Introduction
Non-invasive examination methods are increasingly
important in diagnosing celiac disease (CD). New options for
diagnosing CD have been discovered as a result of progress in
immunology (1). The detection of
circulating autoantibodies is becoming more frequent clinically. As
a result, many new, highly heterogeneous cases of CD have been
diagnosed, and consequently, recognition of the incidence of CD in
the pediatric and adult populations has increased. Detection of
anti-endomysial antibodies (AEA) and anti-tissue transglutaminase
antibodies (t-TG), characteristic for their high sensitivity and
specificity, play important roles in diagnosing and clinically
monitoring CD (2,3). Nevertheless, histopathology remains
the major tool for clinicians, and particularly
gastroenterologists, for definitively diagnosing CD (4,5). A
variety of studies have documented that AEA, and t-TG, are highly
specific in predicting CD (6,7).
There are also seronegative data, which is particularly true for
the adult population (8). The
diagnosis in children is more successful (9), as pediatric CD has clear features in
comparison to adult CD (10). It
is often difficult to diagnose CD quickly, due to the fact that CD
has a wide range of symptoms. However, we do not know if the same
problems exist in the diagnosis of pediatric patients. We decided
to test this hypothesis on a population of children in Eastern
Slovakia. The objective of this study was to compare the levels of
AEA and t-TG (IgA, IgG) in the plasma with that of histological
abnormalities in pediatric patients with CD.
Materials and methods
Patients
Between 2005 and 2010, a program which searched for
CD in the pediatric population was performed. All samples were
obtained from the Teaching Faculty Hospital (Košice, Slovakia). In
total, 4,247 biopsy samples were used. Informed consent was
provided by the parents of the children. This study was approved by
the Ethics Committee of Pavol Jozef Safarik University in Kosice,
Kosice, Slovak Republic.
Serum analysis
Sera from all children with digestive disorders and
chronic non-specific gastroenteritis were sampled during
hospitalization, in addition to routine biochemical investigations.
Blood sera were separated using routine biochemical methodology
(11). Serological testing kits
were provided by INOVA Diagnostics (San Diego, CA, USA). AEA (IgA)
and t-TG (IgA and IgG) were examined using immunofluorescence and
other immunological methods. For AEA examination, binding to human
umbilical cord tissue was used and for t-TG we used the classical
immunological ELISA test. AEA was investigated by indirect
immunofluorescence neutrophil inhibitory factor (NIF), and the
limit value for t-TG was 10 U/ml. Only samples where AEA were
negative were selected. Regardless of the outcome of the serology,
biopsies from the duodenum were subsequently performed.
Biopsies
Each patient underwent between three to five
endoscopic biopsies from the bulb and third duodenal portions and
the samples were fixed in a 10% formalin solution. Duodenal biopsy
specimens were obtained by standard procedures, including
orientation, processing and interpretation in order to avoid, or
minimize, their misinterpretation (12). The samples were hematoxylin &
eosin (H&E) stained and evaluated in accordance with the
Marsh-Oberhuber system (13,14).
