Introduction
Functional appliances are extensively applied in the
treatment of Class II malocclusion by forward mandibular
positioning (FMP). A large number of functional appliances were
demonstrated to achieve the correction of Class II malocclusion by
increasing the mandibular length and rotating the mandibular angle
via functional anterior displacement of the mandible (1,2). FMP
provokes cellular and molecular responses in the temporomandiular
joint and initiates novel bone formation in the condyle (a growth
site) (3). The growth of the
mandibular is a process of endochondral ossification in condylar
cartilage. During endochondral bone formation, the vascular network
in the bone is established by the invasion of capillary sprouts
into the newly forming vascular osseous tissue, through a process
termed sprouting angiogenesis (4,5). It
has been demonstrated that angiopoietin (Ang) is involved in
vascularization (6). Autocrine
Ang-1/Tie-2 modulates blood vessel plasticity and contributes to
vascular maintenance. In addition, Ang-1 enhances survival,
migration and network formation of endothelial cells in
vitro(7), and induces
neovascularization in vivo(8). Ang-2 is a naturally occurring
antagonist of Ang-1 that inhibits Ang-1-induced activation of Tie2
(9). Ang-1 and -2 are located at
sites of endochondral bone formation in the growing skeleton
(10). Moreover,
osteoblast-specific expression of Ang-1 in mice leads to an
increase in bone mass (11). Since
neovascularization is correlated with novel bone formation, it is
important to quantitatively assess and compare the pattern of
expression of Ang and the quantity of novel bone formation induced
by FMP in the condyles with their pattern of expression during
natural growth (12). Since the
involvement of Ang-1 and -2 during the process of FMP remains to be
elucidated, the aim of the present study was to clarify changes in
the Ang-1 and -2 expression patterns in condylar chondrocytes
during FMP.
Materials and methods
Experimental animals
A total of 60 Japanese white rabbits (30 male and 30
female; age, 8 weeks; weight, 1.0–1.5 kg) were purchased from
Zhejiang Experimental Animal Center (Hangzhou, China; Grade II;
Certificate SCXK 2003–0001). The experiment was in accordance with
the Guide for the Care and Use of Laboratory Animals of Zhejiang
University (Hangzhou, China) (13). All rabbits were provided with water
and a standard diet for 1 week prior to the experiment. The rabbits
were randomly assigned to the experimental or control groups (n=30
per group). Food intake and rabbit weight were monitored daily.
Five rabbits in the experimental group and five rabbits in the
control group were sacrificed at 3 days and 1, 2, 4, 8 and 12 weeks
following FMP, respectively. The study was approved by the Ethics
Committee of Sir Run Run Shaw Hospital, Medical College of Zhejiang
University. (Hangzhou, China).
Experimental FMP
The method of experimental FMP was conducted as
described previously by Wu et al(13). Briefly, functional appliances, made
from polymethylmethacrylate with 20–25° inclined planes, were
cemented to the maxillary central incisors of the experimental
group with dental adhesive resin cement [Minnesota Mining and
Manufacturing (3M) Co. Monrovia, CA, USA]. The appliances were worn
for 24 h to produce a continuous forward and downward positioning
of the mandible. The control group was free from the appliance in
order to retain natural growth.
Sample preparation
The right temporomandibular joint (TMJ) samples were
fixed in 4% paraformaldehyde for 3 days and decalcified in 0.5
mol/l ethylenediaminetetraacetic acid (EDTA, pH 7.2) for 4 weeks.
Subsequently, the TMJ samples were dissected into halves and the
connective tissue was removed to expose the areas surrounding the
mandibular condyle. Any excess tissue was removed and specimens
were embedded in paraffin. Serial sections of 3–5 μm were cut
through the TMJ at the parasagittal plane using a rotary microtome
(Leica RM 2155, Leica Mikrosysteme Handelsges m.b.H., Vienna,
Austria) and hematoxylin and eosin staining was applied. The middle
section of the former inclined plane and posterior inclined plane
of the condyle was selected.
Immunohistochemistry
Immunohistochemical analysis was performed using the
ultrasensitive streptavidin-peroxidase method with a
3′3-diaminobenzidine peroxidase (DAB-PO) kit (Maixin Biotechnology
Development Co., Ltd., Fuzhou, China). In brief, for Ang-2
staining, the specimens were treated in 1% citric acid buffer for 5
min at a high temperature (120°C) and pressure (150 kPa), then
cooled to room temperature. Subsequent to this, all specimens,
including those for Ang-1 and -2 staining,, were treated with 0.3%
hydrogen peroxide to inhibit the activity of endogenous peroxidase.
