Introduction
Hodgkin's lymphoma (HL) accounts for ~30% of all
lymphomas, presenting with generalized lymphadenopathy,
hematological abnormalities and B symptoms (including fever, weight
loss and night sweats) (1). Due to
the development of highly active chemotherapy and radiotherapy
strategies, patients with HL have an excellent prognosis following
frontline therapy, and the 5-year progression-free survival rate
can be as high as 75–80% (2).
Myelofibrosis is a chronic myeloproliferative neoplasm
characterized by clonal proliferation of myeloid hematopoietic
cells and intramedullary fibrosis (3). Clinical manifestations of myelofibrosis
include cytopenias, profound splenomegaly, bone pain, night sweats,
weight loss, and fatigue (4).
Secondary MF (SMF) is often observed in a number of hematological
malignancies, including acute megakaryoblastic leukemia (5), chronic myeloid leukemia (6) and hairy cell leukemia (7); however, SMF is rare in lymphoid
neoplasms. The present study reported a case of reversible MF
associated with Hodgkin's lymphoma (HL), which was resolved
following remission of lymphoma. Subsequent to a thorough review of
the literature using the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed), 7 reported cases
of SMF accompanied by HL were identified (Table I); however, no HL cases with SMF have
been previously reported in China. Previously reported lymphomas
associated with MF were of various histological types, including
T-cell lymphoma (8–12), B-cell lymphoma (13) and HL (14–17).
 | Table I.Hematological and clinical features of
patients with HL with myelofibrosis. |
Table I.
Hematological and clinical features of
patients with HL with myelofibrosis.
| Case (Ref.) | Age/sex | WBC
(x109/1) | Hb (g/1) | Platelet
(x109/1) | Lymph node | Liver | Spleen | Bone
marrow/invasion | Histologic type | Treatment | Survival time |
|---|
| 1 (7) | 12/F | 2.3 | 115 | 41 | Yes | No | No | Fibrosis/Yes | LPHL | MOPP | 3 months |
| 2 (7) | 50/M | 11.1 | 141 | 630 | Yes | No | Yes | Fibrosis/No | NSHL | BOPP | 10 years |
| 3 (7) | 31/F | 1.8 | 46 | 100 | Yes | Yes | Yes | Fibrosis/Yes | MCHL | COPP | 20 months |
| 4 (7) | 20/F | 2.15 | 77 | 100 | No | No | No | Fibrosis/No | LPHL | MOPP | 9 years |
| 5 (8) | 37/M | 2.5 | 41 | 50 | Yes | Yes | Yes | Fibrosis/No | NSHL | MOPP/BCVPP | 11 years |
| 6 (9) | 31/M | 6.0 | 118 | 250 | Yes | Yes | Yes | Fibrosis/Yes | NSHL | BCVPP | 4 years |
| 7 (10) | 35/M | 0.8 | 88 | 122 | Yes | Yes | Yes | Fibrosis/Yes | LDHL | MOPP/ABVD/HDAC | 2 years |
Case report
A 30-year-old male presented to the Tianjin Medical
University General Hospital (Tianjin, China) on October 10, 2012
with fever that had persisted for 1 year, night sweats and lumbago
that had persisted for 3 months, and weight loss of 25 kg within 1
year. At the time of admission, the patient exhibited a high
temperature of 40.2°C, a mild degree of pallor and palpable surface
lymph nodes. Marked cervical, supraclavicular, axillary and
inguinal lymphadenopathy had developed, with rubbery lymph nodes
reaching 2–3 cm in size. The patient presented mild hepatomegaly
and tender pain in the section of the lumbar vertebrae; however, no
splenomegaly, adenopathy or skin eruptions were observed. A
complete blood cell count revealed a normal white blood cell count
(10.2×109 cells/l; normal, 4–10×109/l), 88%
neutrophilic granulocytes and 10% lymphocytes, normocytic
normochromic anemia (hemoglobin level, 81 g/l; normal, 120–160 g/l)
and an increased platelet count (410×109/l; normal,
100–300×109/l). No teardrop-shaped red blood cells or
erythroblasts were detected in the peripheral blood. The
prothrombin time and activated partial thromboplastin time were
found to be 12.1 sec and 37.1 sec (normal, 20–40 sec),
respectively, while the fibrinogen level was 511 mg/dl (normal,
1.8–40 mg/dl) and the D-dimer level was 497 µg/l (normal, 0–500
µg/l). Blood chemistry studies demonstrated elevated levels of
lactate dehydrogenase (253 U/l; normal, 94–250 U/l) and
β2-microglobulin (1.41 mg/l; normal, 0.1–0.3 mg/l). The
serum albumin level was 30 g/l (normal, 35–55 g/l), and the hepatic
and renal functions were normal. Direct and indirect Coombs tests
were negative, while hypergammaglobulinemia and monoclonal
gammopathy were not detected and blood sample cultures failed to
reveal any pathogens. The patient was serologically negative for
Epstein-Barr virus, hepatitis virus, cytomegalovirus, parvovirus
B19, toxoplasmosis, coccidiomycosis, brucellosis and human
immunodeficiency virus. In addition, the erythrocyte sedimentation
rate was 64 mm/h (normal, 0–15 mm/h), while immune screening,
including autoantibody and complement, was negative. Finally, the
CD4/CD8 ratio (1.01%; normal, 0.8–2.5%) was within the normal
range.
