Introduction
By damaging injured or unnecessary cells, apoptosis
provides a significant mechanism for maintaining homeostasis and
normal biological functions in vivo, and imbalances between
cell proliferation and cell death can result in the onset of
numerous pathophysiological processes leading to a variety of human
diseases (1,2). Excessive apoptosis is important in a
number of diseases, including chronic degenerative disease
and acquired immune deficiency syndrome. Similarly, inadequate
apoptosis contributes to autoimmune disease and cancer (3). Uncontrolled apoptosis is also
involved in numerous types of renal disease. Acute renal failure
induced by toxic, ischemic or obstructive injury induces apoptosis
and necrosis simultaneously in renal tubular epithelial cells
(4,5). The excessive loss of tubular cells
caused by apoptosis may result in tubular atrophy and
tubulointerstitial fibrosis, subsequently leading to a chronic
decrease in renal function (6,7).
Transforming growth factor-β1 (TGF-β1) regulates
numerous biological functions, including cell proliferation,
differentiation, migration and cell death (3). TGF-β1 is capable of regulating
apoptosis, positively or negatively, in a cell type- and cellular
context-dependent manner as part of a complex signaling system
associated with cell survival and apoptosis (3). TGF-β1 also induces apoptosis in a
variety of cells, including hepatocytes, epithelial cells,
lymphocytes and renal cells (3).
Apoptosis of tubular epithelial cells is correlated with TGF-β1
expression, causing tubular atrophy and progressive renal disease,
including chronic obstructive nephropathy and diabetic kidney
disease (8,9).
Lefty is a novel member of the TGF-β protein
superfamily, consisting of Lefty 1 and Lefty 2 in mice (10,11)
and their homologs Lefty A and Lefty B in humans (12,13).
As one of the most important embryonic signals, Lefty promotes the
development of an asymmetric body plan (10,14–16).
Unlike other members of the TGF-β superfamily, Lefty does not exist
as a dimer. Therefore, it may act as a suppressor of the TGF-β1
signaling pathway (12). Lefty has
been shown to inhibit TGF-β1 signaling via inhibition of Smad2/3
phosphorylation and activation of the TGF-β receptor. Additionally,
Lefty suppresses the downstream events resulting from R-Smad
phosphorylation, including heterodimerization of the R-Smads
(Smad2/3) with Smad4 and nuclear translocation of the R-Smad/Smad4
complex (17). In addition, a
previous study demonstrated that Lefty A/Ebaf (endometrial bleeding
associated factor) significantly reduces the amount of collagen
deposited in tissues via inhibition of connective tissue growth
factor (CTGF) and collagen type I mRNA synthesis, thus increasing
the rates of collagenolysis and elastolysis (18). Therefore, Lefty A may serve as an
antagonist for TGF-β1 to ameliorate TGF-β1-induced apoptosis.
Current studies of Lefty protein focus on its regulation of
individual developmental processes during the embryonic period and
its antifibrotic function in adults. To the best of our knowledge,
no studies examining the role of Lefty protein with regard to
TGF-β1-mediated renal tubular epithelial cell apoptosis have been
performed thus far.
This study aimed to examine the effects of Lefty A
protein on TGF-β1-mediated renal tubular epithelial cell apoptosis
and to investigate the potential mechanisms involved.
Materials and methods
Chemicals and reagents
Human renal tubular epithelial cells (HK-2) were
derived from the Beijing Union Cell Culture Center (Beijing,
China). DMEM, nutrient mixture F-12 (DMEM/F12), FBS and
trypsin/EDTA solution were purchased from Hyclone (Logan, UT, USA).
The pcDNA3.1/Hygro (+) plasmid vector was donated by Professor
Siamak Tabibzadeh (Stony Brook University, Stony Brook, NY, USA).
Chemicals and reagents purchased were: Full-length DNA sequences of
human Lefty A (Wuhan Genesil Biotechnology Company, Hubei, China);
recombinant human TGF-β1 (PeproTech EC Ltd., London, UK); TRIzol
and Lipofectamine™ 2000 (Invitrogen Life Technologies, Carlsbad,
CA, USA); First Strand cDNA Synthesis kit (Fermentas, UAB,
Lithuania); BCA assay kit (Beyotime Institute of Biotechnology,
Jiangsu, China); the monoclonal mouse antibody against Lefty A
(R&D Systems, Minneapolis, MN, USA); the polyclonal antibody
against p-Smad2/3 and the polyclonal anti-actin antibody (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and the Annexin V/PI
Apoptosis kit (MultiSciences Biotech Co., Ltd., Hangzhou,
China).
