Introduction
Malignant glioma is the most common type of cancer
in the central nervous system, with highly invasive characteristics
(1). Research on malignant glioma
invasion has recently gained considerable interest; however, the
exact molecular mechanisms underlying malignant glioma invasion
remain unclear, mainly since invasion involves a variety of complex
processes, as well as regulatory mechanisms (2,3).
microRNAs (miRNAs) are a type of endogenous
non-coding RNA. They can regulate gene expression through binding
to the 3′-untranslated region (3′-UTR) of target mRNAs, resulting
in either mRNA degradation or translational repression (4). Therefore, miRNAs generally act as
endogenous agents of RNA interference. Accumulating evidence has
shown that dysregulation of the expression of some miRNAs plays an
essential role in the development and progression of various
cancers (5), which suggests that
these miRNAs have oncogenic or anti-oncogenic functions. Moreover,
several miRNAs have been shown to be involved in brain physiology
and tumorigenesis, including glioma (6,7).
miR-145 has been reported to function as a tumor-suppressive RNA in
glioma, and several gene targets of miR-145 playing a role in
gliomas have been identified, including Sox9, adducin 3 (ADD3),
ADAM17, and NEDD9 (8–11). However, the mechanism by which
miR-145 regulates glioma has still not been fully uncovered.
The reorganization of the actin cytoskeleton leads
to cellular morphological changes, as well as alterations in
cellular functions, including proliferation, adhesion and migration
(12,13). Rho family proteins interact with
the actin cytoskeleton, and are hence involved in the regulation of
the cytoskeleton reorganization (14). As a downstream effector of Rho, the
Rho-associated protein kinase (ROCK1) is a serine-threonine protein
kinase. It was shown that when ROCK1 binds to the active GTP-bound
form of Rho, it is activated and then interacts with the actin
cytoskeleton to promote the formation of stress fibers, as well as
focal adhesion. Moreover, ROCK1 has been reported to provide a
feedback mechanism and regulate the activity of upstream proteins
Rac1 and RhoA, which act as key regulators in the reorganization of
the actin cytoskeleton (15).
Since the reorganization of the actin cytoskeleton plays a role in
cancer cell migration and invasion, ROCK is believed to be involved
in the process of cancer invasion and metastasis. In fact, ROCK1
has been reported to associate with several types of malignant
tumors, including osteosarcoma, prostate carcinoma, and bladder
cancer (16–18). However, its exact role in glioma
remains unknown.
The present study identified, for the first time to
the best of our knowledge, the ROCK1 gene as a direct target of
miR-145, and showed that this microRNA can inhibit U87 glioma cell
invasion, at least partially via downregulating the RhoA/ROCK1
pathway.
Materials and methods
Reagents and materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), TRIzol, Lipofectamine 2000, TaqMan microRNA
assays, Platinum® RTS SYBR® Green qPCR
SuperMix-UDG and miR-145 mimics were purchased from Life
Technologies (Carlsbad, CA, USA). The QuikChange Site-Directed
Mutagenesis kit was purchased from Stratagene (La Jolla, CA, USA).
The PGL3 Luciferase Reporter vector and the Dual Luciferase Assay
kit were purchased from Promega (Madison, WI, USA). Mouse
monoclonal antibodies anti-ROCK1 and anti-GAPDH, and rabbit
anti-mouse secondary antibody were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
PcDNA3.1+-ROCK1 plasmid was constructed by Sup Biology
(Changsha, China). A 24-well Transwell chamber, pre-coated with
dried extracellular matrix was obtained from Corning Inc. (Corning,
NY, USA). ELISA kits for matrix metalloproteinase (MMP) 2 and 9
were purchased from R&D Systems, Inc. (Minneapolis, MN,
USA).
Collection of tissue specimens
Written informed consent was obtained from all
patients in this study, which was approved by the Ethical Committee
of the First Xiangya Hospital of Central South University
(Changsha, China). Twenty glioma tissues and their matched adjacent
tissues, as well as eight normal brain tissues were obtained from
patients at the Department of Neurosurgery, First Xiangya Hospital
of Central South University, from March to June 2012. Before
surgery, no patient had undergone hormone, radio-, or chemotherapy.
All samples were immediately snap-frozen in liquid nitrogen after
surgical removal, and stored at −80°C until use.
Cell culture
Human glioma cell lines U251 and U87 were purchased
from the Cell Bank of the Chinese Academy of Science and cultured
in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin at
37°C with 5% CO2.
