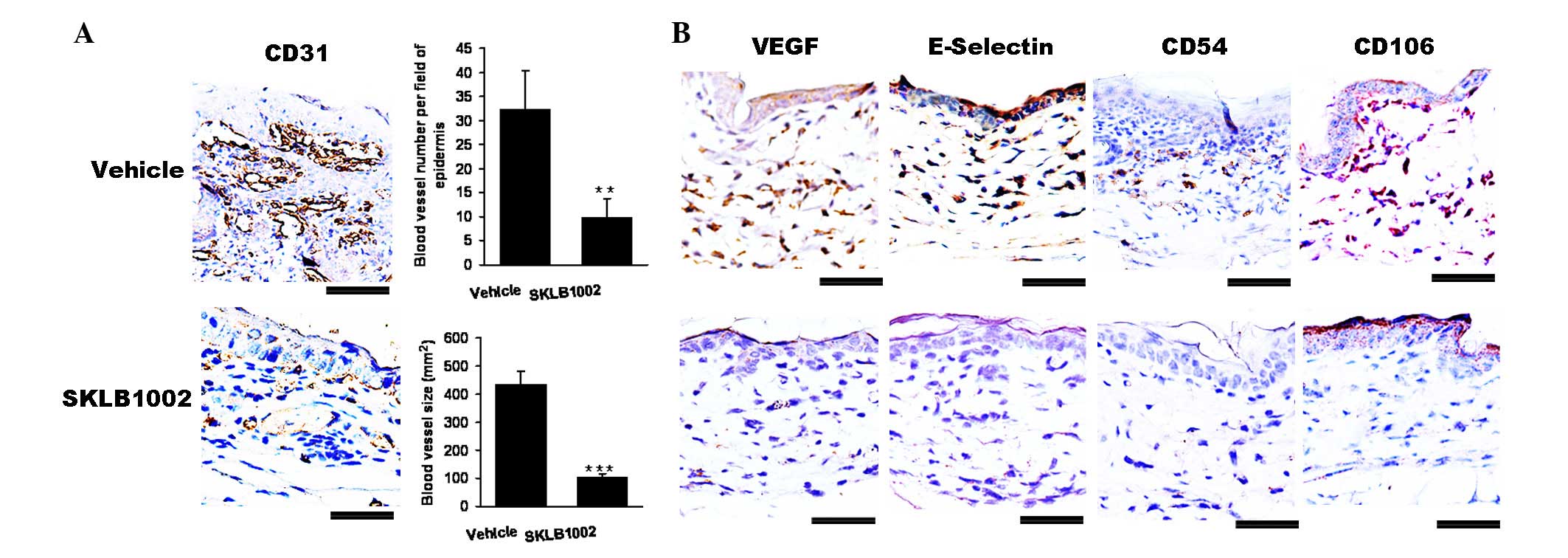
SKLB1002 significantly reduces vascular
abnormalities and permeability in vivo
As shown in Fig.
4A, when compared with the control, SKLB1002 treatment
significantly decreased the expression of VEGF and CD31, which
indicated the suppression of the proliferation and dilation of
blood vessels. Notably, the average number of blood vessels was
significantly reduced (P<0.01) and the average size of blood
vessels (P<0.01) was significantly smaller in SKLB1002-treated
mice than in vehicle control-treated animals.
In particular, the expression of specific
endothelial cell adhesion molecules is a hallmark of the
hyperplastic and inflamed vessels observed in human psoriatic skin
lesions, including the expression of E-selectin, VCAM-1 (CD106) and
ICAM-1 (CD54). Compared with the vehicle treatment group, SKLB1002
treatment significantly inhibited the expression of E-selectin,
CD54 and CD106 (Fig. 4B). Thus,
inhibition of VEGF-dependent pathological angiogenesis normalized
the blood vasculature.
Discussion
Psoriasis is a common inflammatory disease and its
underlying pathogenesis is still not fully understood (20,21).
