Introduction
Mushrooms are a valuable part of obtaining balanced
nutrition, and a number of them contain healthy micronutrients,
including vitamins and mineral nutrients. Fungal polysaccharides
are a type of active organic compound formed by long carbohydrate
molecules of repeating units joined together by glycosidic bonds.
These polysaccharides are located in the fruiting bodies, mycelium
and fermentation broth of large edible and medicinal fungi
(1–2). Fungal polysacchardies are often
linear, but may also be highly branched, and have been reported to
exhibit a variety of biological activities, including antitumor,
immune-stimulation and antioxidation activties (2–8). The
beneficial effects of a number of fungi, including Agaricus
subrufescens (9,10), Ganoderma lucidum (11) and Ophiocordyceps sinensis
(12), have been known since
ancient times and have been used in traditional Chinese medicine.
The lentinan derived from the shiitake mushroom is a source of
clinical drugs used in cancer treatments in certain countries, such
as Japan (13,14). Polysaccharide-K (Krestin) from
Trametes versicolor is also an approved adjuvant for cancer
therapy in Europe and Japan (15).
Boletus speciosus Frost is a type of fungi
that grows in Xiaojin, Sichuan in China at an elevation of 3,400 m.
In our previous study, a novel water-soluble polysaccharide was
purified from the fruiting bodies of Boletus speciosus Frost
using column chromatography, and its chemical structures were
elucidated by various spectroscopic methods (16). The present study aimed to
investigate the pharmacological activities and molecular mechanisms
of BSF-A, the novel polysaccharide isolated from Boletus
speciosus Frost.
Materials and methods
Isolation of the polysaccharide
The fruiting bodies of Boletus speciosus
Frost were collected in Xiaojing, Sichuan, China, and authenticated
by Professor Zhirong Yang (College of Life Sciences, Sichuan
University, Chengdu, China). The novel water-soluble
polysaccharide, BSF-A, was extracted with 95% ethyl alcohol and
purified using a diethylaminoethanol cellulose column and Sephadex
G-100 column chromatography (Sigma Aldrich, St. Louis, MO, USA).
The Sevag method (17) was used
for the deproteination of crude BSF polysaccharide, and the eluate
from the column chromatography was monitored by the phenol-sulfuric
acid method (18). The yield rate
of the purified Boletus speciosus Frost polysaccharide,
named BSF-A, was 0.215% (0.430 g), and the structural features of
BSF-A were studied using total hydrolysis, methylation analysis,
gas chromatography-mass spectrometry, scanning electron microscopy,
infrared spectroscopy, nuclear magnetic resonance spectroscopy and
dynamical analysis of the atomic force microscopy. The results
indicated that BSF-A had a backbone of (1→4)-α-L-mannopyranose
residues that branched at O-6, based on the experimental results.
The branches were mainly composed of one residue which was
→1)-α-D-galactopyranose (Fig.
1).
Microparticle preparation
Microparticles containing purified BSF-A were
prepared by the interracial polymerization method under orthogonal
design experimental conditions. Briefly, BSF-A was emulsified with
5% acacia solution (Scharr and Company, Chicago, IL, USA).
Following this step, interracial polymerization occurred by adding
toluene-2,4-diisocyanate (TDI) and ethylene alcohol (EA; Sigma
Aldrich) into the solution in a thermostat-controlled water bath.
Finally, the particles were stored at 0ºC (19). The encapsulation efficiency was
determined by a previously described method (20,21).
The microcapsules were washed three times with double distilled
water, ground by mixing with diatomite and then extracted by the
Soxhlet extraction method to obtain the encapsulated BSF-A. The
percentage of encapsulation efficiency was calculated using the
following equation: Encapsulation efficiency (%) =
(E/E0) × 100, where E is the amount of encapsulated
BSF-A and E0 is the initial amount of BSF-A.
Animal preparation
In total, 30 male Kunming mice, weighing 25.0±1.0 g,
were purchased from the Institute of Biochemistry and Molecular
Immunology of North Sichuan Medical College (NSMC; Nanchong,
Sichuan, China). S180 tumor cells were maintained in the peritoneal
cavities of the mice. A total of six male Kunming mice were housed
per plastic cage, with wood chip bedding and a 12-h light/12-h dark
cycle at room temperature (25±2ºC). The mice were allowed free
access to standard laboratory diet, which was purchased from NSMC.
The study was approved by the Ethics Committee of China West Normal
University (Nanchong, Sichuan, China).
Assay for detecting antitumor activity in
vivo
S180 tumor cells (3×106) were implanted
subcutaneously into the right hind groins of the 30 mice and they
were randomly divided into five groups. At one day
post-inoculation, the polysaccharide BSF-A was dissolved in
distilled water and administered intra-peritoneally (i.p.) to the
mice at doses of 10, 20, and 40 mg/kg. Positive and negative
controls were set for comparison. In total, 0.2 ml mannatide (10
mg/kg) was administered i.p. for the positive control, while 0.2 ml
physiological saline served as the negative control. The body
weights were measured, and the tumors, spleens and livers were
excised from the mice at sacrifice after 2 weeks. The tumor
inhibitory ratio was calculated by following the formula:
Inhibition ratio (%) = [(A − B)/A] × 100, where A and B are the
average tumor weights of the negative control and treated groups,
respectively.