Nonspecific protein staining was blocked by 1.5% goat serum
(Sigma-Aldrich, St. Louis, MO, USA). The tissues were incubated
with rat anti-rabbit Ang-1 polyclonal antibody (Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) and rat anti-rabbit Ang-2
polyclonal antibody (Santa Cruz Biotechnology Inc.) at 4°C
overnight. The working titer of Ang-1 and -2 was 1:100. Samples
were washed with phosphate-buffered saline (PBS) and incubated at
room temperature with biotinylated secondary antibody for 10 min.
The samples were then washed extensively with PBS, incubated with
the streptavidin-peroxidase complex for 10 min and finally
developed according to the manufacturer’s instructions (Maixin
Biotechnology Development Co., Ltd.). Subsequent to washing in PBS,
fresh DAB (Maixin Biotechnology Development Co., Ltd.) was added
and the specimens were counterstained with hematoxylin. PBS was
used as negative control.
Image and statistical analysis
The immunostained sections were examined using an
Olympus microscope (Olympus Co., Tokyo, Japan; magnification, ×400)
coupled to a video camera (Olympus Co.) and connected to a
computer-aided color video image analysis system [High Resolution
Pathological Image & Word Analysis System (HPIAS-1000); Wuhan
Qianping Image Technology Co. Ltd., Wuhan, China]. When the images
had been captured and digitized onto the video screen, microscopic
images were quantitatively analyzed using an image analysis
software program (HPIAS-1000; Wuhan Qianping Image Technology Co.
Ltd.). Twenty views (1 cm2 per view) in a high power
field (magnification, ×400) were selected randomly. To avoid any
biased analysis, the researchers were blinded to the grouping
information during the sampling and data analysis. The number of
positive cells in the condylar cartilage was calculated and gray