A systemic computed tomography (CT) scan
demonstrated multiple swellings of the bilateral cervical,
supraclavicular, mediastinal, axillary, para-aortic, inguinal and
mesenteric lymph nodes, an enlarged liver and no splenomegaly. A
cranium CT scan demonstrated no lesions. A single-photon emission
computed tomography bone scan revealed numerous areas of increased
tracer uptake, indicating skeleton invasion. Fluorodeoxyglucose
positron emission tomography was not performed at this time.
Biopsy of the cervical lymph node demonstrated
disappearance of the normal architecture and neoplastic cells.
Immunohistochemical staining demonstrated that the cells were
positive for CD30, as well as for CD20 and CD3 in scattered cells,
and negative for CD15. These findings were compatible with a
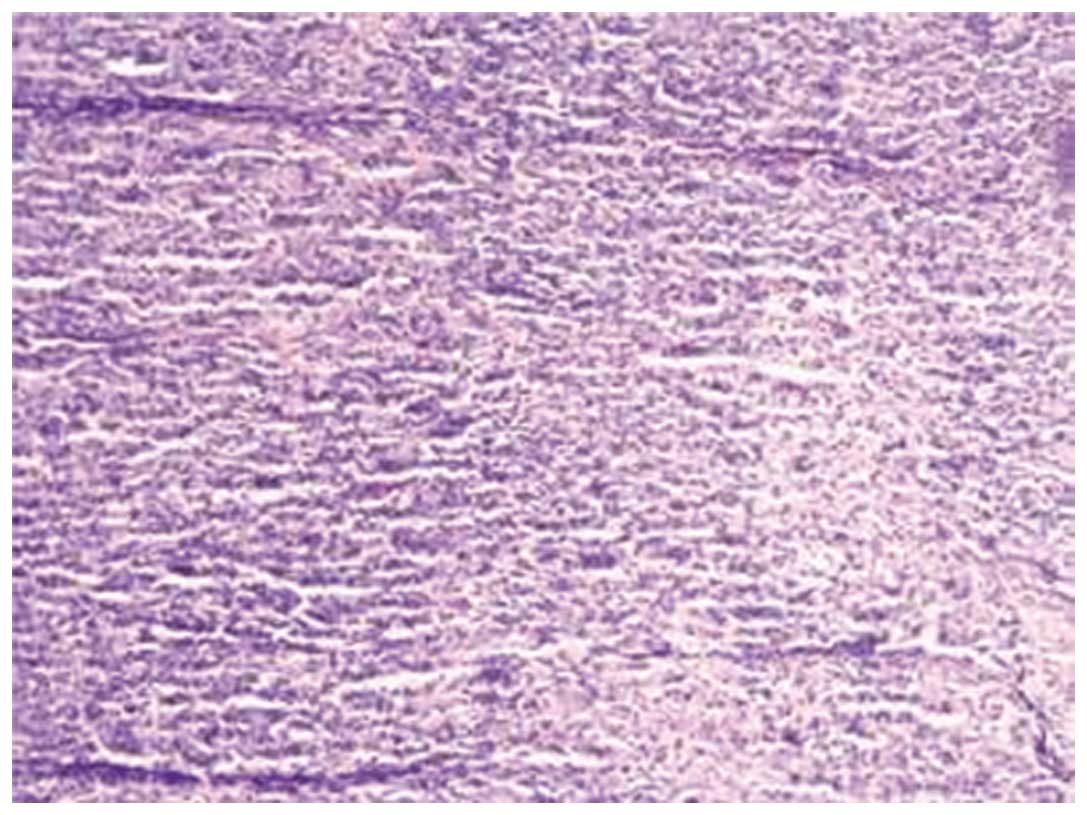
diagnosis of NSHL (Fig. 1). Bone
marrow (BM) aspirations of the sternum and iliac bone were dry
taps, and biopsy of the BM from the iliac bone demonstrated diffuse
MF, showing proliferation of fibroblasts and reticulin fiber,
without an increase in megakaryocytes and without lymphomatous
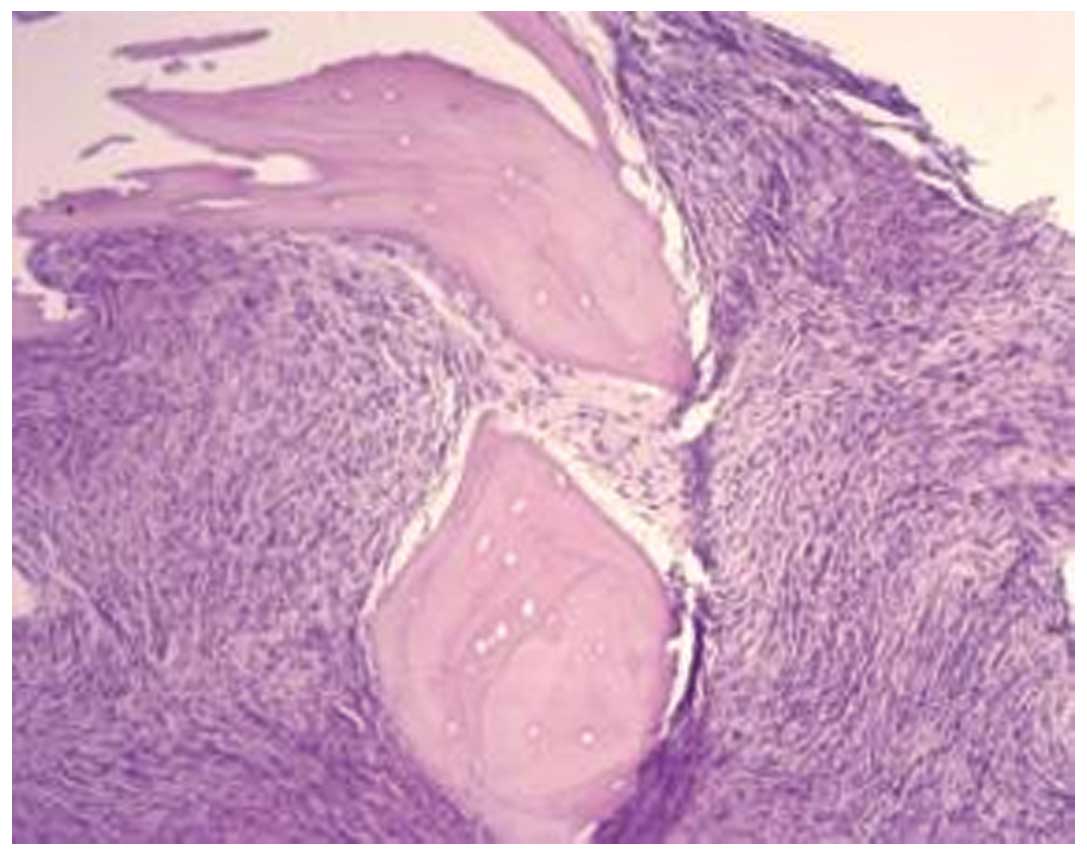
infiltration (Fig. 2).