Histopathological and morphological
observations
Following treatment of the mice with BSF-A as
described, a portion of the tissues were cut into small sections,
fixed in Heidenhain’s Suea Fulid (4.5 g HgCl2; 0.5 g
NaCl; 80.0 ml distilled water; 20.0 ml formalin; 4.0 ml acetic
acid; and 2.0 ml trichloroaceticacid) and stained with HE. The
sections were examined and images under a microscope (Olympus,
Tokyo, Japan) were captured.
Preparation of peritoneal macrophage
cells
Six to eight-week-old BALB/c albino mice were
injected i.p. with 1 ml 3% thioglycollate (Sigma Aldrich). Four
days after injection, the mice were euthanized and peritoneal
exudate cells were collected by lavage with 5 ml sterile cold
D-Hank’s solution. The exudate cells were collected and cultured in
60-mm dishes with RPMI-1640 (Gibco, Carlsbad, CA, USA) containing
10% heat-inactivated fetal bovine serum(FBS), penicillin (100
IU/ml) and streptomycin (100 μg/ml) (RPMI-FBS). Following 1 h of
incubation at 37ºC, the cultures were washed twice with RPMI-1640
to remove non-adherent cells, and the adherent cells were collected
by gentle scraping. The viability of the macrophages was assessed
using trypan blue exclusion.
Pharmacological evaluation for macrophage
stimulation
The macrophage cells (1×106 cells/ml)
were cultured in a 96-well plate (Corning, Inc., Corning, NY, USA)
with phenol red-free RPMI-FBS medium containing increasing
concentrations of BSF-A from 50 to 400 μg/ml (20 μl per well) as
the experimental group and cells cultured with 20 μl
lipopolysaccharide (LPS; 50 μg/ml) as the positive control group.
Eight repeated wells were used for each concentration. Next, 80 μl
phenol red-free RPMI-FBS medium was added to each well to make a
total volume of 200 μl. The cell samples were incubated in a 5%
CO2-air mixture at 37ºC for 4 h and 50 μl neutral red
solution was added to a final concentration of 0.72 mg/l. The
culture was left for 30 min. The neutral red solution was
aspirated, washed three times with PBS and then 200 μl lysis buffer
was added (glacial acetic acid:ethanol, 1:1). The optical density
(OD) was evaluated following complete cell lysis.
Nitric oxide concentration
determination
The amount of stable nitrite and 100 μL culture
supernatants were mixed with an equal volume of Griess reagent (1%
sulfanilamide, 0.1% naphthylethylenediamine dihydrochloride and
2.5% H3PO4). This mixture was incubated at
room temperature for 10 min and the absorbance at 540 nm was read
using a Thermo multiskan ascent reader (Thermo Fisher Scientific,
Waltham, MA, USA). The nitric oxide concentration was determined by
extrapolation based on a standard sodium nitrite curve (22).
Cytokine determination
The amount of TNF-α, IL-6 and IL-1β from the culture
supernatants was determined using an ELISA kit (R&D Systems,
Shanghai, China) following the manufacturer’s instructions.
qPCR detection of related gene
expression
The peritoneal macrophages were harvested following
stimulation by various concentrations of BSF-A for 4 h. Total
cellular RNA was extracted using TRIzol reagent (Invitrogen,
Carlsbad, CA, USA) and reverse transcribed into cDNA using
oligo(dT)18 primers (Invitrogen). Amplification of each
target cDNA was performed using the icycler system (Bio-rad,
Hercules, CA, USA), and the PCR products were quantified using SYBR
Green I. β-actin was used as an endogenous control to normalize
expression levels among samples, and a standard curve of each
primer set was generated using LPS-induced macrophage cDNA. The PCR
primers selected are shown in Table
I. Relative expression abundance was calculated using the
formula: Relative expression abundance = moles of detected
mRNA/moles of β-actin mRNA.
 | Table IResult of primer design. |
Table I
Result of primer design.
| Gene | Antisense
(5′-3′) | Sense (5′-3′) | Tm,ºC | Product size, bp |
|---|
| β-actin |
GCTGTCCCTGTATGCCTCT |
TTGATGTCACGCACGATTT | 55.4 | 222 |
| IL-6 |
GCCTTCTTGGGACTGATGCTGG |
CTCTGGCTTTGTCTTTCTTGTT | 51.7 | 385 |
| TNF-α |
GCCTATGTCTCAGCCTCT |
GGTTGACTTTCTCCTGGTAT | 53.4 | 423 |
| iNOS |
GAGCGAGTTGTGGATTGTC |
GGGAGGAGCTGATGGAGT | 55.2 | 376 |
| IL-1β |
GCCCATCCTCTGTGACTC |
CTGCTTGTGAGGTGCTGA | 52.0 | 434 |
Cell culture
Human laryngeal carcinoma Hep-2 and hepatoma HepG2
cell lines were grown in RPMI-1640 medium supplemented with 10%
heat-inactivated fetal bovine serum, 100 IU/ml penicillin, 100
μg/ml streptomycin and 10 mM 2-[4-(2-hydroxyethyl)-1-piperazinyl]
ethanesulfonic acid (HEPES) at pH 7.4. The cells were kept at 37ºC
in a humidified 5% CO2 incubator.
