Introduction
Colorectal cancer (CRC) is a common cancer worldwide
and is the fourth leading cause of cancer-associated mortality in
China (1). There are >1 million
CRC incidences and 600,000 mortalities occur anually. Survival is
associated with the stage at which the cancer is diagnosed
(2). The TNM and Dukes' staging
systems have greatly improved the rational stratification of CRC
patients and the design of therapeutic strategies (3). Early diagnosis results in a highly
favorable prognosis, stages 1 and 2 CRC have an 80–90% 5-year
survival rate, whereas stages 3 and 4 metastatic diseases have a
5-year survival rate of 60 and 8%, respectively (4). Almost 20% of patients are diagnosed at
an advanced metastatic stage, and >50% ultimately develop
metastases (5).
NVP-BEZ235 as a novel dual phosphatidylinositol
3-kinase (PI3K)/mammalian target of rapamycin (mTOR) inhibitor
currently in phase I/II clinical trials has demonstrated
significant antitumor efficacy in diverse solid tumors, including
CRC (6). NVP-BEZ235 targets PI3K/mTOR
pathways by binding to the ATP-binding pocket and has a dual role
in inhibiting multiple class I PI3K isoforms and mTOR kinase
activity (7). The PI3K/mTOR signaling
pathway is involved in various types of cancer, including CRC, and
this pathway regulates tumorigenesis in many important aspects,
such as cell proliferation, angiogenesis, invasion and cell
motility (8–10). PI3Ks is a family of lipid kinase and
each member is composed of two heterologous subunits. Active PI3Ks
can convert phosphatidylinositol 4, 5-bisphosphate to
phosphatidylinositol 3, 4, 5-triphosphate (PIP3) by phosphorylating
at the 3-position of the inositol ring. PIP3 provides binding sites
for pleckstrin homology-containing proteins such as
3-phosphoinositide-dependent protein kinase-1 (PDK1) and AKT
serine/threonine kinase (11). AKT
activated by PDK1 is able to phosphorylate many protein targets at
the membrane such as caspase-9, tuberin, murine double minute 2,
and mTOR. mTOR is a serine/threonine kinase that regulates cell
proliferation and apoptosis by binding other proteins to form the
mTOR complex 1 (mTORC1) or mTORC2 (12). AKT activation involves the regulation
of cell proliferation, survival, and motility.
Autophagy is an intracellular catabolic process that
recycles unnecessary cell components and damaged organelles in
order to maintain cellular homeostasis and reduce diverse stresses,
commonly through lysosomes (13).
Autophagy has a dual role of tumor promoter and tumor inhibitor in
the cancer cells. It leads to genetic instability by preventing
inflammation and necrosis, otherwise it might provide energy via
recycling mechanism that is vital to tumor progression during the
unfavorable condition, such as starvation or hypoxia (14–18). Many
chemotherapeutic agents, especially drugs effecting through
PI3K/mTOR inhibition such as dual PI3K/mTOR inhibitor are observed
to induce autophagy, and mTOR is the central checkpoint that
negatively regulates autophagy (19,20).
We hypothesized that autophagy inhibition enhance
the therapeutic outcome of NVP-BEZ235 in CRC treatment based on
previous studies. Thus, we examined the anticancer effect of
PI3K/mTOR dual inhibitor NVP-BEZ235 on CRC and assessed whether
autophagy inhibitors were able to enhance NVP-BEZ235 efficacy in
the therapeutic regimen.
Materials and methods
Materials
The antibodies (p-AKT, p-S6, PARP, LC3 and P62) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
NVP-BEZ235 was purchased from LC Laboratories (Woburn, MA, USA).
3-Methyladenine (3-MA), chloroquine (CQ) and
3-(4,5-dimetrylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma-Aldrich China, Inc. (Shanghai,
China).
Cell viability assay
SW480 cells were plated in 96-well plates at a
density of 1.0×104 cells/well in 200 µl of complete
medium. After NVP-BEZ235 treatment, MTT reagent (10 µl, 5 mg/ml)
was added to the wells and incubated for 4 h. MTT treated cells
were then dissolved in 150 µl DMSO and the absorbance was recorded
at a wavelength of 490 nm using ?. Each treatment was performed in
triplicate.