Transfection
Lipofectamine 2000 was used in the transfection
experiments in accordance to the manufacturer’s instructions.
Briefly, 105 cells were harvested, resuspended, seeded
in a 6-well plate and cultured at 37°C, 5% CO2 for 24 h.
The U87 cells were transfected with 200 nM miR-145 mimic or
negative control (NC) miRNA mimic, or 4 μg of the
pcDNA3.1+-ROCK1 plasmid.
RNA extraction and quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
The TRIzol agent was used to extract total RNA from
tissues and cells. To detect mature miR-145 expression, the TaqMan
MicroRNA Assay kit was used in accordance with the manufacturer’s
instructions. All reactions were run in a 7500 Fast Real-Time PCR
system (Life Technologies, Waltham, MA, USA). Independent
experiments were repeated three times for each sample and the
relative expression levels of genes were assessed by using a
comparative Ct method. For mRNA quantification through qRT-PCR
analysis, we followed the manufacturer’s protocol and used
Platinum® RTS SYBR® Green qPCR SuperMix-UDG
and the following specific primers: ROCK1,
5′-GGTGGTCGGTTGGGGTATTTT-3′ (forward) and 5′-CGCCCTAACCTCACTTCCC-3′
(reverse); glyceraldehyde phosphate dehydrogenase (GAPDH),
5′-ACAACTTTGGTATCGTGGAAGG-3′ (forward) and
5′-GCCATCACGCCACAGTTTC-3′ (reverse).
Luciferase activity assay
To determine whether ROCK1 is a direct target of
miR-145, the pGL3 Luciferase Reporter vector was used. The putative
target sequences on the 3′-UTR of ROCK1 were analyzed by TargetScan
(19). The primer sequences for
the wild-type 3′-UTR of ROCK1 were: 5′-CGCG
GCCGCTAGTCTGTGGAATCGTGTGGGAT-3′ (forward) and
5′-CTAGATCCCACACGATTCCACAGACTAGCG GCCGCGAGCT-3′ (reverse). The
QuikChange Site-Directed Mutagenesis kit was used based on the
manufacturer’s protocols to construct the mutant 3′-UTR of ROCK1,
bearing a three-nucleotide substitution (UGG to CAA) within the
miR-145 target sequence (Fig.
2A).
For the luciferase activity assay, we co-transfected
U87 and U251 cells with miR-145 mimic and the pGL3 Luciferase
Reporter vector bearing either the wild-type or the mutant type
3′-UTR of ROCK1. Luciferase activities were determined by the Dual
Luciferase assay 24 h after transfection according to the
manufacturer’s instructions. All experiments were performed in
triplicate.
Invasion assay
Cells were harvested and resuspended in DMEM without
FBS. For the invasion assay, 2×105 cells were added into
the upper Transwell chamber, where DMEM without FBS was added. DMEM
containing 10% FBS was added to the lower chamber. After incubation
for 6 h, cells were fixed with 3.7% formaldehyde and stained with
crystal violet staining solution. The cells that had not passed
through the 8.0-μm pore polycarbonate membrane between the chambers
were removed. Five fields of the lower surface of the membrane were
randomly selected to determine the number of cells that passed
through the membrane.
Western blotting
Glioma tissues or U87 cells were solubilized in cold
radioimmunoprecipitation assay lysis buffer. Then, proteins (15 μg
per lane) were separated with 10% SDS-PAGE, and transferred to a
polyvinylidene difluoride membrane. Membranes were then blocked in
5% non-fat dried milk in phosphate-buffered saline solution with
Tween 20 (PBST) for 4 h and then incubated for 3 h with mouse
anti-ROCK1 monoclonal antibody (1:400), or mouse anti-GAPDH
monoclonal antibody (1:200). After incubation with rabbit
anti-mouse secondary antibody (1:40,000) for 40 min, enhanced
chemiluminescence reagent was used to detect the signals on the
membranes. We then used the Image-Pro® Plus 6.0 software
(Media Cybernetics, Rockville, MD, USA) to perform gray scale
scanning, and calculate the relative values of protein
expression.
Statistical analysis
Statistical analyses were performed with the SPSS
17.0 software (IBM, Armonk, NY, USA). Quantified data are expressed
as mean of at least triplicate samples ± standard deviation.