Testing the effect of BSF-A on human
laryngeal carcinoma Hep-2 and hepatoma HepG2 cell activity by the
MTT method
The two cell lines were seeded into 96-well
microculture plates at appropriate densities to maintain the cells
in an exponential phase of growth throughout the duration of the
experiment. The human laryngeal carcinoma Hep-2 and hepatoma HepG2
cells were exposed to BSF-A at 0, 25, 50, 100, 200 and 400 μg/ml
for 24 h, and each concentration was evaluated in six separate
wells. At the end of the exposure, 20 μl MTT was added to each well
and the plates were incubated for 2–4 h at 37ºC. Next, 150 μl
dimethyl sulfoxide was added to each well and the agitated for 5
min. The OD was read using a plate reader (Bio-rad) at a wavelength
of 490 nm. Media-alone wells and control wells, in which BSF-A was
absent, were included in all the experiments. The degree of
inhibition of cell proliferation was calculated using the following
formula: Growth inhibition (%) = (OD control - OD treated)/OD
control × 100.
Morphological observation of cells
The 96-well microculture plates into which the human
laryngeal carcinoma Hep-2 and hepatoma HepG2 cells had been seeded
were observed by inverted microscopy (Olympus). Images of the
changes in the conformation of the cells were captured and
recorded.
PCR design
This study used the apoptosis PCR array from CT
Bioscience (Changzhou, Jiangsu, China) to compare the expression
profiles of a selected group of genes at various disease stages.
The PCR array employs SYBR Green I-based qPCR to quantify gene
expression levels. Gene specific primers were pre-deposited into
the wells of a 96-well PCR plate in the array. Each PCR array
quantified the mRNA levels of 88 selected target genes and six
reference genes. The reference genes were used to normalize the
target gene expression levels. In addition, each PCR array had one
PCR positive control and one genomic DNA control for quality
control purposes. The PCR positive control contained artificial
templates and PCR primer pairs that specifically amplified this
template. This was designed to monitor the PCR step. The genomic
DNA control measured the residual genomic DNA potentially present
in the RNA samples.
Primer design and selection
Primers were designed to cover all transcripts of a
gene. Primer design also took the following into consideration: All
primers had a similar melting temperature (Tm); the primers did not
contain known single nucleotide polymorphisms; the primers were not
located in genomic repetitive regions; and in the majority of
cases, the primers differed from non-target sequences by three or
more bases, as revealed by a Blast search. The primers were
experimentally selected if they met the following criteria: The
amplification presented a typical amplication curve, and a post-PCR
melting curve analysis demonstrated a single peak.
Sample analysis
Hep-2 cell group total RNA (1 μg) was used for
reverse transcription using a RT kit (Cat no. CTB101, CT
Bioscience) in a 20-μl volume. Following reverse transcription, RT
products were diluted using ddH2O to 1,000 μl. The
diluted RT products were mixed with 1 ml 2× SYBR Green MasterMix
(Cat no. CTB103, CT Bioscience), and 20 μl of the mixture was
aliquoted into each well of the 96-well PCR array. The sealed PCR
plate was loaded onto an ABI 7500 (Applied Biosystems, Grand
Island, NY, USA) instrument. The cycling conditions were as
follows: 95ºC for 5 min to activate hotstart Taq, then 95ºC for 15
sec, 60ºC for 1 min and 72 ºC for 45 sec for 40 cycles. The melting
curve analysis was performed using 95ºC for 5 sec and 65ºC for 1
min.
Data analysis
This study used the classical ΔΔCt method
to perform relative quantification. ΔΔCt = (Ct of target
sample - Ct of reference sample) - (Ct of target control - Ct of
reference control). ΔΔCt was used to calculate the
expression fold change. The formula used was as follows: Expression
fold changes = target gene expression level of experiment
sample/target gene expression level of control sample =
2^(−ΔΔCt). The data are expressed as the mean ± SD. The
significance of the differences were evaluated with a one-way
ANOVA, followed by the Student’s t-test to statistically identify
differences between the control and treated groups. P<0.05 and
P<0.01 were used to indicate statistically significant
differences.
Results and discussion
Antitumor activity of BSF-A in vivo
Microencapsulation was undertaken since the water
solubility of the polysaccharide was poor. Attention was focused on
preserving the biological activity of BSF-A. Microencapsulation of
BSF-A was perfomed by interracial polymerization (23) using poly-TDI-EA (Fig. 2). This procedure avoids the shear
stress produced by sonication that would normally effect the
integrity of the drugs and consequently, their biological activity
(24).
The antitumor activity of a
polysaccharide is believed to be the result of the stimulation of
the cell-mediated immune response (25)
In order to detect the antitumor activity of BSF-A
in vivo, the mice transplanted with S180 cells were used to
evaluate its effects. The results are summarized in Table II. The weight and histological
preparations of the vital organs in each male rat in the control
group were compared with that in the treated group in order to
measure the effect of the drug. BSF-A was able to inhibit the
growth of the tumors (P<0.01) in a dose-dependent manner. The
inhibitory rate in the mice treated with 40 mg/kg BSF-A was 62.449%
and was the highest amongst the three doses administered.