Plasmid construction
The coding sequence of human Lefty A was amplified
from the full-length sequence of Lefty A. The primers used were:
sense, 5′-CCCAAGCTT GCCACCATGTGGCCCCTGTGGC-3′, antisense, 5′-CGCG
GATCCCTATGGCTGGAGCCTCCTT-3′. The PCR products were digested with
restriction endonuclease HindIII and BamHI and
inserted into the HindIII and BamHI sites of a
pcDNA3.1/Hygro (+) vector. The recombinant plasmid was confirmed by
DNA sequencing.
Cell culture and plasmid
transfection
HK-2 cells were maintained in DMEM/F12 supplemented
with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin at
37°C under a humidified 5% CO2 atmosphere. Cells were
seeded in 6-well plates and transfected with 1 μg of either
recombinant plasmid DNA or pcDNA3.1 empty vector using
Lipofectamine™ 2000. Stable transfectants were generated from a
HK-2 cell pool following selection with hygromycin (300 μg/ml).
Overexpression of Lefty A in the stable transfected cell line was
confirmed by western blot analysis. In order to examine the effects
of Lefty A overexpression on TGF-β1-induced apoptosis of human
renal tubular epithelial cells, the stably transfected plasmid cell
line was cultured in serum-free medium for 24 h prior to TGF-β1
treatment. Cell lysates were harvested 6, 12, 24 and 48 h following
treatment with TGF-β1.
Western blotting
The cells were washed with ice-cold PBS and scraped
into lysis buffer (150 mM NaCl, 1% NP40, 50 mM Tris/HCl, 1 mM EGTA,
1 mM PMSF, 10 mM Na4P2O7, 10 mM
NaF, 1 mM Na3VO5, 10 μg/ml leupeptin and 20
μg/ml aprotinin). The protein concentrations were measured using a
BCA assay kit. Following the addition of the protein loading buffer
(50 mM Tris/HCl, 10% glycerol, 0.02% BPB, 2% β-mercaptoethanol and
5% SDS, pH 6.8) and denaturation for 5 min at 95°C, 40 μg of total
protein from each sample was separated by 8% SDS-PAGE, then
transferred onto nitrocellulose membranes. Following blocking in 5%
non-fat milk for 1 h at 37°C, the membranes were incubated with
various primary antibodies for 24 h at 4°C, followed by a
horseradish peroxidase-conjugated secondary antibody for 1 h at
room temperature in blocking buffer. Signals were detected using
the enhanced chemiluminescence method.
Apoptosis detection by flow
cytometry
The samples were washed two or three times and
adjusted to a concentration of 1×106 cells/ml with 4°C
PBS. Suspensions of 100 μg/l were added to each labeled Falcon
tube, and 10 μg/l of annexin V-FITC and 10 μg/l PI (20 μg/ml) were
added into labeled Falcon tubes, and incubated for at least 30 min
in a dark room at room temperature. Following this, 400 μg/l of PBS
buffer was added to each Falcon tube without washing and analyzed
using flow cytometry almost immediately (within 20 min). This assay
was performed in quintuplicate.
Statistical analysis
Data were expressed as the mean ± SEM from at least
three independent experiments. For western blot analysis,
quantification was performed by scanning and analyzing the average
volume density of the hybridization signals corrected for β-actin
using GEL pro3.0 software. Statistical analysis of the data was
performed with the Student’s t-test for comparison of the two
groups or one-way ANOVA for multiple comparisons. P<0.05 was
considered to indicate a statistically significant result.