values of the expression intensity were measured to indicate the
expression of protein. The lower the gray value, the stronger the
intensity of the immunostaining. The data were processed with SPSS
for Windows (version 16.0, SPSS Inc., Chicago, IL, USA) using the
Student’s t-test and analysis of variance. Values are presented as
the mean ± SD. P<0.05 was considered to indicate a statistically
significant difference.
Results
Gross examination of animal behavior
The experimental rabbits exhibited difficulties in
mastication and consumed less food during the first 2 days after
wearing the functional appliance; however, they recovered 2–3 days
following surgery. All animals gained weight during the
experimental period, and no significant differences in weight were
identified between the control and experimental groups.
Expression of Ang-1 and -2 proteins in
condylar chondrocytes
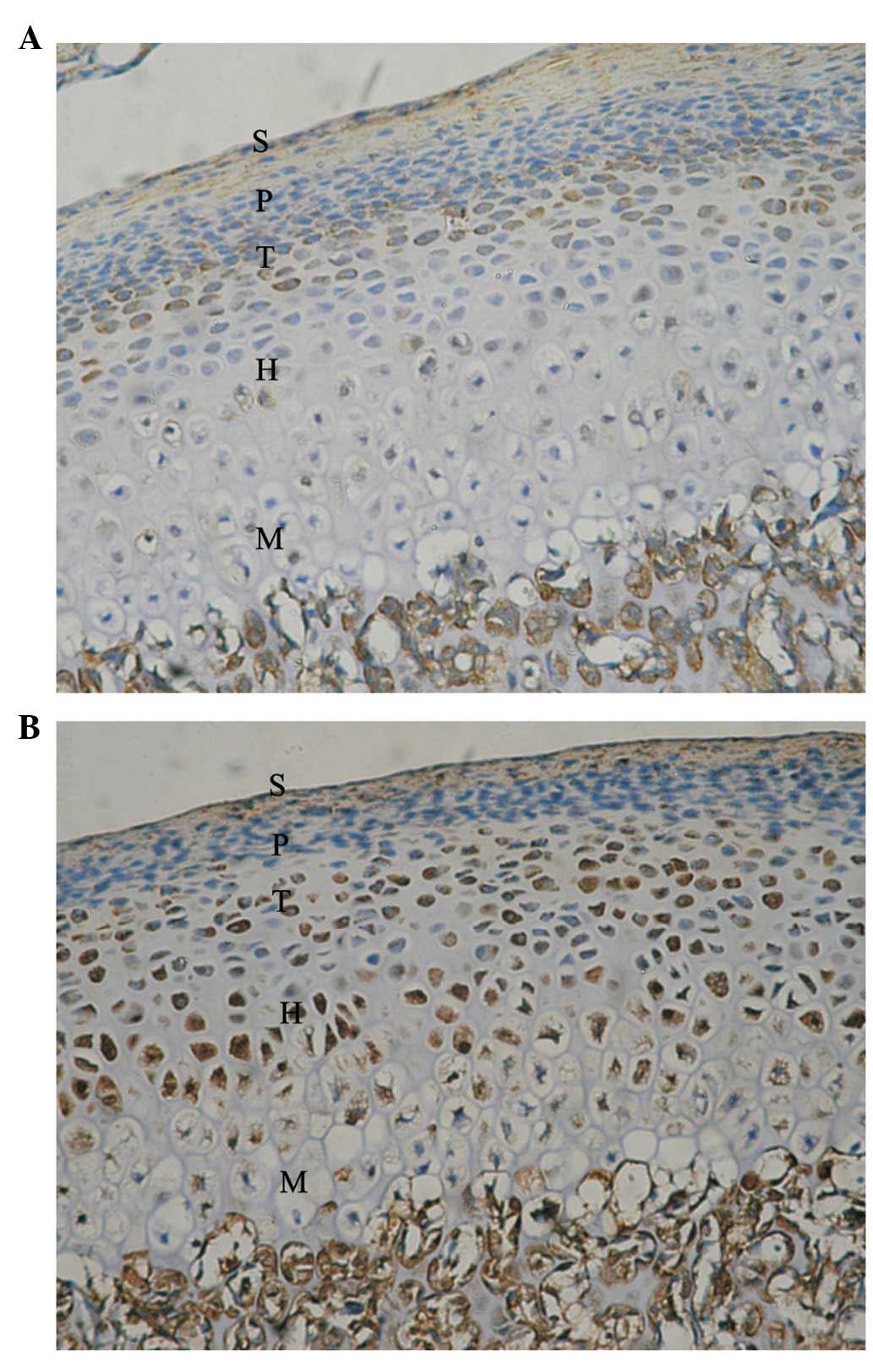
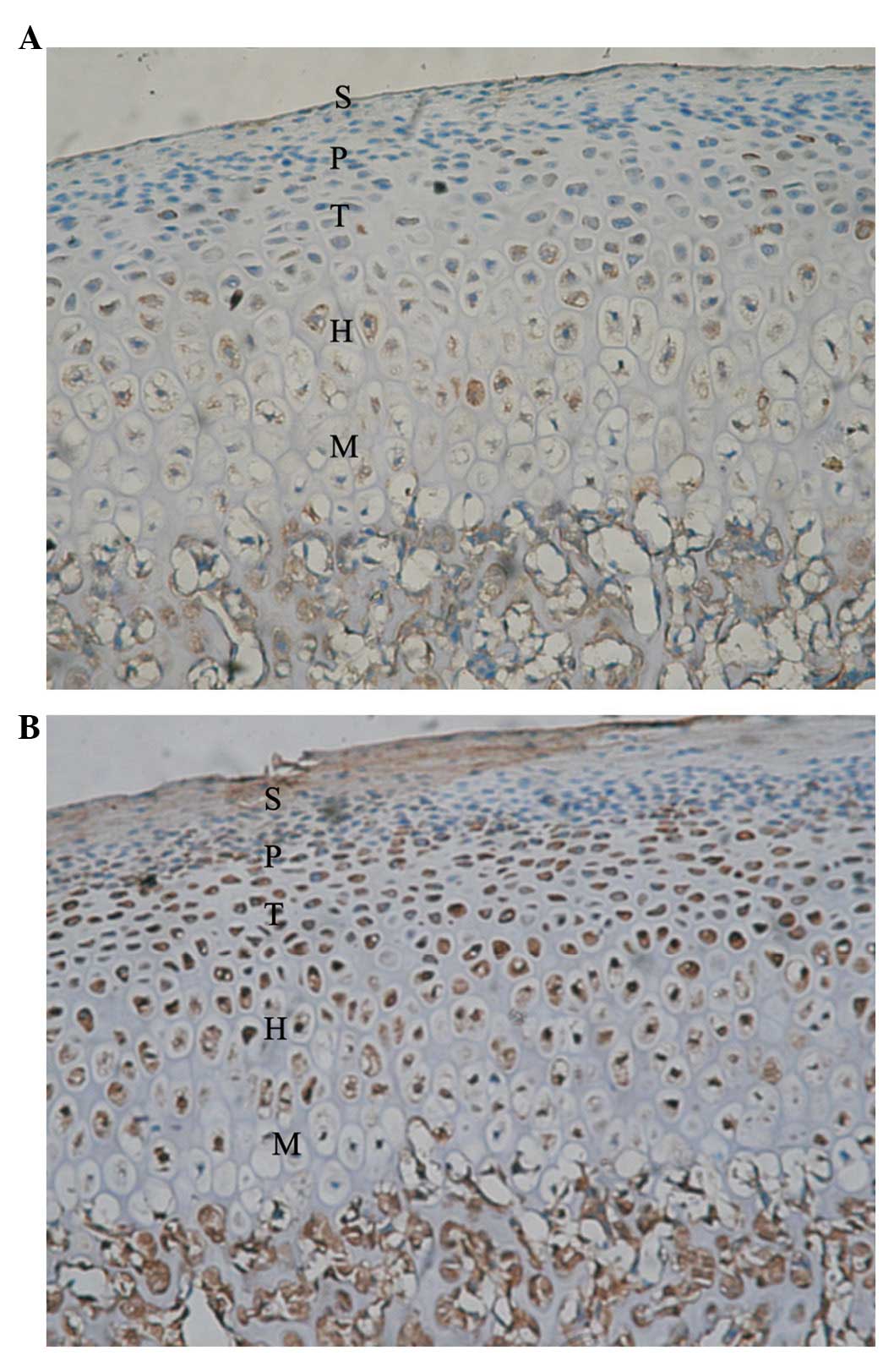
Five zones of condylar cartilage (superficial,
proliferative, transitive, hypertrophic and mineralized zones) were
observed in the control condyle and temporal components. These are
marked with S, P, T, H and M, respectively. In the control condyle,
positive signals of Ang-1 and -2 protein expression were observed
in the P, T, H and M zones; however, these were not observed in the
superficial zone.
Fig. 1 shows the
relative expression of Ang-1 in the experimental and control groups
at 12 weeks. A significant decrease of Ang-1 expression (indicated
by the significant increase of the mean grey value from 77 to 85)
was detected only between 0.5 and 1 week. Thereafter, no
significant changes were observed between each two adjacent
points.. Mandibular advancement resulted in a gradual increase in
the expression of Ang-1 when compared with normal growth. Two weeks
following FMP, the mean gray value of Ang-1 reached the lowest