Furthermore, during the course of the experiments, the appetite,
activity and coat luster of each animal in the BSF-A groups were
improved compared with the mice treated with mannatide. Compared
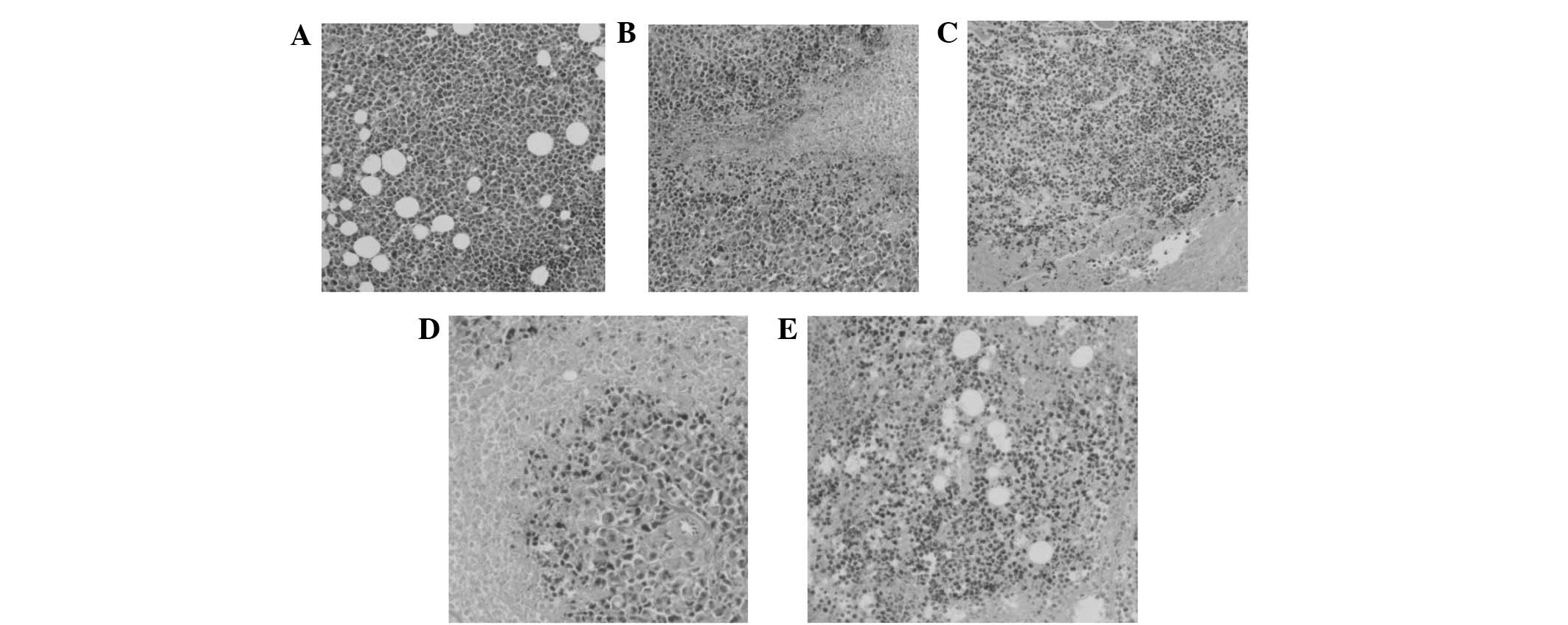
with the vigorous growth of the normal saline group (Fig. 3), massive necrotic areas were
present in the tumor tissue, which was divided into small tumor
cell groups within the BSFA-treated group. As the concentration of
the BSF-A polysaccharide increased, the tumor cell groups were
encapsulated in muscular tissue and the growth of the tumor cells
was inhibited. The results also revealed little change in the
average liver weight of the test groups, which indicated that BSF-A
did not cause serious liver damage. On the 14th day, the average
tumor weight of the negative control mice was 3.707 g, whereas the
average tumor weight of the mice in the 40-mg/kg BSF-A group was
1.392 g. The weight was also significantly reduced to 2.051 g and
1.738 g following treatment with doses of 10 mg/kg and 20 mg/kg,
respectively. It is noteworthy that the average weights of the
spleens and thymus in the test groups were significantly increased
following administration of 20 mg/kg BSF-A compared with the
weights in the mannatide or negative control mice. This indicates
that BSF-A is able to increase the weights of immune organs in
moderate doses (Table II) and
that activating the immune responses in the host may be one of the
mechanisms for the antitumor activity of BSF-A and a number of
other antitumor polysaccharides.
 | Table IIAntitumor activities of BSF-A on S180
tumor (mean ± SD, n=8). |
Table II
Antitumor activities of BSF-A on S180
tumor (mean ± SD, n=8).
| Group | Spleen index,
mg/g | Liver index,
mg/g | Thymus index,
mg/g | Average tumor
weight, g | Inhibitory rate of
tumor, % |
|---|
| N | 5.624±1.912 | 59.509±8.820 | 1.430±0.740 | 3.707±0.361 | - |
| B1 | 7.817±2.405 | 54.519±4.397 | 4.861±0.339 | 2.051±0.727 | 44.672a |
| B2 | 7.801±3.238 | 63.311±7.272 | 7.901±2.803 | 1.738±0.501 | 53.116a |
| B3 | 5.001±0.891 | 59.053±4.407 | 0.801±0.194 | 1.392±0.211 | 62.449a |
| M | 7.022±2.917 | 56.212±4.872 | 2.726±1.137 | 1.612±0.669 | 56.515a |
Immune activity of BSF-A in vitro
The antitumor activity of a polysaccharide is
believed to be the result of the stimulation of the cell-mediated
immune response (25). The present
study revealed the immune activity of BSF-A using a macrophage
stimulation assay. Polysaccharides are good stimulators of
macrophages due to the presence of various receptors on the
macrophage membrane. In the present study, BSF-A was able to
significantly promote the phagocytosis of the mouse peritoneal
macrophages within the dose range of 100–400 μg/ml compared with
the control group (P<0.01; Fig.