Results
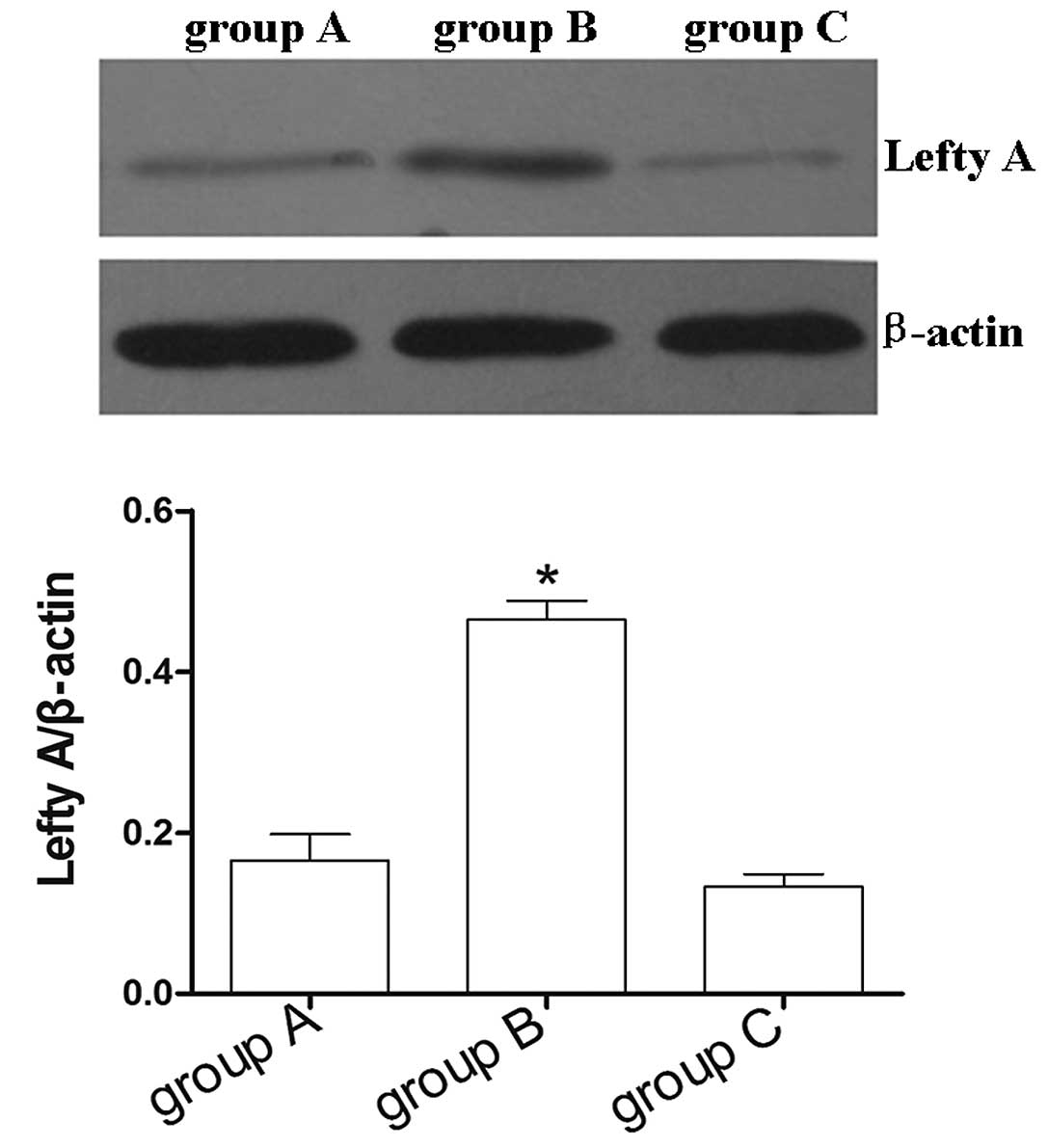
Expression of Lefty A in HK-2 cells
stably transfected with Lefty A
Following a 2 week screening with hygromycin B,
non-transfected cells gradually died off. Lefty-positive clone cell
islands were observed. The abundance of the Lefty A protein in
normal HK-2 cells was extremely low. To examine whether
overexpression of Lefty A affects the rate of apoptosis in human
renal tubular epithelial cells, a recombinant of the Lefty A
plasmid construct was stably transfected into HK-2 cells, and
overexpression of the Lefty A was confirmed by western blot
analysis. The results demonstrated that the expression levels of
Lefty A in HK-2 cells stably transfected with Lefty A were
significantly higher compared with normal untransfected HK-2 cell
controls and HK-2 cells stably transfected with pcDNA3.1 empty
vector (P<0.05; Fig. 1). No
evident difference in the expression levels of Lefty A was observed
between normal untransfected HK-2 cell controls and HK-2 cells
stably transfected with pcDNA3.1 empty vector (Fig. 1).