Western blott analysis
SW480 cells were washed with cold phosphate-buffered
saline twice following treatment with NVP-BEZ235.
Radioimmunoprecipitation assay buffer (200 µl) was added and the
cell lysates were agitated and centrifuged for 3,000 × g for 20
min, at 4°C. Protein concentration was detected by bicinchoninic
acid protein assay. Proteins (45 µg) were loaded and run through
12% (w/v) sodium dodecyl sulphate-polyacrylamide gel
electrophoresis. Proteins were then transferred onto a
polyvinylidene difluoride (PVDF) membrane. The membrane was then
blocked with 5% (w/v) skimmed milk for 120 min at room temperature
(20°C) and primary antibodies were added and incubated overnight at
4°C. The following day, PVDF membranes were incubated with
secondary antibodies for 60 min. Tanon Gel Imaging System (Tanon
Co., Shanghai, China) was used for the semi-quantitative analysis
of proteins.
Flow cytometry
Following the treatment of SW480 cells with
NVP-BEZ235, SW480 cells were incubated at 37°C in the dark for 45
min with Annexin V-fluorescein isothiocyanate (Invitrogen-Life
Technologies, Carlsbad, CA, USA) and propidium iodide (Invitrogen
Life Technologies) to detect the apoptotic rate. The cells were
analyzed using a FACScan flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA).
Statistical analysis
Data were presented as mean ± standard deviation.
Data were representative of three independent experiments performed
in triplicate. P<0.05 was considered to indicate a statistically
significant difference.
Results
NVP-BEZ235 inhibits the growth of
SW480 cells and PI3K/AKT/mTOR signaling pathway
The NVP-BEZ235 treatment of SW480 cells reduced cell
viability in a dose- and time-dependent manner (Fig. 1A and B). NVP-BEZ235 also caused a
decrease in the expression of p-AKT and p-S6 proteins in SW480
cells (Fig. 1C and D) and this
decrease was dose-dependent.
NVP-BEZ235 induces apoptosis in SW480
cells
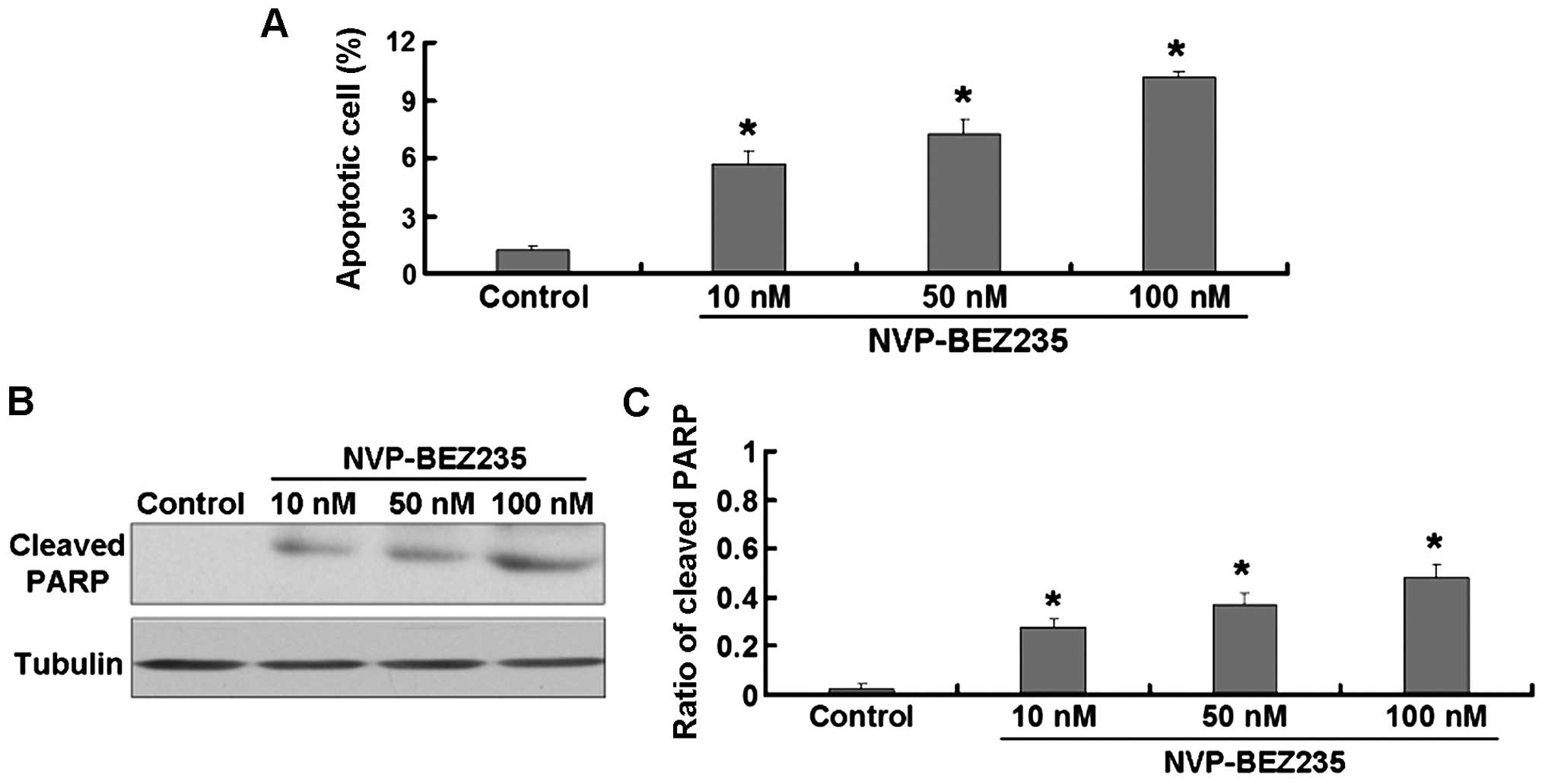
The flow cytometric analysis revealed that, the
NVP-BEZ235 treatment increased the rate of apoptosis in SW480 cells