4) and the best stimulatory capacity of BSF-A was at a dose of
400 μg/ml with an OD value of 0.36.
The stimulatory capacity of BSF-A on macrophage
cells and the concentration of BSF-A were positively
correlated.
BSF-A enhances cytokine production in
peritoneal macrophage cells
Using the ELISA method, the level of IL-6 and TNF-α
secreted by the BSF-A-activated macrophages was compared with the
control. The levels of the two cytokines secreted by the
BSF-A-stimulated macrophages was much higher than that secreted by
the medium-treated macrophages. LPS (50 μg/ml) was used as the
positive control. The level of cytokines induced by BSF-A treatment
at varying concentrations was similar to the level induced by LPS.
It is notable that the production of IL-6 was stimulated at high
levels when the concentration of BSF-A was only 50 μg/ml (Table III).
 | Table IIIProduction of NO and cytokines in PM
stimulated by BSF-A. (mean ± SD, n=6). |
Table III
Production of NO and cytokines in PM
stimulated by BSF-A. (mean ± SD, n=6).
| Group | N | A1 | A2 | A3 | L |
|---|
| TNF-α, pg/ml | 120.4±8.8 | 158.5±5.4a | 167.4±4.1a | 174.0±2.6a | 176.3±4.7a |
| NO, μm | 2.4±0.2 | 8.5±0.1a | 10.5±0.3b | 11.4±0.6b | 9.5±0.5b |
| IL-6, pg/ml | 201.7±11.4 | 832.4±9.7b | 843.1±14.7b | 877.3±11.7b | 884.9±15.8b |
| IL-1β, pg/ml | 104.4±7.9 | 151.2±7.3b | 154.3±5.9b | 157.4±4.5b | 163.2±5.4b |
BSF-A stimulates the expression of TNF-α,
IL-6, IL-1β and inducible nitric oxide synthase (iNOS) mRNA
The qPCR results showed a significant increase in
the levels of TNF-α, IL-6, IL-1β and iNOS mRNA in the BSF-A-treated
peritoneal macrophages compared with those that were untreated. The
positive control, LPS 50 μg/ml, also promoted the expression of
these genes. The expression of all the genes studied in the
untreated macrophage was weak, but increased significantly in a
dose-dependent manner in the BSF-A-treated cells (Table IV).
 | Table IVExpression of TNF-α, iNOS, IL-6 and
IL-1β mRNA in PM stimulated by BSF-A (mean ± SD, n=6). |
Table IV
Expression of TNF-α, iNOS, IL-6 and
IL-1β mRNA in PM stimulated by BSF-A (mean ± SD, n=6).
| Group | N | A1 | A2 | A3 | L |
|---|
| TNF-α | 0.01±0.00 | 0.17±0.01a | 0.28±0.02a | 0.47±0.04a | 0.14±0.02a |
| NO | 0.12±0.01 | 0.69±0.03a | 1.36±0.21a | 1.54±0.14a | 1.27±0.26a |
| IL-1β | 0.01±0.01 | 0.13±0.02a | 0.37±0.02a | 0.43±0.01a | 0.11±0.01a |
| IL-6 | 0.01±0.00 | 0.23±0.01a | 0.59±0.02a | 1.03±0.08a | 0.12±0.01a |
The role of activated macrophages in the defense
against tumor cells has been investigated extensively over the last
few decades (26–28). Accumulated evidence indicates that
activated macrophages are able to recognize and lyse tumor cells,
including those that are resistant to cytostatic drugs. Therefore,
macrophage activation may play a role in novel immunotherapeutic
approaches to the treatment of cancer (28). Macrophages are able to kill tumor
cells either by macrophage-mediated tumor cytotoxicity or
antibody-dependent cellular cytotoxicity. These processes are
likely to result in the same manner of release as the cytotoxic
mediators, including TNF-α, NO and reactive oxygen intermediates,
or phagocytosis (29). TNF-α is
one of the most significant mediators involved in tumor cell death
by the induction of multiple intracellular pathways, including the
generation of reactive oxygen intermediates in the mitochondria
preceding plasma membrane permeabilization (30) and the induction of iNOS expression.
Ultimately, these processes may lead to cell death. BSF-A increases
the secretion of the TNF-α of macrophages and the expression of
TNF-α mRNA in vitro. The toxic effects of NO and its
derivatives on target cells are due to several mechanisms (31). The results of the present study
demonstrated that BSF-A was able to increase the release of NO and
induce the expression of the iNOS gene to several folds higher
in vitro. Taken together, these results indicate that it is
reasonable to assume that the release of TNF-α and NO, which is
activated by BSF-A, may be a significant mechanism of the antitumor
effect of BSF-A in macrophages. However, further evidence is
required. It is well known that IL-6 is also considered to be major
immune and inflammatory mediator in cancer. The present study
indicated that the macrophages were induced to enhance the
secretion and expression of the IL-6 cytokines by BSF-A. These
results indicate that these cytokines may be involved in the
antitumor effect of BSF-A.