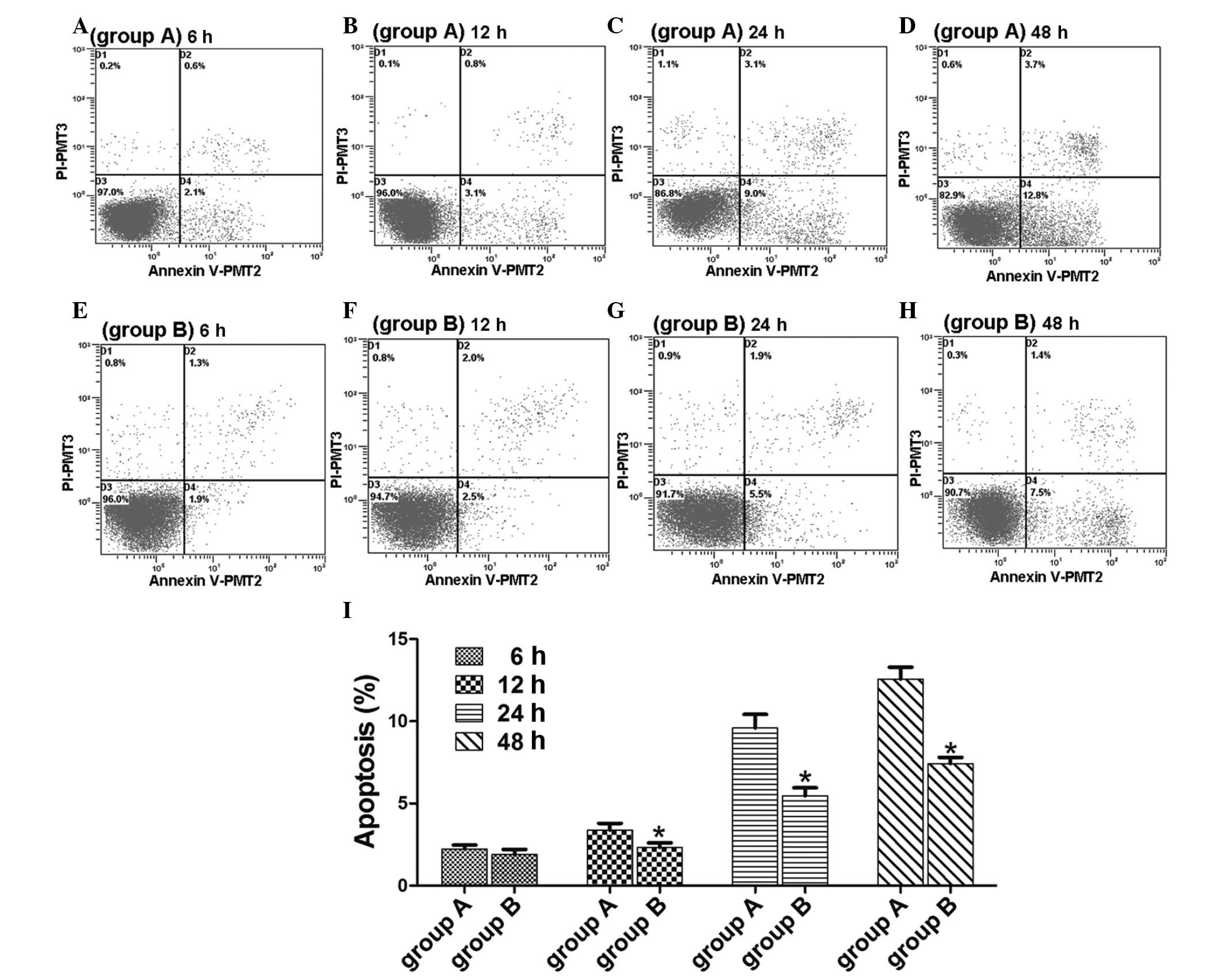
The apoptotic rate of HK-2 cells stably
transfected with Lefty A
No evident cell apoptosis was observed in group A
(normal untransfected HK-2 cell control) and group B (HK-2 cells
stably transfected with Lefty A) at 6 h following treatment with
TGF-β1 and at 12 h, apoptotic rates began to increase (Fig. 2A-H). The apoptotic rate of HK-2
cells stably transfected with Lefty A was significantly lower at
each time point than the normal untransfected HK-2 cell controls
(P<0.05; Fig. 2I).