level of 52.84±0.32 in the experimental group, particularly in the
H and M zones (Fig. 2).
Subsequently, the gray value of Ang-1 gradually increased. Compared
with the control group, the experimental group exhibited a
significantly increased expression of Ang-1 at all time points
(P<0.05).
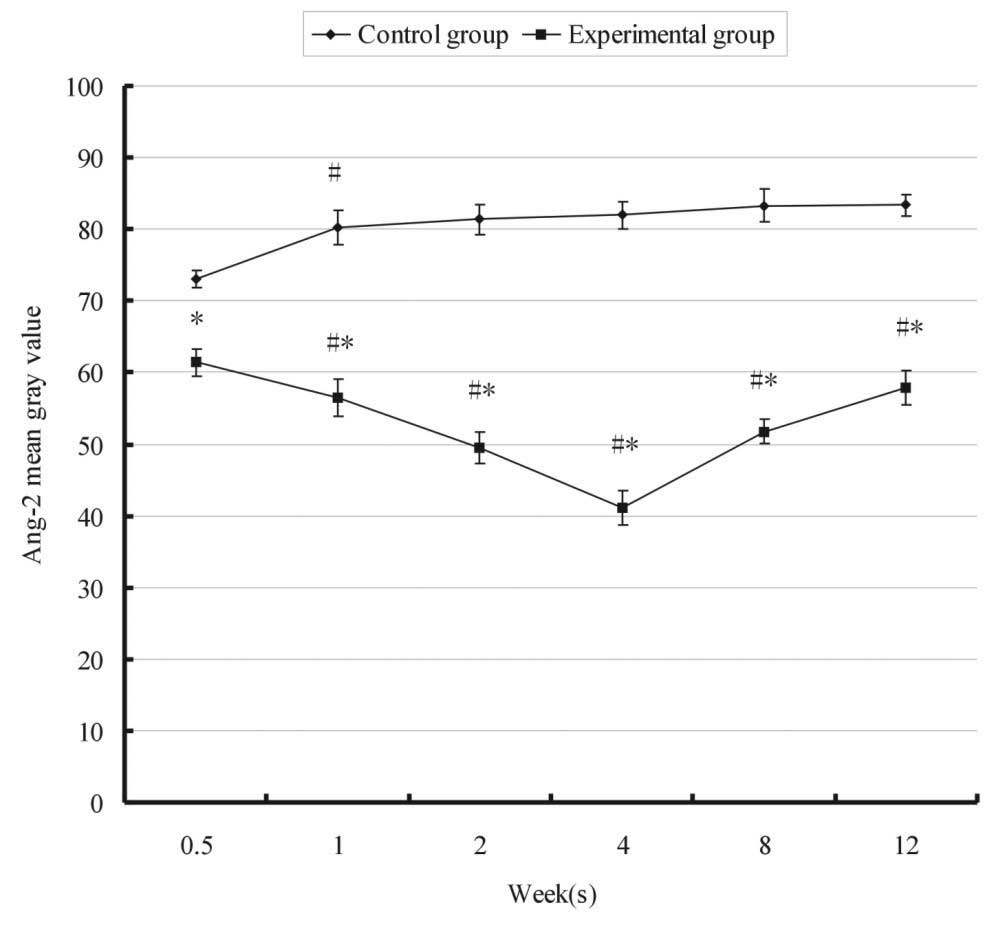
The relative expression of Ang-2 over time in the
control and experimental groups is shown in Fig. 3. In the control group, the mean
gray value of Ang-2 was significantly increased following 1 week.
However, in the experimental group, a significant decrease in the
gray value of Ang-2 was detected from 61.44±0.21 to 56.51±0.57 at 3
days and 1 week, respectively, following FMP. Ang-2 was
continuously upregulated and the gray value reached the lowest
level 4 weeks after FMP, particularly in the M zone (Fig. 4). Statistical analysis demonstrated
that the expression of Ang-2 in the experimental group at each time
point was stronger than that in the control group (P<0.05). The
expression of Ang-1 and -2 predominantly increased in the posterior
region of the condyle.
Discussion
Ang-1 and -2, members of the angiopoietin family,
were demonstrated to be associated with angiogenesis in the present
study. The expression of Ang-1 and -2 in the control group
marginally fluctuated due to the natural growth of the rabbits.
Condyles from the experimental group began to exhibit a progressive
increase in the expression of Ang-1 and -2 three days following
FMP. In a previous study, Ang-1 was constitutively expressed at low
levels in adult vasculature (14).
However, pro-angiogenic stimuli, including nitric oxide, hypoxia,
tumor necrosis factor and vascular endothelial growth factor
(VEGF), led to Ang-1 upregulation (15–17).
Ang-2 is a naturally occurring antagonist of Ang-1 and inhibits