Results
Samples
From the 4,247 samples, we found 44 new cases of CD
in children with AEA negativity, but these instances always
coexisted with histological abnormalities of the small intestine
(Table I). A t-TG (IgA) value
>10 U/ml was detected in 4 patients, and a t-TG (IgG) value
>10 U/ml was detected in another 4 patients. All others t-TG
(IgA) and t-TG (IgG) values were <10 U/ml. There was elevated
t-TG (IgA) or t-TG (IgG), but none of the patients exhibited
increases in both values. All values are in the context of the AEA
negativity. As shown in the Table
I, AEA negativity is always visible in Marsh I histological
grade, as well as in the advanced grade of histological
abnormalities, MIII. Values of t-TG (IgA) and t-TG (IgG) >10
U/ml were visible only in the advanced Marsh III grade of
histological abnormalities, remaining <10 U/ml in histological
grade Marsh I. Of the 44 samples, nine were diagnosed as Marsh I,
two as Marsh IIIA, 14 as Marsh IIIB and 19 as Marsh IIIC. Variable
damage, including inflammatory lesions with fine, or total, villous
atrophy of the small intestine was visible in our investigated
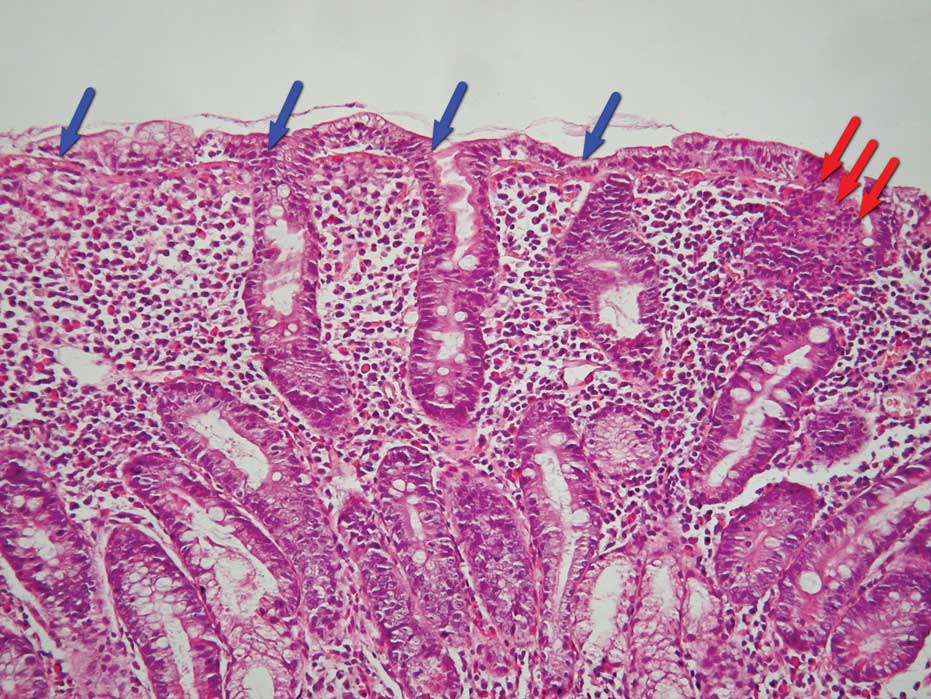
materials. In the cases with partial villous atrophy, the
differentiated intraepithelial inflammatory florid process
(Fig. 1) was visible as well. In
cases where it was difficult to obtain a morphological image, which
prevented the exact evaluation of mucous membrane characteristics,
we were able to find only residual structures of crypts with
compensatory cryptic hyperplasia and hyperplasia of Brunner’s
glands. Regarding the material characteristics, the main feature
was severe structural disorder, resulting in completely smoothed
mucous membrane relief, which consisted of mixed dense inflammation
infiltrate with predominant intraepithelial lymphocytes and plasma
cells (Fig. 2).
 | Table IOverview of immunological evaluation
values and associated histopathological description in pediatric
CD. |
Table I
Overview of immunological evaluation
values and associated histopathological description in pediatric
CD.
| Patient | t-TG IgA | t-TG IgG | AEA | Marsh |
|---|
| 1 | 2.6 | 3.8 | Negative | I |
| 2 | 2.7 | 2.4 | Negative | I |
| 3 | 1.0 | 1.6 | Negative | I |
| 4 | 4.4 | 2.8 | Negative | I |
| 5 | 2.7 | 2.5 | Negative | I |
| 6 | 3.2 | 4.4 | Negative | I |
| 7 | 1.0 | 5.4 | Negative | I |
| 8 | 2.6 | 3.8 | Negative | I |
| 9 | 2.7 | 2.4 | Negative | I |
| 10 | 7.2 | 6.8 | Negative | M3A |
| 11 | 7.1 | 4.2 | Negative | M3A |
| 12 | 3.0 | 3.5 | Negative | M3B |
| 13 | 2.6 | 4.9 | Negative | M3B |
| 14 | 3.4 | 9.1 | Negative | M3B |
| 15 | 2.5 | 1.4 | Negative | M3B |
| 16 | 11.2 | 2.7 | Negative | M3B |
| 17 | 7.8 | 7.3 | Negative | M3B |
| 18 | 4.1 | 4.5 | Negative | M3B |
| 19 | 1.0 | 1.8 | Negative | M3B |
| 20 | 1.0 | 4.0 | Negative | M3B |
| 21 | 3.0 | 3.5 | Negative | M3B |
| 22 | 2.6 | 4.9 | Negative | M3B |
| 23 | 3.4 | 9.1 | Negative | M3B |
| 24 | 2.5 | 1.4 | Negative | M3B |
| 25 | 12.2 | 2.7 | Negative | M3B |
| 26 | 1.7 | 89.3 | Negative | M3C |
| 27 | 2.5 | 4.8 | Negative | M3C |
| 28 | 5.1 | 10.1 | Negative | M3C |
| 29 | 6.1 | 3.5 | Negative | M3C |
| 30 | 2.5 | 2.6 | Negative | M3C |
| 31 | 2.4 | 6.9 | Negative | M3C |
| 32 | 5.1 | 4.0 | Negative | M3C |
| 33 | 17.2 | 4.8 | Negative | M3C |
| 34 | 3.8 | 5.9 | Negative | M3C |
| 35 | 8.3 | 8.0 | Negative | M3C |