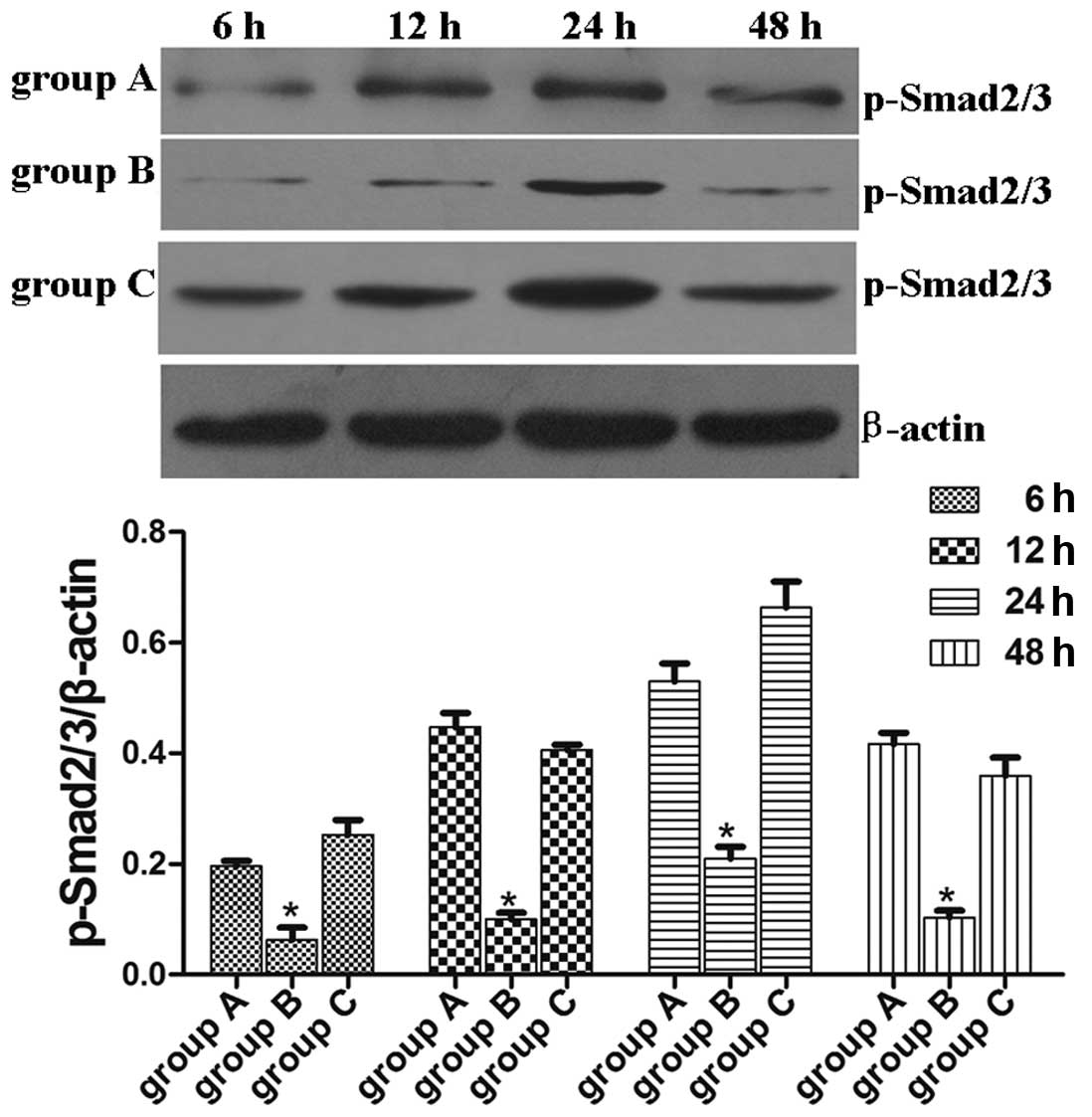
Lefty A inhibits the TGF-β1-induced Smad
signal transduction pathway
The present study indicates that overexpression of
the Lefty A protein notably alleviates TGF-β1-mediated apoptosis in
human renal tubular epithelial cells, however, the mechanism by
which this occurs remains unknown. As previously observed in stem
cells, Lefty A inhibits the TGF-β1-induced Smad signal transduction
pathway (2). We investigated
whether TGF-β1-induced Smad2/3 activation in HK-2 cells are able to
be inhibited by Lefty A. The abundance of p-Smad2/3 protein in
normal HK-2 cells was extremely low and TGF-β1 induced activation
of p-Smad2/3 in a time-dependent manner with a peak at 24 h.