Antitumor activity of BSF-A in vitro
The majority of laryngeal cancers, also called
cancers of the larynx or laryngeal carcinomas, are squamous cell
carcinomas. Their features reflect their origin from squamous cells
prior to forming the majority of the laryngeal epithelium.
Laryngeal cancer may spread to adjacent structures by direct
extension, to regional cervical lymph nodes by metastasis or to
further regions, such as the lung, through the blood stream
(32). As a robust cell line,
Hep-2 is able to resist temperature, nutritional and environmental
changes and maintain viability. Hep-2 has been broadly used for
tumor production studies in rats, mice, hamsters, embryonated eggs
and volunteer terminal cancer patients (33). Human hepatocellular carcinoma HepG2
cells are epithelial in morphology and are capable of secreting
various types of plasma proteins, including albumin, acute phase
proteins, fibrinogen, transferrin, plasminogen, α1-antitrypsin and
α2-macroglobulin transferrin (34). The present experiments demonstrated
variability in the results due to the differences between these two
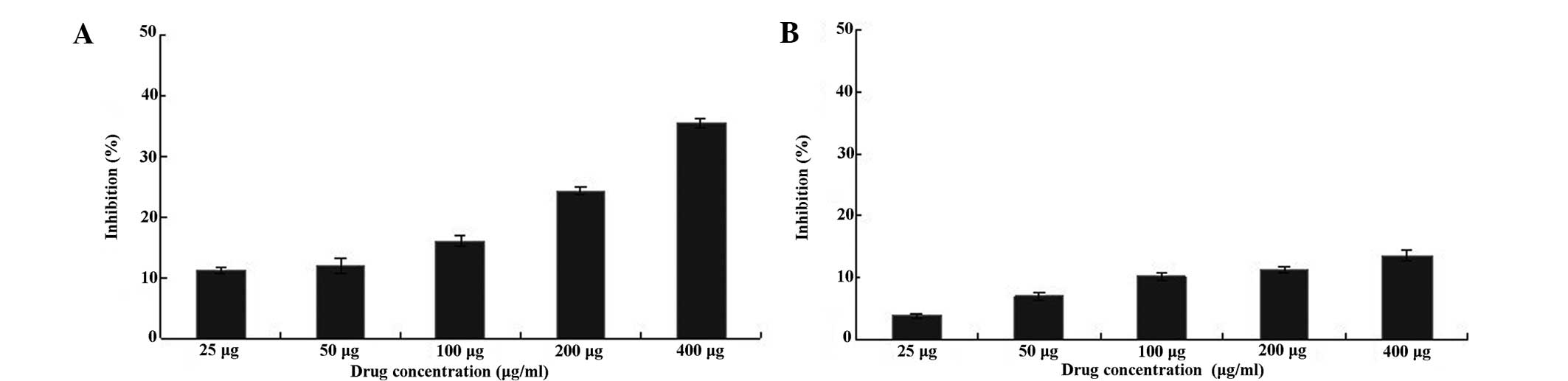
types of cancer cells. As shown in Fig. 5, the human laryngeal carcinoma
Hep-2 cells treated with 25–600 μg/ml BSF-A for 24 h exhibited
significant cell growth inhibition (P<0.01, n=6) compared with
the control (untreated) cells when assayed using MTT. For
comparison, human hepatoma HepG2 cells exhibited no significant
change (P>0.01, n=6) when compared with the control (untreated)
cells (Fig. 5). BSF-A had a time-
and dose-dependent effect on Hep-2 and HepG2 cell growth
inhibition, and a concentration of 400 μg/ml had the best rate of
growth inhibition, which were 35.9 and 13.3%, respectively.
The 96-well plates were placed under an inverted
microscope. A camera recorded the changes in cell morphology for
the varying concentration in order to measure the effect that BSF-A
exhibited on the high anticancer activity. This could be observed
from the cell morphology, which broke up and even fragmented in the
Hep-2 group, while the HepG2 cells exhibited no significant changes
compared with the control group (Fig.
6).
Apoptosis is a genetically controlled mechanism for
cell death that is also involved in the regulation of tissue
homeostasis. There are two major pathways of apoptosis that may be
observed in the cytoplasm. The extrinsic pathways include fas and
other tumor necrosis factor receptor superfamily members and
ligands, and the intrinsic pathways are mitochondria-associated.
The mitogen-activated protein kinase (MAPK) cascade is a
highly-conserved module involved in various cellular functions,
including cell proliferation, migration and differentiation. qPCR
array analysis of the gene expression profile of the Hep-2 cells
indicated that the anticancer activities of BSF-A on these cells
may involve the MAPK signaling pathway for cell apoptosis (Table V). The complete signaling pathway
for apoptosis of Hep-2 cells requires further study. The results
obtained in the present study indicate that the purified
polysaccharide of Boletus speciosus Frost may be a potential
source of natural anticancer substances.
 | Table VqPCR array analysis of the gene
expression profile of the Hep-2 cells. |
Table V
qPCR array analysis of the gene
expression profile of the Hep-2 cells.