| 36 | 3.3 | 1.7 | Negative | M3C |
| 37 | 2.7 | 3.9 | Negative | M3C |
| 38 | 1.7 | 89.3 | Negative | M3C |
| 39 | 2.5 | 4.8 | Negative | M3C |
| 40 | 5.1 | 10.1 | Negative | M3C |
| 41 | 15.6 | 8.2 | Negative | M3C |
| 42 | 8.3 | 8.0 | Negative | M3C |
| 43 | 3.3 | 1.7 | Negative | M3C |
| 44 | 2.7 | 3.9 | Negative | M3C |
Discussion
In this study, we wanted to demonstrate the lack of
association between autoantibodies and lesions in certain cases of
pediatric CD. This may justify the necessity of biopsies for the
accurate diagnosis of CD. We show lesions, found in our samples,
that are diagnostically important. The findings of Rostami et
al(15) demonstrated that AEA
have limited value in CD screening programs. Their sensitivity in
patients with total villous atrophy (Marsh IIIC) was 100%, but with
partial atrophy (Marsh IIIA) the sensitivity of AEA decreased to
3l%. Furthermore, a high IgA t-TG titer correlates with the
histopathological finding of Marsh IIIC (16,17).
Another study revealed that positive AEA are observed mostly in CD
patients with severe tissue damage due to partial or total villous
atrophy, but they have a low sensitivity in CD patients with
partial villous atrophy in spite of the use of different substrates
(18,19). Additionally, a study by Tărmure
et al(20) demonstrated
that in patients with Marsh I and Marsh II, the sensitivity of AEA
was lower. These results demonstrated that the autoantibody titers
may correlate with the damage to the small intestine. In our study,
there have been negative antibodies with Marsh IIIC, demonstrating
that serological screening is not 100% accurate for the diagnosis
of CD. That serological tests would be negative was known for adult
CD patients but was not previously known for pediatric CD patients.
It is possible that clinical symptomatology is poor in certain
children, while the disease remains active, and these diagnostic
problems require a solution. Therefore, a biopsy must always be
performed, so that an expert pathologist may accurately distinguish
CD from other diseases (21).
The literature has provided evidence that AEA titers
are the first to disappear following the introduction of a
gluten-free diet, as CD is induced by gluten and related cereal
proteins. Midhagen et al(22) demonstrated that antibody titers
decreased sharply within the first month following the introduction
of a gluten-free diet. It is possible that weak reactions of AEA or
t-TG are caused by the ingestion of gluten-free food, but the
degree of histological damage remains high. Fernández-Salazar et
al(23) documented that AEA
negativity is not rare in patients with CD. These results
demonstrate that sensitivity to serology varies according to the
intestinal damage and the introduction of a gluten-free diet. The
results of other studies also support this hypothesis (24,25).
Therefore, we recommend multiple endoscopic biopsies from the
proximal and distal duodenum (26,27).
Furthermore, in cases of low IgA t-TG titers or in patients with
IgA deficiency, intestinal biopsies should remain mandatory
(28). The results of certain
studies have revealed that only patients with a IgA t-TG level ≥100
U/ml and whose symptoms improve upon the introduction of a
gluten-free diet may not require a biopsy of the small intestine to
confirm CD (29,30). Although these results are
promising, in our opinion, biopsies should not be rejected.