Overexpression of the Lefty A protein inhibited TGF-β1-induced
Smad2/3 activation in HK-2 cells, demonstrating that Lefty A is
capable of inhibiting the TGF-β1/Smad signaling pathway in the
process of TGF-β1-mediated apoptosis in human renal tubular
epithelial cells. p-Smad2/3 protein expression levels in group B at
each time point were lower than that in groups A and C (HK-2 cells
stably transfected with pcDNA3.1 empty vector; P<0.05; Fig. 3). No significant difference in the
p-Smad2/3 protein expression levels were observed at each time
point between normal untransfected HK-2 cell controls and HK-2
cells stably transfected with pcDNA3.1 empty vector (Fig. 3).
Discussion
As a multifunctional cytokine, TGF-β1 is involved in
regulating the cell cycle, cell growth, cell differentiation,
matrix formation and cell-mediated apoptosis in a wide range of
cells and tissues. A number of studies examining the molecular
mechanism involved in renal obstruction disease revealed that
TGF-β1 is the key cytokine that mediates cell apoptosis and renal
fibrosis. In animal models of unilateral ureteral obstruction
(UUO), TGF-β1 antibody reduces the rate of apoptosis in renal
tubular epithelial cells (19).
In vitro and in vivo studies confirm that TGF-β1 may
trigger apoptosis in renal injury (19,20),
and Smad proteins are important downstream signal transduction
factors for TGF-β1. The Lefty protein was first identifed by Meno
et al(11) in the mouse
embryo with a left-right asymmetry of protein expression. Lefty is
capable of inhibiting the TGF-β1/Smad pathway, particularly the
phosphorylation of receptor-activated Smads (R-Smads) in the
TGF-β1/Smad pathway and the formation of the R-Smad/co-Smad
copolymer.
In the present study, coculture of normal HK-2 cells
with TGF-β1 induced elevated expression levels of p-Smad2/3. The
phosphorylation of the Smad2/3 proteins forms a key step in the
activation of the TGF-β1/Smad pathway. The increased levels of
p-Smad2/3 suggest that the activation of the TGF-β1/Smad pathway
and higher rates of apoptosis occur simultaneously. No significant
difference in p-Smad2/3 expression levels and rate of apoptosis was
detected between groups A and C. In group B, liposomes were used to
transfect human Lefty A plasmids into HK-2 cells in order to induce
the stable expression of endogenous Lefty protein. Following
treatment with TGF-β1, the expression levels of p-Smad2/3 and rate
of cell apoptosis decreased in group B to a greater degree compared
with group A. In vivo and in vitro experiments
demonstrate that the TGF-β1/Smad signaling pathway is closely
correlated with the rate of apoptosis (21–23).
Our study provides consistent results which support this
hypothesis. In UUO mouse models, the rate of apoptosis in renal
tubular epithelial cells in Smad3-knockout mice was greatly reduced
compared with wild-type mice (24). Huang et al(25) observed that high expression levels
of Smad7 (TGF-β1/Smad pathway inhibitor) are capable of inhibiting
TGF-β1-induced renal tubular cell growth arrest and apoptosis in
renal tubular cells transfected with the Smad7 gene.
Taken together, these results indicate that the
TGF-β1/Smad signaling pathway is most likely responsible for the
regulation of apoptosis in renal tubular epithelial cells. In
addition, the Lefty A protein is capable of inhibiting the
TGF-β1/Smad pathway in order to reduce TGF-β1/Smad-mediated
apoptosis in renal tubular epithelial cells. This study may provide
novel insights into the prevention and treatment of urinary tract
obstruction disease using the Lefty A protein.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81170710).
References
|
1
|
Padanilam BJ: Cell death induced by acute
renal injury: a perspective on the contributions of apoptosis and
necrosis. Am J Physiol Renal Physiol. 284:F608–F627. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carson DA and Ribeiro JM: Apoptosis and
disease. Lancet. 341:1251–1254. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sánchez-Capelo A: Dual role for TGF-beta1
in apoptosis. Cytokine Growth Factor Rev. 16:15–34. 2005.
|
|
4
|
Rana A, Sathyanarayana P and Lieberthal W:
Role of apoptosis of renal tubular cells in acute renal failure:
therapeutic implications. Apoptosis. 6:83–102. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Havasi A and Borkan SC: Apoptosis and
acute kidney injury. Kidney Int. 80:29–40. 2011. View Article : Google Scholar
|
|
6
|
Razzaque MS, Ahsan N and Taguchi T: Role
of apoptosis in fibrogenesis. Nephron. 90:365–372. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeisberg M, Strutz F and Müller GA: Renal
fibrosis: an update. Curr Opin Nephrol Hypertens. 10:315–320. 2001.