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|
| 1 | 1.62 | 0.02 | 0.01 | 1.67 | 2.11 | 1.73 | 0.32 | 1.03 |
| ACIN1 | CASP10 | CD27 | E2F1 | IKBKB | MADD | NFKB1 | RELA |
| 2 | 0.01 | 1.23 | 2.34 | 0.23 | 0.78 | 0.74 | 0.02 | 0.76 |
| AIFM1 | CASP2 | CD40LG | E2F2 | IKBKG | MAP3K1 | NFKBIA | RIPK1 |
| 3 | 0.01 | 0.06 | 1.09 | 7.78 | 0.87 | 2.81 | 1.89 | 0.55 |
| AIFM2 | CASP3 | CD5 | EGFR | IL10 | MAP3K10 | NTRK1 | STAT1 |
| 4 | 1.88 | 0.04 | 0.00 | 2.23 | 0.07 | 0.46 | 0.88 | 0.85 |
| AKT1 | CASP4 | CD70 | E40OG | IL1A | MAP3K11 | PDCD1 | STAT5A |
| 5 | 3.25 | 0.03 | 0.01 | 0.54 | 0.13 | 5.35 | 0.04 | 1.44 |
| AKT2 | CASP5 | CDC2 | ERBB3 | IL1B | MAP3K14 | PDCD4 | STAT5B |
| 6 | 0.03 | 0.04 | 0.17 | 2.48 | 0.01 | 0.11 | 0.13 | 2.92 |
| AKT3 | CASP6 | HLA-A | FADD | IL2 | MAP3K5 | PDCD7 | TGFB1 |
| 7 | 0.57 | 0.19 | 2.67 | 0.40 | 0.16 | 0.14 | 0.02 | 0.00 |
| APAF1 | CASP7 | CIDEB | FASLG | IL4 | MAPK1 | PIK3CA | TNF |
| 8 | 0.00 | 0.67 | 3.23 | 0.37 | 1.13 | 1.78 | 0.00 | 0.35 |
| API5 | CASP8 | DAPK1 | IFNA2 | IL6R | MAPK3 | PIK3CG | TNFRSF10A |
| 9 | 0.01 | 0.09 | 0.01 | 0.46 | 0.19 | 0.23 | 1.22 | 1.12 |
| BCL2 | CASP9 | HLA-B | IFNB1 | IL7 | MAPK8 | PIK3R2 | TNFRSF10B |
| 10 | 0.02 | 0.35 | 5.11 | 0.47 | 4.13 | 0.04 | 0.01 | 0.41 |
| BIRC2 | CD226 | DAPK3 | IGF1 | IRAK1 | MAPK9 | PPP3CC | TP53 |
| 11 | 0.02 | 0.12 | 0.12 | 1.12 | 0.05 | 1.88 | 0.37 | 0.45 |
| BIRC3 | CD24 | DFFA | IGF1R | JAK2 | MYD88 | PPP3R1 | XIAP |
Acknowledgements
This project was supported by the National Natural
Science Foundation of China (31200012), the Application Foundation
Project of Sichuan Province (2013JY0094), the Science and
Technology Support Project of Sichuan Province (2014SZ0020 and
2014FZ0024), the Cultivate Major Projects of Sichuan Province
(14CZ0016) and the Doctor Startup Foundation Project of China West
Normal University (11B019 and 11B020).
References
|
1
|
Hibbett DS, Binder M, Bischoff JF,
Blackwell M, Cannon PF, Eriksson OE, et al: A higher level
phylogenetic classification of the Fungi. Mycol Res. 111:509–547.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertozzi CR and Kiessling LL: Chemical
glycobiology. Science. 291:2357–2364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wasser SP: Medicinal mushrooms as a source
of antitumor and immunomodulating polysaccharides. Appl Microbiol
Biotechnol. 60:258–274. 2002.PubMed/NCBI
|
|
4
|
Borchers AT, Stern JS, Hackman RM, Keen CL
and Gershwin ME: Mushrooms, tumors, and immunity. Proc Soc Exp Biol
Med. 221:281–293. 1999. View Article : Google Scholar
|
|
5
|
Rudd PM, Elliott T, Cresswell P, Wilson IA
and Dwek RA: Glycosylation and the immune system. Science.
291:2370–2376. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Y, Xie MY, Nie SP, Li C and Wang YX:
Purification, composition analysis and antioxidant activity of a
polysaccharide from the fruiting bodies of Ganoderma atrum.
Food Chem. 107:231–241. 2008. View Article : Google Scholar
|
|
7
|
Angeli JP, Ribeiro LR, Gonzaga ML, de
Soares SA, Ricardo MP, Tsuboy MS, Stidl R, Knasmueller S, Linhares
RE and Mantovani MS: Protective effects of beta-glucan extracted
from Agaricus brasiliensis against chemically induced DNA
damage in human lymphocytes. Cell Biol Toxicol. 22:285–291.