However, there are children who are AEA positive and exhibit a
normal mucosal villous morphology of the small intestine, which
indicates that the diagnostic criteria for CD should be
re-evaluated (31). These
disparities were described for other antibodies [e.g., AGA (IgA),
ARA (IgA) and JAB (IgA)], which is also important for the
pathologist to avoid rejection of biopsies based on insufficient
evidence (32). Certain studies
report that an accurate diagnosis is supported by the genetic
analysis of HLADQ2 or HLADQ8 (33,34),
but these tests are not yet standard practice in all workplaces,
and HLA association alone is inadequate for providing an
explanation of the inheritance pattern. For children with a biopsy
consistent with CD, in addition to a positive response to a
gluten-free diet, HLA testing is not required (35). Di Osdado et al(36) found variations in the expression
levels of 109 genes by comparing Marsh III and Marsh 0 lesions,
which have roles in the proliferation and differentiation pathways.
These results are crucial and encouraging for medical practice, as
they may lead to alternative future genetic analyses and the
discovery of combinations with new candidate genes for CD. Recent
studies have focused on ways to speed up the diagnosis of CD
(37,38), but according to our study, biopsies
remain necessary. Until we find a combination of diagnostic methods
that are 100% reliable, we should continue to perform biopsies. We
believe that currently biopsies are the optimum way for the
gastroenterologist to accurately diagnose CD in at risk pediatric
groups, as well as in children with unspecified long-term related
digestive problems. Histological results are important for
clinicians, but particularly for the gastroenterologist, in order
to establish tests and interpret all available data (39,40).
It has been many years since CD was considered to be
a rare disease and experts have agreed that the incidence of CD
will increase, since more programs have started to investigate the
‘celiac iceburg’ (41).
Autoantibodies are considered to only be helpful in clinical
practice, with regards to CD screening. In accordance with this, a
biopsy of the small intestine is always required for the accurate
diagnosis of CD. We recommend a minimum of four endoscopic biopsies
to be obtained from the bulb and third duodenal portions, as a
section of the biopsy may be damaged, and the absence of certain
material that should be investigated may result in incorrect
histopathological interpretation. Slice descriptions should include
all relevant information, and the histopathological report should
be clear and conform to the recommended standards for intestinal
biopsies. We consider this information to be particularly important
for pathologists, which is in accordance with the views expressed
in other studies (42). We
recommend the use of autoantibodies only as a supportive method, as
a control in gluten-free diets or as an early predictor of CD with
necessary further follow-up. Recent European Society of Pediatric
Gastroenterology, Hepatology and Nutrition (ESPGHAN) guidelines
state that in cases where a child manifests clear symptoms of CD
and demonstrates particularly high levels of t-TG, with positive
HLA testing, the physician may consider omitting a biopsy. However,
there are some instances where pediatric CD has remained
undiagnosed, providing support for a revision to the diagnostic
criteria in a clinical, endoscopic and serological context.
However, since there are no reliable data on the incidence of CD in
children in the East region of the Slovak Republic, our results may
be considered to be valid for other countries as well, given that
many countries have no screening, only local screening or use
different diagnosis methods. The incidence of CD in pediatric
patients is higher than previously assumed.
Recent and current literature indicates that celiac
disease is underdiagnosed. From our results we conclude that the
screening program should be considerably more intense, particluarly
in pediatrics. In numerous cases it is difficult to diagnose only
from the serum. Biopsies should be part of the investigation,
regardless of the outcome of serology.
Acknowledgements
This study was supported by the IGA MZCR: NT
13424-4/2012 grant.
References
|
1
|
Samaşca G, Sur G and Lupan I: Current
trends and investigative developments in celiac disease. Immunol
Invest. 42:273–284. 2013.PubMed/NCBI
|
|
2
|
Fasano A, Araya M, Bhatnagar S, et al:
Federation of International Societies of Paediatric
Gastroenterology, Hepatology, and Nutrition consensus report on
celiac disease. J Pediatr Gastroenterol Nutr. 47:214–219. 2008.