(Fig. 2A). To further confirm this
result, we detected the expression of cleaved PARP protein which is
widely considered as an apoptosis indicator. The NVP-BEZ235
treatment significantly increased the expression of cleaved PARP
protein in SW480 cells (Fig. 2B and
C).
Autophagy occurs in SW480 cells
treated with NVP-BEZ235
The treatment of SW480 cells with NVP-BEZ235
affected the expression of light chain 3 (LC3) (LC3-I and LC3-II)
and p62 proteins involved in the process of cellular autophagy. As
shown in Fig. 3A and B, NVP-BEZ235
treatment increased the expression of LC3-II, whereas it decreased
the expression of p62 (Fig. 3C and
D).
Autophagy synergistically increases
apoptosis induced by NVP-BEZ235
Autophagy can lead to a pro-survival or pro-death
effect through apoptosis that is induced by the antitumor agent
depending on different circumstances. In the present study, the
autophagy inhibitors 3-MA and CQ were used to examine the role of
autophagy in NVP-BEZ235-induced apoptosis in SW480 cells. The
combination NVP-BEZ235+3-MA showed a higher growth inhibition in
SW480 cells than NVP-BEZ235 alone (Fig.
4A). A similar result was observed in SW480 treated with the
combination NVP-BEZ235+CQ (Fig. 4B).
We also assessed the level of expression of cleaved PARP protein in
SW480 cells treated with the combination NVP-BEZ235+3-MA and
NVP-BEZ235+CQ. As shown in Fig. 4C and
D, NVP-BEZ235 in combination with 3-MA enhanced the expression
of cleaved PARP as combined to NVP-BEZ235 alone in SW480 cells.
Similarly, the expression of cleaved PARP protein was higher in
SW480 cells treated with the combination of NVP-BEZ235 and CQ as
combined to NVP-BEZ235 alone (Fig. 4E and
F).
Discussion
CRC is a heterogeneous disease and its development
commonly spans approximately 10–15 years as a result of the
accumulation of diverse genetic and epigenetic alterations. Early
detected CRC is potentially curable via surgical resection followed
with adjuvant chemotherapy or radiotherapy. However, the treatment
is of limited efficacy for advanced CRC, which increases the
mortality to a high level (21,22).