Therefore, therapeutic interventions are currently limited and
restricted to the treatment of symptoms rather than targeting the
mechanisms underlying the disease. In the present study, we
investigated the activity of a new small molecule VEGF inhibitor in
a psoriasis-like mouse model. The three major components of
psoriasis pathogenesis, infiltration of leukocytes,
hyperproliferation of epidermal keratinocytes, and occurrence of
vascular abnormalities, were markedly improved following treatment
with SKLB1002. These findings indicate that therapeutic
intervention at the level of the vasculature may be sufficient to
reduce the immune-mediated and epidermal components of the
disease.
SKLB1002 is a new and selective VEGFR2 inhibitor
that potently inhibits VEGFR2 with a half maximal inhibitory
concentration (IC50) of 32 nmol/l (16). SKLB1002 significantly inhibits
HUVEC proliferation, migration, invasion and tube formation in
vitro(16). In this study,
vascular abnormalities and secretion of VEGF were reduced by
treatment with SKLB1002 in K14/VEGF transgenic mice. Besides its
role in angiogenesis, VEGF induces hyperpermeability of blood
vessels, leading to tissue edema during inflammation (22–24).
Furthermore, chronic overexpression of VEGF in the skin of K14-VEGF
transgenic mice promoted leukocyte rolling and adhesion in skin
microvessels, most likely resulting from the increased expression
of adhesion molecules such as E-selectin, CD106 and CD54 (18,25).
Thus, the inhibition of these additional activities of VEGF related
to attracting and activating T lymphocytes by systemic treatment
with VEGFR inhibitor most likely contributed to the diminished
inflammatory cell infiltration observed in the skin.
In conclusion, the new and potent VEGFR2 inhibitor
SKLB1002 effectively alleviates skin inflammation or completely
cures psoriasis in a mouse model. Mechanistic studies indicated
that SKLB1002 treatment significantly decreased the number of blood
vessels and reduced the tortuosity of epidermal blood, prevented
vascular leakage and T lymphocyte infiltration in skin, which may
promote skin inflammation. These findings establish a solid basis
for future clinical studies of the new selective VEGFR2 inhibitor
for the treatment of psoriasis.
Acknowledgements
This study was supported by the Veterinary Medicine
Discipline Program of Southwest University for Nationalities
(2011XWD-S0906), and the Applied Basic Research Program of Sichuan
Province (2011JY0033). We would like to thank Dr Sheng-Yong Yang
from the State Key Laboratory of Biotherapy and Cancer Center, West
China Hospital, West China Medical School, Sichuan University for
donating SKLB1002.
References
|
1
|
Christensen TE, Callis KP, Papenfuss J, et
al: Observations of psoriasis in the absence of therapeutic
intervention identifies two unappreciated morphologic variants,
thin-plaque and thick-plaque psoriasis, and their associated
phenotypes. J Invest Dermatol. 126:2397–2403. 2006. View Article : Google Scholar
|
|
2
|
Bowcock AM and Krueger JG: Getting under
the skin: the immunogenetics of psoriasis. Nat Rev Immunol.
5:699–711. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Creamer D, Allen MH, Sousa A, Poston R and
Barker JN: Localization of endothelial proliferation and
microvascular expansion in active plaque psoriasis. Br J Dermatol.
136:859–865. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pinkus H and Mehregan AH: The primary
histologic lesion of seborrheic dermatitis and psoriasis. J Invest
Dermatol. 46:109–116. 1966.PubMed/NCBI
|
|
5
|
Liew SC, Das-Gupta E, Chakravarthi S, et
al: Differential expression of the angiogenesis growth factors in
psoriasis vulgaris. BMC Res Notes. 5:2012012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Detmar M, Brown LF, Claffey KP, et al:
Overexpression of vascular permeability factor/vascular endothelial
growth factor and its receptors in psoriasis. J Exp Med.