Based on the aforementioned findings, the patient
was diagnosed with an NSHL variant with MF. The lymphoma was
classified as clinical stage IV (18). Subsequently, the patient underwent 4
courses of chemotherapy with ABVD (doxorubicin, 120 mg, days 1 and
15; bleomycin, 15 mg, days 1 and 15; vinblastine, 3 mg, days 1 and
15; and dacarbazine, 600 mg, days 1 and 15) achieving a partial
response. The patient showed resolution of B symptoms with anemia
improvement, and reduction in the size of the lymph nodes and
liver. Aspiration of BM became possible and a BM biopsy
demonstrated a hypocellular marrow without evidence of lymphoma and
a reduction in fibrosis. Flow cytometric analyses of BM lymphocytes
revealed that they were positive for CD7, CD3, CD5 and CD38, and
negative for CD20, CD22, CD23, CD10, CD9, ZAP70 and CD79a. In
addition, chromosome analysis of BM cells demonstrated a normal
karyotype (46XY). A JAK2 mutation, V617F, was detected and
immunoglobulin gene rearrangement was negative. However, small
lymph nodes (1–2 cm in diameter) remained in the neck and
supraclavicular after 4 courses of chemotherapy. Therefore, the
patient was treated with 2 courses of BEACOPP (bleomycin, 15 mg,
day 8; etoposide, 200 mg, days 1–3; doxorubicin, 80 mg, day 1;
cyclophosphamide, 1,200 mg, day 1; vincristine, 4 mg, day 8;
procarbazine, 700 mg, days 1 and 15; and prednisone, 80 mg, days
1–14) combined with local radiotherapy (2 Gy, days 1–20). Residual
lesions were not observed on CT scans, and a BM biopsy demonstrated
recovery of hematopoiesis and a disappearance of fibrosis. The
patient was followed-up until relapse occurred after 2 years, and
he was subsequently subjected to stem cell transplantation. Written
informed consent was obtained from the patient prior to publication
of this study.
Discussion
Primary or idiopathic MF is a myeloproliferative
disorder characterized by myeloid metaplasia, evident splenomegaly,
pancytopenia and a leukoerythroblastic peripheral blood smear. SMF
occurs in a variety of systemic diseases, including tuberculosis,
metastatic carcinoma, osteopetrosis and toxic marrow injury
following irradiation or chemical exposure (19,20). SMF
is also observed in a variety of hematological malignancies,
including acute megakaryoblastic leukemia, chronic myeloid leukemia
and hairy cell leukemia. However, SMF is uncommon in malignant
lymphoma and reported cases of MF associated with HL are extremely
rare.
In the present study, at the time of NSHL diagnosis,
the patient's BM aspiration was a dry tap. A BM biopsy specimen
demonstrated MF representing the proliferation of fibroblasts and
reticulin fiber. No apparent increase in megakaryocytes was
observed. Infiltration by lymphoma cells was not confirmed in the
BM biopsy specimen or in the BM aspiration smear, when it became
available. The fibrosis was reversible following successful
chemotherapy for HL.
To the best of our knowledge, only 7 previous
reports of MF associated with HL exist (Table I) (8–11). The
patient ages were variable (median, 31 years; range, 12–50 years)
and no significant difference in incidence based on gender was
identified (male, 4; female, 3). The patients, with the exception
of 1 case, exhibited pancytopenia without BM invasion by the
lymphoma cells; in addition, 5 patients experienced bicytopenia and
3 patients had BM invasion by lymphoma cells. Furthermore, 6 of the
7 patients presented lymph node swelling, 5 demonstrated
splenomegaly and 4 had hepatomegaly. The histological type of HL
was reported to be of the nodular sclerosis subtype in 3 cases,
lymphocyte-depleted in 2 cases, and of mixed cellularity or
lymphocyte-predominant in one case each. All the patients were
treated with chemotherapy, but survival times differed widely.
Patients without marrow involvement presented a relatively good
prognosis (survival, 9–11 years). However, marrow involvement was
common, and those patients had an extremely poor prognosis
(survival, 3 months to 4 years).
No specific association is known between MF and
lymphoma, despite a few reported cases of MF complicated by
concomitant or subsequent lymphoma (21). However, lymphoma is a recognized cause
of MF, although it is uncommon. Gisselbrecht et al (22) reported that there were no patients
with MF in a series of 1,883 patients with diffuse aggressive NHL.