One-way analysis of variance or Student’s t-tests were applied to
assess the statistical significance of observed differences.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-145 expression is significantly
reduced in glioma tissues and U87 and U251 cells
To investigate the biological significance of
miR-145, we first applied qRT-PCR to determine its expression in 20
glioma and their matched adjacent tissue samples, and found that
the miR-145 level is significantly reduced in glioma tissues,
compared to the matched adjacent tissues (Fig. 1A). Next, the expression level of
miR-145 was examined in the glioma cell lines U87 and U251, as well
as in normal brain tissues. As demonstrated in Fig. 1B, the expression of miR-145 was
markedly reduced in U87 and U251 cells compared to normal brain
tissues. These findings suggest that miR-145 might act as a tumor
suppressor in malignant glioma.
miR-145 directly targets ROCK1 in U87 and
U251 cells
To further investigate the regulatory role of
miR-145 in glioma, we applied bioinformatic analysis to predict its
targets, and the TargetScan software analysis identified the gene
ROCK1 as a putative target of miR-145 (Fig. 2A). The ROCK1 protein has been
suggested to play a role in cancer cell migration, and the
combination of ROCK1 inhibition and an anti-neoplastic agent has
been recommended for glioma treatment (20,21).
Thus, we hypothesized that miR-145 may be involved in the
regulation of glioma cell invasion by directly targeting ROCK1. To
verify this hypothesis, we transfected U87 and U251 cells with
miR-145 mimic, and determined the expression level of ROCK1 by
western blotting. As shown in Fig.
2B, miR-145 significantly reduced the ROCK1 protein level
compared to the control group (non-transfected cells), while
transfection with the NC miRNA had no effect. To further
investigate whether a direct interaction exists between miR-145 and
ROCK1, the luciferase activity assay was performed. As demonstrated
in Fig. 2C, co-transfection of U87
and U251 cells with miR-145 along with the wild-type 3′-UTR of
ROCK1 caused a notable decrease in luciferase activity; while this
activity was not affected by co-transfection with miR-145 and the
mutant 3′-UTR of ROCK1. Based on these findings, we conclude that
ROCK1 is a direct target of miR-145, and miR-145 can inhibit ROCK1
protein expression.
The expression of ROCK1 is markedly
increased in glioma tissue and in U87 and U251 cells
We further performed qRT-PCR and western blotting
assays to determine the mRNA and protein levels of ROCK1 in glioma
tissues, their matched adjacent tissues, as well as in U87 and U251
cells. As demonstrated in Fig. 3A,
the ROCK1 mRNA level in glioma tissues was higher than that