View Article : Google Scholar
|
|
3
|
Fric P, Gabrovska D and Nevoral J: Celiac
disease, gluten-free diet, and oats. Nutr Rev. 69:107–115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bao F and Bhagat G: Histopathology of
celiac disease. Gastrointest Endosc Clin N Am. 22:679–694. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Sabatino A and Corazza GR: Coeliac
disease. Lancet. 373:1480–1493. 2009.
|
|
6
|
Chorzelski TP, Sulej J, Tchorzewska H,
Jablonska S, Beutner EH and Kumar V: IgA class endomysium
antibodies in dermatitis herpetiformis and coeliac disease. Ann NY
Acad Sci. 420:325–334. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dieterich W, Ehnis T, Bauer M, Donner P,
Volta U, Riecken EO and Schuppan D: Identification of tissue
transglutaminase as the autoantigen of celiac disease. Nat Med.
3:797–801. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lindfors K, Koskinen O, Kurppa K, et al:
Serodiagnostic assays for celiac disease based on the open or
closed conformation of the autoantigen, transglutaminase 2. J Clin
Immunol. 31:436–442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mariné M, Farre C, Alsina M, et al: The
prevalence of celiac disease is significantly higher in children
compared with adults. Aliment Pharmacol Ther. 33:477–486.
2011.PubMed/NCBI
|
|
10
|
Rodrigo-Sáez L, Fuentes-Álvarez D,
Pérez-Martínez I, et al: Differences between pediatric and adult
celiac disease. Rev Esp Enferm Dig. 103:238–244. 2011.(Article in
English and Spanish).
|
|
11
|
Samaşca G, Iancu M, Farcău D, et al: IgA
anti-tissue transglutaminase antibodies, first line in the
diagnosis of celiac disease. Clin Lab. 57:695–701. 2011.PubMed/NCBI
|
|
12
|
Ravelli A and Villanacci V: Tricks of the
trade: How to avoid histological pitfalls in celiac disease. Pathol
Res Pract. 208:197–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marsh MN: Gluten, major histocompatibility
complex, and the small intestine. A molecular and immunobiologic
approach to the spectrum of gluten sensitivity (‘celiac sprue’).
Gastroenterol. 102:330–354. 1992.PubMed/NCBI
|
|
14
|
Oberhuber G, Granditsch G and Vogelsang H:
The histopathology of coeliac disease: time for a standardized
report scheme for pathologists. Eur J Gastroenterol Hepatol.
11:1185–1194. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rostami K, Kerckhaert J, Tiemessen R,
Blomberg BM, Meijer JW and Mulder CJ: Sensitivity of antiendomysium
and antigliadin antibodies in untreated celiac disease:
disappointing in clinical practice. Am J Gastroenterol. 94:888–894.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arshad H and Ahmad Z: Histologic findings
in biopsies/resection specimens from the small intestine with
special emphasis on celiac disease: experience from a developing
country in South Asia. Ann Diagn Pathol. 16:436–440. 2012.
View Article : Google Scholar
|
|
17
|
Vivas S, Ruiz de Morales JG, Riestra S, et
al: Duodenal biopsy may be avoided when high transglutaminase
antibody titers are present. World J Gastroenterol. 15:4775–4780.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rostami K, Kerckhaert J, Tiemessen R,
Meijer JW and Mulder CJ: The relationship between anti-endomysium
antibodies and villous atrophy in coeliac disease using both monkey
and human substrate. Eur J Gastroenterol Hepatol. 11:439–442. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rostami K, Mulder CJ, Stapel S, et al:
Autoantibodies and histogenesis of celiac disease. Rom J
Gastroenterol. 12:101–106. 2003.PubMed/NCBI
|
|
20
|
Tărmure S, Cristea A, Sămpelean D, Negrean
V and Alexescu T: Serological and histological correlations in
celiac disease. Rom J Intern Med. 45:263–268. 2007.PubMed/NCBI
|
|
21
|
Anderson RP: Coeliac disease: current
approach and future prospects. Intern Med J. 10:790–799. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Midhagen G, Aberg AK, Olcen P, et al:
Antibody levels in adult patients with coeliac disease during
gluten-free diet: a rapid initial decrease of clinical importance.
J Intern Med. 256:519–524. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fernández-Salazar LI, de la Torre Ferrera
N, Velayos Jiménez B, et al: Diagnostic problems in adult celiac
disease. Rev Esp Enferm Dig. 100:24–28. 2008.(In Spanish).
|
|
24
|
Abrams JA, Diamond B, Rotterdam H and
Green PH: Seronegative celiac disease: increased prevalence with
lesser degrees of villous atrophy. Dig Dis Sci. 49:546–550. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nachman F, Sugai E, Vázquez H, et al:
Serological tests for celiac disease as indicators of long-term
compliance with the gluten-free diet. Eur J Gastroenterol Hepatol.