The PI3K/mTOR signaling pathway is involved in
tumorigenesis in many important aspects, such as cell
proliferation, angiogenesis, invasion, as well as cell survival and
motility (10). Previous studies
identified PI3K/mTOR signaling downregulation in various types of
cancer, including breast cancer (23), hepatocellular cancer (24), lung cancer (25), pancreatic adenocarcinoma (26), and CRC (27). Thus, targeting the PI3K/mTOR pathway
has become a promising and intense research field over the last 10
years aiming to develop effective novel anticancer agents. The
PI3K/mTOR inhibitor has experienced two generations of the drugs
LY294002, wortmannin and rapamycin, and its derivatives. These
drugs belong to the first-generation inhibitor with low efficacy
and has severe side effects (28–30).
NVP-BEZ235 is one of the second generation inhibitors with improved
pharmacological properties currently under evaluation in a phase
I/II clinical trial (6,31,32). In
the present study, we firstly examined the impact of NVP-BEZ235 on
SW480 CRC cells and our results supported those of previous
studies, in which NVP-BEZ235 effectively inhibited the survival of
CRC cells in a time- and dose-dependent manner.
As mentioned earlier, AKT is the key node of the
PI3K/mTOR signaling pathway and its activation by PDK1 is able to
phosphorylate many protein targets, including mTOR, at the membrane
thereby regulating cell proliferation, apoptosis, survival, and
motility (8–12). AKT phosphorylation involves the
activity of PI3K and AKT kinases, whereas S6K phosphorylation
directly reflects mTOR kinase activity (33). Thus, we further investigated the
abundance of phosphorylated AKT and S6K in SW480 cells after
NVP-BEZ235 treatment. The results were in accordance with studies
of other tumors in which NVP-BEZ235 inhibited the PI3K/mTOR
signaling pathway (6,31,34–36).
Our study also showed that, NVP-BEZ235 induced
apoptosis and autophagy in a dose-dependent manner in SW480 CRC
cells. Autophagy is known as the basic catabolic mechanism that
degrades cellular components, which are unnecessary or
dysfunctional, through the lysosomal pathway (13). In recent studies, autophagy is well
demonstrated as a tumor promoter via a recycling mechanism that is
vital to tumor progression during chemotherapy with antitumor
agents (14–18). Previous findings have revealed that,
the PI3K/mTOR pathway inhibition induced autophagy as a mechanism
of cell death or drug resistance (37,38).
Autophagy is reported to have a crosstalk with apoptosis that under
certain circumstances may suppress apoptosis serving as a cell
survival pathway (39). On the other
hand, autophagy blockage may enhance the pro-apoptotic effects of
PI3K/mTOR inhibitors in preclinical studies (26,40) and it
is evident that our results support the latter.
Taking the above findings into account, it was found
that inhibiting autophagy enhanced the efficacy of NVP-BEZ235 in
CRC. The synergistic effect of the autophagy inhibitor observed in
the present study was also observed in glioma (41) and malignant peripheral nerve sheath
tumors in previous studies (42).
Collectively, our findings suggest autophagy
inhibition as a potential strategy to enhance the therapeutic
efficacy of dual PI3K/mTOR inhibitor in CRC treatment.
References
|
1
|
Li Q, Wang D, Li J and Chen P:
Clinicopathological and prognostic significance of HER-2/neu and
VEGF expression in colon carcinomas. BMC Cancer. 11:2772011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Atkin WS, Edwards R, Kralj-Hans I,
Wooldrage K, Hart AR, Northover JM, Parkin DM, Wardle J, Duffy SW
and Cuzick J: UK Flexible Sigmoidoscopy Trial Investigators:
Once-only flexible sigmoidoscopy screening in prevention of
colorectal cancer: A multicentre randomised controlled trial.
Lancet. 375:1624–1633. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li MX, Liu XM, Zhang XF, Zhang JF, Wang
WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L, et al: Prognostic role
of neutrophil-to-lymphocyte ratio in colorectal cancer: A
systematic review and meta-analysis. Int J Cancer. 134:2403–2413.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mathur A, Ware C, Davis L, Gazdar A, Pan
BS and Lutterbach B: FGFR2 is amplified in the NCI-H716 colorectal
cancer cell line and is required for growth and survival. PLoS One.
9:e985152014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zimonin FS: Reliable protection of chicks
against pullorum disease. Veterinariia. 8:69–70. 1973.(In Russian).