observed in their matched adjacent tissues. In addition, ROCK1 mRNA
expression was upregulated in U87 and U251 cells compared to normal
brain tissues (Fig. 3B). The
qRT-PCR results were confirmed by western blotting analysis, which
demonstrated that ROCK1 is also upregulated at the protein level in
glioma compared to matched adjacent tissues, as well as in U87 and
U251 cells compared to normal brain tissues (Fig. 3C and D).
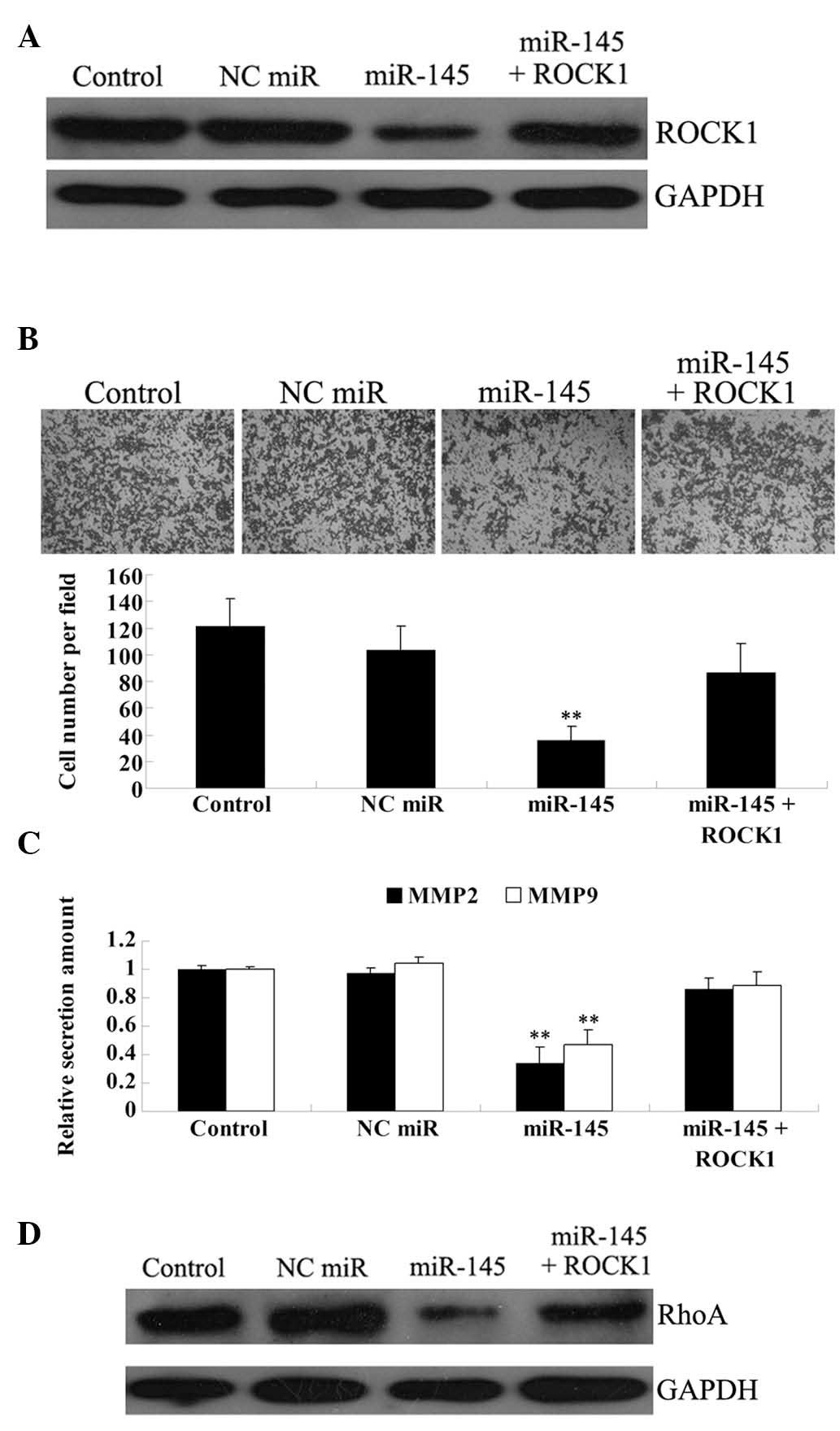
Overexpression of ROCK1 attenuates the
suppressive effect of miR-145 on U87 cell invasion
To verify our hypothesis that miR-145 inhibits the
invasive ability of U87 cells in a ROCK1-dependent manner, we
transfected U87 cells with miR-145 mimic and the
pcDNA3.1+-ROCK1 plasmid. The effect of transfection was
confirmed by western blotting (Fig.
4A). The invasion assay showed that miR-145 significantly
reduces the invasive ability of U87 cells, while ROCK1
overexpression partially, but not significantly, rescues this
phenotype (Fig. 4B).
It has been well established that MMP2 and MMP9,
secreted by cancer cells, play crucial roles in cancer invasion and
metastasis (22). Thus, we used an
ELISA assay to examine the protein levels of the two proteins in
the culture medium. As shown in Fig.
4C, the protein levels of MMP2 and MMP9 were markedly and
significantly reduced in U87 cells transfected with miR-145 mimic,
as compared with the control group. ROCK1 overexpression also
attenuated the inhibitory effect of miR-145 on MMP2 and MMP9
secretion, albeit again not in a significant manner.
To further investigate the molecular mechanism
underlying the miR-145/ROCK1-mediated reduction in U87 cell
invasion, we focused on the Rho/ROCK pathway, which has been found
to associate with cancer metastasis by regulating the actin
cytoskeleton reorganization. As shown in Fig. 4D, enhanced expression of miR-145
notably reduced the protein expression of RhoA in U87 cells, while
ROCK1 overexpression attenuated this effect.
Discussion
Accumulating evidence has recently revealed the
crucial suppressive role of miR-145 in various types of cancer,
including bladder, urothelial, breast, prostate and colon carcinoma
(23–25). In this study, we found that in
glioma tissues classified as high grade based on World Health
Organization (WHO) standards, as well as in the glioblastoma cell
lines U87 and U251, the expression level of miR-145 is
significantly reduced compared to normal brain tissues. Our
findings are consistent with other studies (8,26).
For instance, Lee et al showed that miR-145 is downregulated
in gliomas, and its low expression in glioblastomas predicts poor
prognosis (6). Based on these and
our findings, we suggest that miR-145 may play an essential role in
glioma progression.