23:473–480. 2011.PubMed/NCBI
|
|
26
|
Prasad KK, Thapa BR, Nain CK and Singh K:
Assessment of the diagnostic value of duodenal bulb histology in
patients with celiac disease, using multiple biopsy sites. J Clin
Gastroenterol. 43:307–311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Webb C, Halvarsson B, Norström F, et al:
Accuracy in celiac disease diagnostics by controlling the
small-bowel biopsy process. J Pediatr Gastroenterol Nutr.
52:549–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Clouzead-Girard H, Rebouissoux L, Taupin
JL, et al: HLA-DQ genotyping combined with serological markers for
the diagnosis of celiac disease: Is intestinal biopsy still
mandatory? J Pediatr Gastroenterol Nutr. 52:729–733. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Donaldson MR, Book LS, Leiferman M, Zone
JJ and Neuhausen SL: Strongly positive tissue transglutaminase
antibodies are associated with Marsh 3 histopathology in adult and
pediatric celiac disease. J Clin Gastroenterol. 42:256–260.
2008.PubMed/NCBI
|
|
30
|
Mubarak A, Wolters VM, Gerritsen SA,
Gmelig-Meyling FH, Ten Kate FJ and Houwen RH: A biopsy is not
always necessary to diagnose celiac disease. J Pediatr
Gastroenterol Nutr. 52:554–557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kurppa K, Ashorn M, Iltanen S, Koskinen
LL, Saavalainen P, Koskinen O, Mäki M and Kaukinen K: Celiac
disease without villous atrophy in children: a prospective study. J
Pediatr. 157:373–348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vega F, Chang CC, Schwartz MR, Preti HA,
Younas M, Ewton A, Verm R and Jaffe ES: Atypical NK-cell
proliferation of the gastrointestinal tract in a patient with
antigliadin antibodies but not celiac disease. Am J Surg Pathol.
30:539–544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tikkakoski S, Savilahti E and Kolho KL:
Undiagnosed coeliac disease and nutritional deficiencies in adults
screened in primary health care. Scand J Gastroenterol. 42:60–65.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lowichik A and Book L: Pediatric celiac
disease: clinicopathologic and genetic aspects. Pediatr Dev Pathol.
6:470–483. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rizkalla NR, Dixit R, Simpson S and Green
PH: Celiac disease in children: an old disease with new features.
Minerva Pediatr. 64:71–81. 2012.PubMed/NCBI
|
|
36
|
Di Osdado B, Wapenaar MC, Franke L, et al:
A microarray green for novel candidate genes in coeliac disease
pathogenesis. Gut. 53:944–951. 2004.PubMed/NCBI
|
|
37
|
Brusca I, Carroccio A, Tonutti E, et al:
The old and new tests for celiac disease: which is the best test
combination to diagnose celiac disease in pediatric patients? Clin
Chem Lab Med. 50:111–117. 2011.PubMed/NCBI
|
|
38
|
Högberg L and Stenhammar L: Celiac
disease: Pediatric celiac disease - is a diagnostic biopsy
necessary? Nat Rev Gastroenterol Hepatol. 9:127–128.
2012.PubMed/NCBI
|
|
39
|
Steele R and CRF: Diagnosis and management
of coeliac disease in children. Postgrad Med J. 87:19–25. 2011.
View Article : Google Scholar
|
|
40
|
Villanacci V, Ceppa P, Tavani E, et al:
Coeliac disease: the histology report. Dig Liver Dis. 43:S385–S395.
2011. View Article : Google Scholar
|
|
41
|
Sapone A, Bai JC, Ciacci C, et al:
Spectrum of gluten-related disorders: consensus of new nomenclature
and classification. BMC Med. 10:132012. View Article : Google Scholar
|
|
42
|
Bao F, Green PH and Bhagat G: An update on
celiac disease histopathology and the the road ahead. Arch Pathol
Lab Med. 136:735–745. 2012. View Article : Google Scholar : PubMed/NCBI
|