180:1141–1146. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Flisiak I, Zaniewski P, Rogalska M,
Mysliwiec H, Jaroszewicz J and Chodynicka B: Effect of psoriasis
activity on VEGF and its soluble receptors concentrations in serum
and plaque scales. Cytokine. 52:225–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cordiali-Fei P, Trento E, D’Agosto G, et
al: Effective therapy with anti-TNF-alpha in patients with
psoriatic arthritis is associated with decreased levels of
metalloproteinases and angiogenic cytokines in the sera and skin
lesions. Ann NY Acad Sci. 1110:578–589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nielsen HJ, Christensen IJ, Svendsen MN,
et al: Elevated plasma levels of vascular endothelial growth factor
and plasminogen activator inhibitor-1 decrease during improvement
of psoriasis. Inflamm Res. 51:563–567. 2002. View Article : Google Scholar
|
|
10
|
Detmar M: The role of VEGF and
thrombospondins in skin angiogenesis. J Dermatol Sci. 24(Suppl 1):
S78–S84. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yano K, Kajiya K, Ishiwata M, Hong YK,
Miyakawa T and Detmar M: Ultraviolet B-induced skin angiogenesis is
associated with a switch in the balance of vascular endothelial
growth factor and thrombospondin-1 expression. J Invest Dermatol.
122:201–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Man XY, Yang XH, Cai SQ, Yao YG and Zheng
M: Immunolocalization and expression of vascular endothelial growth
factor receptors (VEGFRs) and neuropilins (NRPs) on keratinocytes
in human epidermis. Mol Med. 12:127–136. 2006.PubMed/NCBI
|
|
13
|
Elias PM, Arbiser J, Brown BE, et al:
Epidermal vascular endothelial growth factor production is required
for permeability barrier homeostasis, dermal angiogenesis, and the
development of epidermal hyperplasia: implications for the
pathogenesis of psoriasis. Am J Pathol. 173:689–699. 2008.
View Article : Google Scholar
|
|
14
|
Canavese M, Altruda F, Ruzicka T and
Schauber J: Vascular endothelial growth factor (VEGF) in the
pathogenesis of psoriasis - a possible target for novel therapies?
J Dermatol Sci. 58:171–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao Y: Opinion: emerging mechanisms of
tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer.
5:735–743. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang S, Cao Z, Tian H, et al: SKLB1002, a
novel potent inhibitor of VEGF receptor 2 signaling, inhibits
angiogenesis and tumor growth in vivo. Clin Cancer Res.
17:4439–4450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hedlund EM, Yang X, Zhang Y, et al: Tumor
cell-derived placental growth factor sensitizes antiangiogenic and
antitumor effects of anti-VEGF drugs. Proc Natl Acad Sci.
110:654–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia YP, Li B, Hylton D, Detmar M,
Yancopoulos GD and Rudge JS: Transgenic delivery of VEGF to mouse
skin leads to an inflammatory condition resembling human psoriasis.
Blood. 102:161–168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baker BS, Brent L, Valdimarsson H, et al:
Is epidermal cell proliferation in psoriatic skin grafts on nude
mice driven by T-cell derived cytokines? Br J Dermatol.
126:105–110. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lowes MA, Chamian F, Abello MV, et al:
Increase in TNF-alpha and inducible nitric oxide
synthase-expressing dendritic cells in psoriasis and reduction with
efalizumab (anti-CD11a). Proc Natl Acad Sci USA. 102:19057–19062.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schonthaler HB, Huggenberger R, Wculek SK,
Detmar M and Wagner EF: Systemic anti-VEGF treatment strongly
reduces skin inflammation in a mouse model of psoriasis. Proc Natl
Acad Sci USA. 106:21264–21269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kunstfeld R, Hirakawa S, Hong YK, et al:
Induction of cutaneous delayed-type hypersensitivity reactions in
VEGF-A transgenic mice results in chronic skin inflammation
associated with persistent lymphatic hyperplasia. Blood.
104:1048–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dvorak HF, Brown LF, Detmar M and Dvorak
AM: Vascular permeability factor/vascular endothelial growth
factor, microvascular hyperpermeability, and angiogenesis. Am J
Pathol. 146:1029–1039. 1995.PubMed/NCBI
|
|
25
|
Detmar M, Brown LF, Schon MP, et al:
Increased microvascular density and enhanced leukocyte rolling and
adhesion in the skin of VEGF transgenic mice. J Invest Dermatol.
111:1–6. 1998. View Article : Google Scholar : PubMed/NCBI
|