Ang-1-induced activation of Tie2. An increase in the expression of
Ang-2 is an early marker of angiogenesis, as Ang-2 promotes early
vascular degeneration and also promotes angiogenesis (18).
The direction of blood supply in the condylar bone
plate is radial, pointing towards the mineralized layer of
articular cartilage (19). In the
present study, the expression of Ang-1 and -2 increased with the
maturation of the chondrocytes, particularly in the hypertrophic
zone, thus indicating that Ang-1 and -2 may be involved in inducing
blood vessel growth into the hypertrophic zone of the condylar
cartilage. This expression pattern of Ang-1 and -2 suggests that
the invasion of the vasculature is important in bone growth. The
expression of Ang-1 and -2 began to increase from day 3, and the
expression of Ang-1 reached a peak at 2 weeks, while the expression
of Ang-2 reached the highest level at 4 weeks. We have previously
demonstrated that the peak of bone remodeling during FMP occurred
at 8 weeks (20). The expression
of Ang-1 and Ang-2 was much earlier than the bone remodeling
process. Angiogenesis in bone occurs earlier than endochondral
ossification due to the length of the process, which includes
vascular invasion, the aggregation and differentiation of
mesenchymal cells and endochondral ossification (21). The expression of Ang-2 was stronger
and exhibited a longer duration than Ang-1, which may indicate that
Ang-2 exhibits a dominant role in the process. Ang-2 was expressed
predominantly in the mineralized zone, the site of proliferation
for vascular remodeling, and blocked the blood vessel stability
effect of Ang-1. Ang-2 has been shown to result in the significant
contraction of blood vessels without VEGF; however, with a high
concentration of VEGF, the antivascular stabilization effect of
Ang-2 promoted novel blood vessel formation (22,23).