PubMed/NCBI
|
|
6
|
Maira SM, Stauffer F, Brueggen J, Furet P,
Schnell C, Fritsch C, Brachmann S, Chène P, De Pover A, Schoemaker
K, et al: Identification and characterization of NVP-BEZ235, a new
orally available dual phosphatidylinositol 3-kinase/mammalian
target of rapamycin inhibitor with potent in vivo antitumor
activity. Mol Cancer Ther. 7:1851–1863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Samuels Y, Diaz LA Jr, Schmidt-Kittler O,
et al: Mutant PIK3CA promotes cell growth and invasion of human
cancer cells. Cancer Cell. 7:561–573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carracedo A, Ma L, Teruya-Feldstein J,
Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma
SC, et al: Inhibition of mTORC1 leads to MAPK pathway activation
through a PI3K-dependent feedback loop in human cancer. J Clin
Invest. 118:3065–3074. 2008.PubMed/NCBI
|
|
9
|
Carpten JD, Faber AL, Horn C, Donoho GP,
Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage
S, et al: A transforming mutation in the pleckstrin homology domain
of AKT1 in cancer. Nature. 448:439–444. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Samuels Y, Wang Z, Bardelli A, Silliman N,
Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al:
High frequency of mutations of the PIK3CA gene in human cancers.
Science. 304:5542004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
From growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Filomeni G, De Zio D and Cecconi F:
Oxidative stress and autophagy: the clash between damage and
metabolic needs. Cell Death Differ. 22:377–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: Apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Denton D, Nicolson S and Kumar S: Cell
death by autophagy: Facts and apparent artefacts. Cell Death
Differ. 19:87–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scarlatti F, Granata R, Meijer AJ and
Codogno P: Does autophagy have a license to kill mammalian cells?
Cell Death Differ. 16:12–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen HY and White E: Role of autophagy in
cancer prevention. Cancer Prev Res (Phila). 4:973–983. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murrow L and Debnath J: Autophagy as a
stress-response and quality-control mechanism: Implications for
cell injury and human disease. Annu Rev Pathol. 8:105–137. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Janku F, McConkey DJ, Hong DS and Kurzrock
R: Autophagy as a target for anticancer therapy. Nat Rev Clin
Oncol. 8:528–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rubinsztein DC, Codogno P and Levine B:
Autophagy modulation as a potential therapeutic target for diverse
diseases. Nat Rev Drug Discov. 11:709–730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ogino S and Goel A: Molecular
classification and correlates in colorectal cancer. J Mol Diagn.
10:13–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lao VV and Grady WM: Epigenetics and
colorectal cancer. Nat Rev Gastroenterol Hepatol. 8:686–700. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Generali D, Fox SB, Brizzi MP, Allevi G,
Bonardi S, Aguggini S, Milani M, Bersiga A, Campo L, Dionisio R, et
al: Down-regulation of phosphatidylinositol 3′-kinase/AKT/molecular
target of rapamycin metabolic pathway by primary letrozole-based
therapy in human breast cancer. Clin Cancer Res. 14:2673–2680.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Masuda M, Shimomura M, Kobayashi K, Kojima
S and Nakatsura T: Growth inhibition by NVP-BEZ235, a dual
PI3K/mTOR inhibitor, in hepatocellular carcinoma cell lines. Oncol
Rep. 26:1273–1279. 2011.PubMed/NCBI
|
|
25
|
Herrera VA, Zeindl-Eberhart E, Jung A,
Huber RM and Bergner A: The dual PI3K/mTOR inhibitor BEZ235 is
effective in lung cancer cell lines. Anticancer Res. 31:849–854.
2011.PubMed/NCBI
|
|
26
|
Mirzoeva OK, Hann B, Hom YK, Debnath J,
Aftab D, Shokat K and Korn WM: Autophagy suppression promotes
apoptotic cell death in response to inhibition of the PI3K-mTOR
pathway in pancreatic adenocarcinoma. J Mol Med Berl. 89:877–889.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Potter DS, Kelly P, Denneny O, Juvin V,
Stephens LR, Dive C and Morrow CJ: BMX acts downstream of PI3K to
promote colorectal cancer cell survival and pathway inhibition
sensitizes to the BH3 mimetic ABT-737. Neoplasia. 16:147–157. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Markman B, Dienstmann R and Tabernero J:
Targeting the PI3K/Akt/mTOR pathway - beyond rapalogs. Oncotarget.