2006.PubMed/NCBI
|
|
8
|
Li SP, Zhao KJ, Ji ZN, Song ZH, Dong TT,
Lo CK, Cheung JK, Zhu SQ and Tsim KW: A polysaccharide isolated
from Cordyceps sinensis, a traditional Chinese medicine,
protects PC12 cells against hydrogen peroxide-induced injury. Life
Sci. 73:2503–2513. 2003.
|
|
9
|
Hetland G, Johnson E, Lyberg T,
Bernardshaw S, Tryggestad AM and Grinde B: Effects of the medicinal
mushroom Agaricus blazei Murill on immunity, infection and
cancer. Scand J Immunol. 68:363–370. 2008.
|
|
10
|
Firenzuoli F, Gori L and Lombardo G: The
medicinal mushroom Agaricus blazei Murrill: Review of literature
and pharmaco-toxicological problems. Evid Based Complement Alternat
Med. 5:3–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paterson RR: Ganoderma - a therapeutic
fungal biofactory. Phytochemistry. 67:1985–2001. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paterson RR: Cordyceps: a traditional
Chinese medicine and another fungal therapeutic biofactory?
Phytochemistry. 69:1469–1495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sullivan R, Smith JE and Rowan NJ:
Medicinal mushrooms and cancer therapy: translating a traditional
practice into Western medicine. Perspect Biol Med. 49:159–170.
2006. View Article : Google Scholar
|
|
14
|
Yang P, Liang M, Zhang Y and Shen B:
Clinical application of a combination therapy of lentinan,
multi-electrode RFA and TACE in HCC. Adv Ther. 25:787–794. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fisher M and Yang LX: Anticancer effects
and mechanisms of polysaccharide-K (PSK): implications of cancer
immunotherapy. Anticancer Res. 22:1737–1754. 2002.PubMed/NCBI
|
|
16
|
Ding X, Hou YL and Hou WR: Structure
elucidation and antioxidant activity of a novel polysaccharide
isolated from Boletus speciosus Frost. Int J Biol Macromol.
50:613–618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Staub AM: Removal of proteins - Sevag
method. Methods Carbohydr Chem. 5:5–6. 1965.
|
|
18
|
Dubois M, Gillis KA, Hamilton JK, Rebers
PA and Smith F: Colorimetric method for determination of sugars and
related substances. Anal Chem. 28:350–356. 1956. View Article : Google Scholar
|
|
19
|
Shantha LK, Lynette D and Andrew L:
Preparation and characterisation of chitosan microspheres for
antioxidant delivery. Carbohydr Poly. 64:163–167. 2006. View Article : Google Scholar
|
|
20
|
Yin Z, Jia R, Gao P, Gao R, Jiang D, Liu K
and Liu S: Preparation of contraceptive pill microcapsule and its
anti-fertility effect. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi.
21:979–982. 2004.(In Chinese).
|
|
21
|
Cho JS, Kwon A and Cho CG:
Microencapsulation of octadecane as a phase-change material by
interfacial polymerization in an emulsion system. Colloid Polym
Sci. 280:260–266. 2002. View Article : Google Scholar
|
|
22
|
Keller R, Geiges M and Keist R:
L-arginine-dependent reactive nitrogen intermediates as mediators
of tumor cell killing by activated macrophages. Cancer Res.
50:1421–1425. 1990.PubMed/NCBI
|
|
23
|
Anna S, Harald DH and Stöver X: Polymer
microcapsules by interfacial polyaddition between styrene-maleic
anhydride copolymers and amines. J Membr Sci. 209:421–432. 2002.
View Article : Google Scholar
|
|
24
|
Rasenack N, Hartenhauer H and Müller BW:
Microcrystals for dissolution rate enhancement of poorly
water-soluble drugs. Int J Pharm. 254:137–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ooi VE and Liu F: Immunomodulation and
anti-cancer activity of polysaccharide-protein complexes. Curr Med
Chem. 7:715–729. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fidler IJ and Kleinerman ES: Therapy of
cancer metastasis by systemic activation of macrophages; from bench
to the clinic. Res Immunol. 144:284–287; discussion 294–298. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Flick DA and Gifford GE: Comparison of in
vitro cell cytotoxicity assays for tumor necrosis factor. J Immunol
Methods. 68:167–175. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Klostergaard J: Macrophage tumoricidal
mechanisms. Res Immunol. 144:274–276. 1993. View Article : Google Scholar
|
|
29
|
Klimp AH, deVries EG, Scherphof GL and
Daemen T: A potential role of macrophage activation in the
treatment of cancer. Crit Rev Oncol Hematol. 44:143–161. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goossens V, Grooten J, De Vos K and Fiers
W: Direct evidence for tumor necrosis factor-induced mitochondrial
reactive oxygen intermediates and their involvement in
cytotoxicity. Proc Natl Acad Sci USA. 92:8115–8119. 1995.
View Article : Google Scholar
|
|
31
|
Kröncke KD, Fehsel K and Kolb-Bachofen V:
Inducible nitric oxide synthase and its product nitric oxide, a
small molecule with complex biological activities. Biol Chem Hoppe
Seyler. 376:327–343. 1995.PubMed/NCBI
|
|
32
|
Yu L, Li HZ, Lu SM, Liu WW, Li JF, Wang HB
and Xu W: Alteration in TWIST expression: possible role in
paclitaxel-induced apoptosis in human laryngeal carcinoma Hep-2
cell line. Croat Med J. 50:536–542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nutting CM, Robinson M and Birchall M:
Survival from laryngeal cancer in England and Wales up to 2001. Br
J Cancer. 99(Suppl 1): S38–S39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen CC and Chan WH: Inhibition of
citrinin-induced apoptotic biochemical signaling in human hepatoma
G2 cells by resveratrol. Int J Mol Sci. 10:3338–3357. 2009.
View Article : Google Scholar : PubMed/NCBI
|