In addition, the pathogenesis of the fibrotic change in the BM of
HL patients is unknown. Fibroblasts have been reported to be
stimulated by certain cytokines, such as transforming growth
factor-β (TGF-β), platelet-derived growth factor (PDGF) and basic
fibroblast growth factor (bFGF) (23). These cytokines are known to play an
important role in the development of stromal proliferation. PDGF
induces the proliferation of fibroblasts, while TGF-β induces the
synthesis and accumulation of extracellular matrix proteins,
including fibronectin and type I and III collagens (24). Megakaryocytes and monocytes have been
reported to be sources of these cytokines. In cases of lymphoma
with SMF, PDGF is expressed in monocytes (25). T cells are not known to secrete PDGF,
which causes MF in myeloproliferative diseases; however, T cells
secrete TGF, which can cause fibrosis. Plasma TGF-β levels were
found to be elevated in cases of MF associated with peripheral
T-cell (8), cytotoxic T-cell
(12) and splenic marginal zone
lymphomas (13). In addition, there
is evidence that the nodular sclerosis of certain cases of
Hodgkin's disease is due to the increased TGF levels (26,27).
In the present case, the patient developed MF and
lymphoma simultaneously, and these regressed completely following
chemotherapy. In conclusion, the disease status of MF was similar
with that of HL, suggesting that HL plays an important role in the
pathogenesis of MF. Certain cytokines are hypothesized to stimulate
the growth of fibroblasts and synthesis of collagen in BM
fibroblasts. Further studies and additional case reports will be
required to clarify the pathogenesis of SMF and improve our
understanding of the immunological dysregulation associated with
lymphoma.
Acknowledgements
This study was supported by a grant from the Tianjin
Cancer major special project (no. 12ZCDZSY18000).
References
|
1
|
Younes A: Novel treatment strategies for
patients with relapsed classical Hodgkin lymphoma. Hematology.
2009:507–519. 2009. View Article : Google Scholar
|
|
2
|
Eichenauer DA, Böll B and Diehl V:
Pharmacotherapy of Hodgkin lymphoma: standard approaches and future
perspectives. Expert Opin Pharmacother. 15:1139–1151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tefferi A: Pathogenesis of myelofibrosis
with myeloid metaplasia. J Clin Oncol. 23:8520–8530. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tefferi A: Primary myelofibrosis: 2014
update on diagnosis, risk-stratification, and management. Am J
Hematol. 89:915–925. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niino D, Tsuchiya T, Tomonaga M, Miyazaki
Y and Ohshima K: Clinicopathological features of acute
megakaryoblastic leukaemia: Relationship between fibrosis and
platelet-derived growth factor. Pathol Int. 63:141–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aljinovic N, Bogusz AM, Kantarci S, Buck
TP and Dewar R: An unusual case of Philadelphia chromosome-positive
chronic myelogenous leukemia with trisomy 19 presenting with
megakaryoblastosis and myelofibrosis. Arch Pathol Lab Med.
137:1147–1151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Araki H, Matsunaga T, Murase K, Kuroda H,
Kuribayashi K, Terui T and Niitsu Y: Successful induction and
complete improvement of myelofibrosis and erythema nosodum with
cladribine in a case of hairy cell leukemia. Gan To Kagaku Ryoho.
31:965–969. 2004.(In Japanese). PubMed/NCBI
|
|
8
|
Okabe S, Miyazawa K, Lguchi T, Sumi M,
Takaku T, Ito Y, Kimura Y, Serizawa H, Mukai K and Ohyashiki K:
Peripheral T-cell lymphoma together with myelofibrosis with
elevated plasma transforming growth factor-β1. Leuk Lymphoma.