View Article : Google Scholar
|
|
8
|
Böttinger EP and Bitzer M: TGF-beta
signaling in renal disease. J Am Soc Nephrol. 13:2600–2610.
2002.
|
|
9
|
Kopp JB: TGF-beta signaling and the renal
tubular epithelial cell: too much, too little, and just right. J Am
Soc Nephrol. 21:1241–1243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meno C, Ito Y, Saijoh Y, et al: Two
closely-related left-right asymmetrically expressed genes, lefty-1
and lefty-2: their distinct expression domains, chromosomal linkage
and direct neuralizing activity in Xenopus embryos. Genes
Cells. 2:513–524. 1997. View Article : Google Scholar
|
|
11
|
Meno C, Saijoh Y, Fujii H, et al:
Left-right asymmetric expression of the TGF beta-family member
lefty in mouse embryos. Nature. 381:151–155. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kothapalli R, Buyuksal I, Wu SQ, Chegini N
and Tabibzadeh S: Detection of ebaf, a novel human gene of the
transforming growth factor beta superfamily association of gene
expression with endometrial bleeding. J Clin Invest. 99:2342–2350.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kosaki K, Bassi MT, Kosaki R, et al:
Characterization and mutation analysis of human LEFTY A and LEFTY
B, homologues of murine genes implicated in left-right axis
development. Am J Hum Genet. 64:712–721. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hamada H, Meno C, Watanabe D and Saijoh Y:
Establishment of vertebrate left-right asymmetry. Nat Rev Genet.
3:103–113. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dvash T, Mayshar Y, Darr H, et al:
Temporal gene expression during differentiation of human embryonic
stem cells and embryoid bodies. Hum Reprod. 19:2875–2883. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marjoram L and Wright C: Rapid
differential transport of Nodal and Lefty on sulfated
proteoglycan-rich extracellular matrix regulates left-right
asymmetry in Xenopus. Development. 138:475–485. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ulloa L and Tabibzadeh S: Lefty inhibits
receptor-regulated Smad phosphorylation induced by the activated
transforming growth factor-beta receptor. J Biol Chem.
276:21397–21404. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mason JM, Xu HP, Rao SK, et al: Lefty
contributes to the remodeling of extracellular matrix by inhibition
of connective tissue growth factor and collagen mRNA expression and
increased proteolytic activity in a fibrosarcoma model. J Biol
Chem. 277:407–415. 2002. View Article : Google Scholar
|
|
19
|
Miyajima A, Chen J, Lawrence C, et al:
Antibody to transforming growth factor-beta ameliorates tubular
apoptosis in unilateral ureteral obstruction. Kidney Int.
58:2301–2313. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai C, Yang J and Liu Y: Transforming
growth factor-beta1 potentiates renal tubular epithelial cell death
by a mechanism independent of Smad signaling. J Biol Chem.
278:12537–12545. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Ge Y, Liu FY, et al: Norcantharidin,
a protective therapeutic agent in renal tubulointerstitial
fibrosis. Mol Cell Biochem. 361:79–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Sun W, Zhang C, et al: TGF-beta1
inhibits the growth and metastasis of tongue squamous carcinoma
cells through Smad4. Gene. 485:160–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Deane JA, Campanale NV, Bertram JF
and Ricardo SD: Blockade of p38 mitogen-activated protein kinase
and TGF-beta1/Smad signaling pathways rescues bone marrow-derived
peritubular capillary endothelial cells in adriamycin-induced
nephrosis. J Am Soc Nephrol. 17:2799–2811. 2006. View Article : Google Scholar
|
|
24
|
Inazaki K, Kanamaru Y, Kojima Y, et al:
Smad3 deficiency attenuates renal fibrosis, inflammation, and
apoptosis after unilateral ureteral obstruction. Kidney Int.
66:597–604. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang YJ, Mei YM, Wang YQ, et al:
Transfection and expression of Smad7 inhibits transforming growth
factor-β1 effects on renal tubular cells (Chinese). J Third
Military Medical University. 25:1049–1052. 2003.
|