Recently, Rani and colleagues reported that miR-145
inhibits the proliferation, adhesion and invasion of glioblastoma
cells by targeting the oncogenic proteins Sox9 and ADD3 (8). Lee et al showed that miR-145
regulates glioma cell migration by targeting CTGF, which further
reduces the expression of SPARC, an important extracellular protein
involved in cancer cell migration (26). In addition, NEDD9 and ADAM17 were
also recently identified as targets of miR-145, and might become
important biomarkers of glioma progression (11). Despite these findings, the detailed
role of miR-145 in glioma progression still needs to be fully
investigated.
Since miR-145 has been shown to act as a suppressor
in glioma, mainly via inhibiting the protein expression of its
targets, we hypothesized that a few of its targets that participate
in miR-145-dependent regulation of biological processes in glioma
cells might not have been identified yet. As ROCK1 has been
suggested to associate with several types of cancer through its
regulatory role in cytoskeleton reorganization (27), we investigated whether ROCK1 is a
miR-145 target in U87 cells, as suggested the bioinformatic
prediction based on the 3′-UTR sequence of this gene. Western blot
analysis, as well as the luciferase reporter assay data, confirmed
that ROCK1 is a novel, direct target of miR-145. We further showed
that ROCK1 is upregulated in malignant glioma tissues and in the
U87 and U251 cell lines, while transfection with miR-145 led to a
decrease in its protein level in U87 cells. These findings indicate
that ROCK1, directly regulated by miR-145, may be associated with
glioma progression.
As the Rho/ROCK pathway has been found to be
involved in the progression and metastasis of several types of
cancer (28,29), we further investigated its
implication and showed that miR-145 has a suppressive effect on U87
cell invasion, at least partially by downregulating the RhoA/ROCK1
pathway. Notably, we found that miR-145 not only inhibits ROCK1
gene and protein expression, but also downregulates the expression
of the upstream small GTPase RhoA at the protein level, indicating
that ROCK1 may have diverse effects on the functional balance of
small GTPases. Tang et al recently also proposed a feedback
regulation mechanism for ROCK1 and RhoA (30). Moreover, the specific ROCK
inhibitor Y-27632 was shown to inhibit tumor growth and metastasis
(31), which renders this
inhibitor a promising agent for the targeted therapy of glioma.
In conclusion, the present study provided evidence,
for the first time to the best of our knowledge, that miR-145 can
inhibit invasion of glioma cells, at least partially through its
inhibitory effect on the Rho/ROCK1 pathway. More importantly, since
some compounds have been reported to act as ROCK1 inhibitors, ROCK1
may constitute the new molecular target for the treatment of
glioma.
References
|
1
|
Wang Y and Jiang T: Understanding high
grade glioma: molecular mechanism, therapy and comprehensive
management. Cancer Lett. 331:139–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakada M, Kita D, Teng L, et al: Receptor
tyrosine kinases: principles and functions in glioma invasion. Adv
Exp Med Biol. 986:143–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kwiatkowska A and Symons M: Signaling
determinants of glioma cell invasion. Adv Exp Med Biol.
986:121–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagadia R, Pandit P, Coman WB,
Cooper-White J and Punyadeera C: miRNAs in head and neck cancer
revisited. Cell Oncol (Dordr). 36:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baer C, Claus R and Plass C: Genome-wide
epigenetic regulation of miRNAs in cancer. Cancer Res. 73:473–477.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Q, Li P, Li A, et al: Plasma specific
miRNAs as predictive biomarkers for diagnosis and prognosis of
glioma. J Exp Clin Cancer Res. 31:972012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asadi-Moghaddam K, Chiocca EA and Lawler
SE: Potential role of miRNAs and their inhibitors in glioma
treatment. Expert Rev Anticancer Ther. 10:1753–1762. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rani SB, Rathod SS, Karthik S, Kaur N,
Muzumdar D and Shiras AS: MiR-145 functions as a tumor-suppressive
RNA by targeting Sox9 and adducin 3 in human glioma cells. Neuro
Oncol. 15:1302–1316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu Y, Chopp M, Zheng X, Katakowski M,
Buller B and Jiang F: MiR-145 reduces ADAM17 expression and
inhibits in vitro migration and invasion of glioma cells.