46:599–602. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kikukawa M, Umahara T, Kikawada M, Kanaya
K, Sakurai H, Shin K, Mori M and Iwamoto T: Peripheral T-cell
lymphoma presenting as myelofibrosis with the expression of basic
fibroblast growth factor. Geriatr Gerontol Int. 9:395–398. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsui K, Adachi M, Tominaga T, Shinohara
S and Kamei T: Angioimmunoblastic T cell lymphoma associated with
reversible myelofibrosis. Int Med. 47:1921–1924. 2008. View Article : Google Scholar
|
|
11
|
Rao SA, Gottesman SR, Nguyen MC and
Braverman AS: T cell lymphoma associated with myelofibrosis. Leuk
Lymphoma. 44:715–718. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abe Y, Ohshima K, Shiratsuchi M, Honda K,
Nishimura J, Nawata H and Muta K: Cytotoxic T-cell lymphoma
presenting as secondary myelofibrosis with high levels of PDGF and
TGF-beta. Eur J Haematol. 66:210–212. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsunaga T, Takemoto N, Miyajima N, Okuda
T, Nagashima H, Sato T, Terui T, Sasaki H, Ohmi N, et al: Splenic
marginal zone lymphoma presenting as myelofibrosis associated with
bone marrow involvement of lymphoma cells which secrete a large
amount of TGF-beta. Ann Hematol. 83:322–325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meadows LM, Rosse WR, Moore JO, Crawford
J, Laszlo J and Kaufman RE: Hodgkin's disease presenting as
myelofibrosis. Cancer. 64:1720–1726. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carroll WL, Berberich FR and Glader BE:
Pancytopenia with myelofibrosis. An unusual presentation of
childhood Hodgkin's disease. Clin Pediatr (Phila). 25:106–108.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vukelja SJ, Krishnan J, Ward FT and
Redmond J III: Synchronous Hodgkin's disease and myelofibrosis
terminating with granulocytic sarcoma and acute megakaryocytic
leukemia. South Med J. 83:1317–1320. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Viola MV, Kovi J and Nukhopadhyay M:
Reversal of myelofibrosis in Hodgkin disease. JAMA. 223:1145–1146.
1973. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Izumi T and Ozawa K: Clinical staging
classification of non-Hodgkin's lymphoma. Nihon Rinsho. 58:598–601.
2000.(In Japanese). PubMed/NCBI
|
|
19
|
Viallard JF, Parrens M, Boiron JM, Texier
J, Mercie P and Pellegrin JL: Reversible myelofibrosis induced by
tuberculosis. Clin Infect Dis. 34:1641–1643. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Atasever T, Vural G, Yenidünya S, Ataoğlu
O, Atavci S and Unlü M: Tc-99m MIBI bone marrow uptake in bone
marrow fibrosis secondary to metastatic breast carcinoma. Clin Nucl
Med. 22:655–656. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gabali AM, Jazaerly T, Chang CC, Cleveland
R and Kass L: Simultaneous hepatosplenic T-cell lymphoma and
myelofibrosis. Avicenna J Med. 4:34–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gisselbrecht C, Gaulard P, Lepage E,
Coiffier B, Brière J, Haioun C, Cazals-Hatem D, Bosly A, Xerri L,
Tilly H, et al: Prognostic significance of T-cell phenotype in
aggressive non-Hodgkin's lymphomas. Groupe d'Etudes des Lymphomes
de l'Adulte (GELA). Blood. 92:76–82. 1998.PubMed/NCBI
|
|
23
|
Tefferi A: Myelofibrosis with myeloid
metaplasia. N Engl J Med. 342:1255–1265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Charni Chaabane S, Coomans de Brachène A,
Essaghir A, Velghe A, Lo Re S, Stockis J, Lucas S, Khachigian LM,
Huaux F and Demoulin JB: PDGF-D expression is down-regulated by
TGFβ in fibroblasts. PLoS One. 9:e1086562014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang JC, Chang TH, Goldberg A, Novetsky
AD, Lichter S and Lipton J: Quantitative analysis of growth factor
production in the mechanism of fibrosis in agnogenic myeloid
metaplasia. Exp Hematol. 34:1617–1623. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kadin ME, Agnarsson BA, Ellingsworth LR
and Newcom SR: Immunohistochemical evidence of a role for
transforming growth factor beta in the pathogenesis of nodular
sclerosing Hodgkin's disease. Am J Pathol. 136:1209–1214.
1990.PubMed/NCBI
|
|
27
|
Newcom SR and Tagra KK: High molecular
weight transforming growth factor beta is excreted in the urine in
active nodular sclerosing Hodgkin's disease. Cancer Res.
52:6768–6773. 1992.PubMed/NCBI
|