Rabie et al(24) observed
the high expression of VEGF in the mandibular condyle following
FMP. The interaction of Ang-2 and VEGF promoted coordination for
the formation of blood vessels. A change in Ang-2 expression
resulted in vascular instability. This instability in the blood
vessels in the presence of VEGF may be involved in promoting
angiogenesis, inducing cell differentiation for osteogenesis and
promoting the ossification of condylar cartilage and subchondral
bone remodeling.
In the present study, in the experimental group, the
expression of Ang-1 and -2 predominantly increased in the posterior
region of condyle, but not in the anterior and middle regions,
which is consistent with the growth direction of novel bone
(25). Studies using imaging
examination, such as CT and MRI, have demonstrated that the
posterior region of condylar growth was markedly greater than the
growth in the middle or anterior regions following FMP, and the
metabolism in the posterior condyle increased (26,27).
Thus, the change of the stress and strain direction in the condyle
may have enhanced the expression of Ang-1 and-2 and other related
factors, enhanced active osteogenic function and changed the
condylar growth direction (28,29).
In conclusion, biomechanical stimulation by FMP induced an increase
in Ang-1 and -2 expression in condylar chondrocytes. These changes
may lead to angiogenesis, which may be involved in the remodeling
of the condyle during the treatment of Class II malocclusion.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81170979).
References
|
1
|
Bishara SE and Ziaja RR: Functional
appliances: a review. Am J Orthod Dentofacial Orthop. 95:250–258.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Bialy T, El-Shamy I and Graber TM:
Growth modification of the rabbit mandible using therapeutic
ultrasound: is it possible to enhance functional appliance results?
Angle Orthod. 73:631–639. 2003.
|
|
3
|
Ma B, Sampson W, Wilson D, Wiebkin O and
Fazzalari N: A histomorphometric study of adaptive responses of
cancellous bone in different regions in the sheep mandibular
condyle following experimental forward mandibular displacement.
Arch Oral Biol. 47:519–527. 2002. View Article : Google Scholar
|
|
4
|
Shen G and Darendeliler MA: The adaptive
remodeling of condylar cartilage - a transition from chondrogenesis
to osteogenesis. J Dent Res. 84:691–699. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sundaramurthy S and Mao JJ: Modulation of
endochondral development of the distal femoral condyle by
mechanical loading. J Orthop Res. 24:229–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ribatti D, Vacca A and Presta M: The
discovery of angiogenic factors: a historical review. Gen
Pharmacol. 35:227–231. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cascone I, Audero E, Giraudo E, Napione L,
Maniero F, Philips MR, Collard JG, Serini G and Bussolino F:
Tie-2-dependent activation of RhoA and Rac1 participates in
endothelial cell motility triggered by angiopoietin-1. Blood.
102:2482–2490. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho CH, Sung HK, Kim KT, Cheon HG, Oh GT,
Hong HJ, Yoo OJ and Koh GY: COMP-angiopoietin-1 promotes wound
healing through enhanced angiogenesis, lymphangiogenesis, and blood
flow in a diabetic mouse model. Proc Natl Acad Sci USA.
103:4946–4951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Escobar E, Rodríguez-Reyna TS, Arrieta O
and Sotelo J: Angiotensin II, cell proliferation and angiogenesis
regulator: biologic and therapeutic implications in cancer. Curr
Vasc Pharmacol. 2:385–399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horner A, Bord S, Kelsall AW, Coleman N
and Compston JE: Tie2 ligands angiopoietin-1 and angiopoietin-2 are
coexpressed with vascular endothelial cell growth factor in growing
human bone. Bone. 28:65–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suzuki T, Miyamoto T, Fujita N, Ninomiya
K, Iwasaki R, Toyama Y and Suda T: Osteoblast-specific Angiopoietin
1 overexpression increases bone mass. Biochem Biophys Res Commun.