Oncol Rep. 29:67–72. 2013.PubMed/NCBI
|
|
10
|
Lee SJ, Kim SJ, Seo HH, et al:
Over-expression of miR-145 enhances the effectiveness of HSVtk gene
therapy for malignant glioma. Cancer Lett. 320:72–80. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Speranza MC, Frattini V, Pisati F, et al:
NEDD9, a novel target of miR-145, increases the invasiveness of
glioblastoma. Oncotarget. 3:723–734. 2012.PubMed/NCBI
|
|
12
|
Chen RH, Wang WJ and Kuo JC: The tumor
suppressor DAP-kinase links cell adhesion and cytoskeleton
reorganization to cell death regulation. J Biomed Sci. 13:193–199.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Becart S and Altman A: SWAP-70-like
adapter of T cells: a novel Lck-regulated guanine nucleotide
exchange factor coordinating actin cytoskeleton reorganization and
Ca2+ signaling in T cells. Immunol Rev. 232:319–333.
2009. View Article : Google Scholar
|
|
14
|
Hall A: Rho family GTPases. Biochem Soc
Trans. 40:1378–1382. 2012. View Article : Google Scholar
|
|
15
|
Schofield AV and Bernard O: Rho-associated
coiled-coil kinase (ROCK) signaling and disease. Crit Rev Biochem
Mol Biol. 48:301–316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou X, Wei M and Wang W: MicroRNA-340
suppresses osteosarcoma tumor growth and metastasis by directly
targeting ROCK1. Biochem Biophys Res Commun. 437:653–658. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bu Q, Tang HM, Tan J, Hu X and Wang DW:
Expression of RhoC and ROCK-1 and their effects on MAPK and Akt
proteins in prostate carcinoma. Zhonghua Zhong Liu Za Zhi.
33:202–206. 2011.(In Chinese).
|
|
18
|
Majid S, Dar AA, Saini S, et al:
MicroRNA-1280 inhibits invasion and metastasis by targeting ROCK1
in bladder cancer. PLoS One. 7:e467432012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oellers P, Schroer U, Senner V, Paulus W
and Thanos S: ROCKs are expressed in brain tumors and are required
for glioma-cell migration on myelinated axons. Glia. 57:499–509.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inaba N, Ishizawa S, Kimura M, et al:
Effect of inhibition of the ROCK isoform on RT2 malignant glioma
cells. Anticancer Res. 30:3509–3514. 2010.PubMed/NCBI
|
|
22
|
Hadler-Olsen E, Winberg JO and
Uhlin-Hansen L: Matrix metalloproteinases in cancer: their value as
diagnostic and prognostic markers and therapeutic targets. Tumour
Biol. 34:2041–2051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Villadsen SB, Bramsen JB, Ostenfeld MS, et
al: The miR-143/-145 cluster regulates plasminogen activator
inhibitor-1 in bladder cancer. Br J Cancer. 106:366–374. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chiyomaru T, Tatarano S, Kawakami K, et
al: SWAP70, actin-binding protein, function as an oncogene
targeting tumor-suppressive miR-145 in prostate cancer. Prostate.
71:1559–1567. 2011.PubMed/NCBI
|
|
25
|
Zhang J, Guo H, Zhang H, et al: Putative
tumor suppressor miR-145 inhibits colon cancer cell growth by
targeting oncogene Friend leukemia virus integration 1 gene.
Cancer. 117:86–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee HK, Bier A, Cazacu S, et al:
MicroRNA-145 is downregulated in glial tumors and regulates glioma
cell migration by targeting connective tissue growth factor. PLoS
One. 8:e546522013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morgan-Fisher M, Wewer UM and Yoneda A:
Regulation of ROCK activity in cancer. J Histochem Cytochem.
61:185–198. 2013. View Article : Google Scholar
|
|
28
|
Wen S, Shang Z, Zhu S, Chang C and Niu Y:
Androgen receptor enhances entosis, a non-apoptotic cell death,
through modulation of Rho/ROCK pathway in prostate cancer cells.
Prostate. 73:1306–1315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Sun L, Yang M, et al: DEK
depletion negatively regulates Rho/ROCK/MLC pathway in non-small
cell lung cancer. J Histochem Cytochem. 61:510–521. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang AT, Campbell WB and Nithipatikom K:
ROCK1 feedback regulation of the upstream small GTPase RhoA. Cell
Signal. 24:1375–1380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Imamura F, Mukai M, Ayaki M and Akedo H:
Y-27632, an inhibitor of rho-associated protein kinase, suppresses
tumor cell invasion via regulation of focal adhesion and focal
adhesion kinase. Jpn J Cancer Res. 91:811–816. 2000. View Article : Google Scholar : PubMed/NCBI
|