1:530–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prevo R, Deutsch E, Sampson O, Diplexcito
J, Cengel K, Harper J, O'Neill P, McKenna WG, Patel S and Bernhard
EJ: Class I PI3 kinase inhibition by the pyridinylfuranopyrimidine
inhibitor PI-103 enhances tumor radiosensitivity. Cancer Res.
68:5915–5923. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wan X, Harkavy B, Shen N, Grohar P and
Helman LJ: Rapamycin induces feedback activation of Akt signaling
through an IGF-1R-dependent mechanism. Oncogene. 26:1932–1940.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan QW, Knight ZA, Goldenberg DD, Yu W,
Mostov KE, Stokoe D, Shokat KM and Weiss WA: A dual PI3 kinase/mTOR
inhibitor reveals emergent efficacy in glioma. Cancer Cell.
9:341–349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kurtz JE and Ray-Coquard I: PI3 kinase
inhibitors in the clinic: an update. Anticancer Res. 32:2463–2470.
2012.PubMed/NCBI
|
|
33
|
Yang F, Qian XJ, Qin W, Deng R, Wu XQ, Qin
J, Feng GK and Zhu XF: Dual phosphoinositide 3-kinase/mammalian
target of rapamycin inhibitor NVP-BEZ235 has a therapeutic
potential and sensitizes cisplatin in nasopharyngeal carcinoma.
PLoS One. 8:e598792013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu TJ, Koul D, LaFortune T, Tiao N, Shen
RJ, Maira SM, Garcia-Echevrria C and Yung WK: NVP-BEZ235, a novel
dual phosphatidylinositol 3-kinase/mammalian target of rapamycin
inhibitor, elicits multifaceted antitumor activities in human
gliomas. Mol Cancer Ther. 8:2204–2210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brachmann SM, Hofmann I, Schnell C,
Fritsch C, Wee S, Lane H, Wang S, Garcia-Echeverria C and Maira SM:
Specific apoptosis induction by the dual PI3K/mTor inhibitor
NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells.
Proc Natl Acad Sci USA. 106:22299–22304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McMillin DW, Ooi M, Delmore J, Negri J,
Hayden P, Mitsiades N, Jakubikova J, Maira SM, Garcia-Echeverria C,
Schlossman R, et al: Antimyeloma activity of the orally
bioavailable dual phosphatidylinositol 3-kinase/mammalian target of
rapamycin inhibitor NVP-BEZ235. Cancer Res. 69:5835–5842. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fujiwara K, Iwado E, Mills GB, Sawaya R,
Kondo S and Kondo Y: Akt inhibitor shows anticancer and
radiosensitizing effects in malignant glioma cells by inducing
autophagy. Int J Oncol. 31:753–760. 2007.PubMed/NCBI
|
|
38
|
Yang S, Xiao X, Meng X and Leslie KK: A
mechanism for synergy with combined mTOR and PI3 kinase inhibitors.
PLoS One. 6:e263432011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang Y, Xing D, Zhou F and Chen Q:
Mitochondrial autophagy protects against heat shock-induced
apoptosis through reducing cytosolic cytochrome c release and
downstream caspase-3 activation. Biochem Biophys Res Commun.
395:190–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu CX, Zhao L, Yue P, Fang G, Tao H,
Owonikoko TK, Ramalingam SS, Khuri FR and Sun SY: Augmentation of
NVP-BEZ235′s anticancer activity against human lung cancer cells by
blockage of autophagy. Cancer Biol Ther. 12:549–555. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cerniglia GJ, Karar J, Tyagi S,
Christofidou-Solomidou M, Rengan R, Koumenis C and Maity A:
Inhibition of autophagy as a strategy to augment radiosensitization
by the dual phosphatidylinositol 3-kinase/mammalian target of
rapamycin inhibitor NVP-BEZ235. Mol Pharmacol. 82:1230–1240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ghadimi MP, Lopez G, Torres KE, Belousov
R, Young ED, Liu J, Brewer KJ, Hoffman A, Lusby K, Lazar AJ, et al:
Targeting the PI3K/mTOR axis, alone and in combination with
autophagy blockade, for the treatment of malignant peripheral nerve
sheath tumors. Mol Cancer Ther. 11:1758–1769. 2012. View Article : Google Scholar : PubMed/NCBI
|