362:1019–1025. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li QF and Rabie AB: A new approach to
control condylar growth by regulating angiogenesis. Arch Oral Biol.
52:1009–1017. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu MJ, Zhan J and Gu ZY: Time course of
expression of bcl-2 and bax in rabbit condylar chondrocytes
following forward mandibular positioning. Angle Orthod. 78:453–459.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fiedler U and Augustin HG: Angiopoietins:
a link between angiogenesis and inflammation. Trends Immunol.
27:552–558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scott BB, Zaratin PF, Gilmartin AG,
Hansbury MJ, Colombo A, Belpasso C, Winkler JD and Jackson JR:
TNF-alpha modulates angiopoietin-1 expression in rheumatoid
synovial fibroblasts via the NF-kappa B signalling pathway. Biochem
Biophys Res Commun. 328:409–414. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park YS, Kim NH and Jo I: Hypoxia and
vascular endothelial growth factor acutely up-regulate
angiopoietin-1 and Tie2 mRNA in bovine retinal pericytes. Microvasc
Res. 65:125–131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zacharek A, Chen J, Zhang C, Cui X,
Roberts C, Jiang H, Teng H and Chopp M: Nitric oxide regulates
Angiopoietin1/Tie2 expression after stroke. Neurosci Lett.
404:28–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oike Y, Yasunaga K and Suda T:
Angiopoietin-related/angiopoietin-like proteins regulate
angiogenesis. Int J Hematol. 80:21–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aoyama J, Tanaka E, Miyauchi M, Takata T,
Hanaoka K, Hattori Y, Sasaki A, Watanabe M and Tanne K:
Immunolocalization of vascular endothelial growth factor in rat
condylar cartilage during postnatal development. Histochem Cell
Biol. 122:35–40. 2004.PubMed/NCBI
|
|
20
|
Zhan J and Gu ZY: Expression of bone
histomorphometry parameters in rabbit condyle during mandibular
forward positioning. Zhonghua Kou Qiang Yi Xue Za Zhi. 48:303–307.
2013.PubMed/NCBI
|
|
21
|
De Spiegelaere W, Cornillie P, Casteleyn
C, Burvenich C and Van den Broeck W: Detection of hypoxia inducible
factors and angiogenic growth factors during foetal endochondral
and intramembranous ossification. Anat Histol Embryol. 39:376–384.
2010.PubMed/NCBI
|
|
22
|
Yee G, Yu Y, Walsh WR, Lindeman R and
Poole MD: The immunolocalisation of VEGF in the articular cartilage
of sheep mandibular condyles. J Craniomaxillofac Surg. 31:244–251.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lobov IB, Brooks PC and Lang RA:
Angiopoietin-2 displays VEGF-dependent modulation of capillary
structure and endothelial cell survival in vivo. Proc Natl Acad Sci
USA. 99:11205–11210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rabie AB, Leung FY, Chayanupatkul A and
Hägg U: The correlation between neovascularization and bone
formation in the condyle during forward mandibular positioning.
Angle Orthod. 72:431–438. 2002.
|
|
25
|
Gu Z, Feng J, Shibata T, Hu J and Zhang Z:
Type II collagen and aggrecan mRNA expression by in situ
hybridization in rabbit temporomandibular joint posterior
attachment following disc displacement. Arch Oral Biol. 48:55–62.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muto T, Kawakami J, Kanazawa M, Ishii H,
Uga S, Yokoyama K and Takeuchi M: Relationship between disc
displacement and morphologic features of skeletal Class III
malocclusion. Int J Adult Orthodon Orthognath Surg. 13:145–151.
1998.PubMed/NCBI
|
|
27
|
Ishimaru J, Handa Y, Kurita K and Goss AN:
The effect of occlusal loss on normal and pathological
temporomandibular joints: an animal study. J Craniomaxillofac Surg.
22:95–102. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang Q, Opstelten D, Samman N and Tideman
H: Experimentally induced unilateral tooth loss: histochemical
studies of the temporomandibular joint. J Dent Res. 81:209–213.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hajjar D, Santos MF and Kimura ET:
Propulsive appliance stimulates the synthesis of insulin-like
growth factors I and II in the mandibular condylar cartilage of
young rats. Arch Oral Biol. 48:635–642. 2003. View Article : Google Scholar
|