Introduction
The central nervous system is comprised of the brain
and the spinal cord, which regulate the majority of bodily
functions. Glioma arises as a result of abnormal glial cell
proliferation resulting in the formation of an aggressive mass;
this occurs most frequently in the brain and less commonly in the
spinal cord. Patients with aggressive forms of glioma have an
average survival time of 15 months (1,2). Treatment
for the disease consists of surgery, in addition to radiation or
chemotherapy, and usually only prolongs patient survival for a few
more months (3). Early-stage glioma
rapidly develops into the advanced stages of disease, due to an
invasive nature and fast growth that is associated with a vigorous
pattern of intra-tumor blood vessel formation (4). Other complications of glioma are
associated with the stage and treatment of the tumor, with efficacy
often depending on the location of the tumor within the brain
(5,6).
Surgical dissection of the malignancy is not recommended if it has
developed within an important structure of the brain. Despite large
improvements in microsurgical procedures and the increased
availability of advanced chemotherapy, the improvement to patient
survival has been controversial. Therefore, detailed investigation
is required to establish the underlying mechanisms of glioma
pathogenesis and identify novel therapeutic approaches to treat
glioma (7).
The Egl-9 family hypoxia-inducible factor (Egln)
hydroxylases, including Egln1, Egln2 and Egln3, are proteins that
are associated with the oxygen-sensing pathway, and are also
involved in the proline hydroxylation of specific targets (8–11). As the
hydroxylation reaction requires molecular oxygen (12), any changes in oxygen requirement are
monitored through an oxygen sensing pathway. When this pathway is
disturbed, it becomes associated with different forms of cancer
(13–15). Egln3 was identified to be an α-subunit
hydroxylase for hypoxia-inducible factors (Hifs) in an
O2-dependent manner, triggering their proteasomal
degradation to retract tumor progression (16–18).
Additionally, Egln3 exhibits a high affinity for Hif-2α (19), a protein that regulates genes
associated with cell proliferation, angiogenesis and cell
metabolism (16,20,21). The
upregulation of Hif-2α is also associated with proapoptotic
activity (22). However, the role of
Egln hydroxylases in cancer biology is poorly understood, with two
varying hypotheses currently presented; one suggesting that they
function as tumor suppressors and the other favoring their
contribution towards tumor aggressiveness (23–28).
The present study developed a mouse model of glioma
and investigated the function of Egln3 by analyzing its expression
in the presence and absence of an Egln3 inhibitor within the
context of apoptosis.
Materials and methods
Experimental animals
Male 4-month-old NSG mice were purchased from The
Jackson Laboratory (Bar Harbor, ME, USA), and were monitored for a
week in a host institute West China Hospital of Sichuan University,
Chengdu, China) under laboratory conditions. The work plan and
protocol for the present study were approved by the institutional
ethical committee of West China Hospital of Sichuan University
(Chengdu, China). Following regular supervision for 1 week, active
healthy mice were selected for further procedures. The mice were
anesthetized and located in the Lab Standard™ Stereotaxic
Instrument (Stoelting Co., Wood Dale, IL, USA) in a position that
allowed easy access for cerebral cortex injection. The mice were
then injected intracranially with 1 µl C6 rat glioma cells (30,000
cells; Sigma-Aldrich, St. Louis, MO, USA), to deliver the cells to
the cerebral cortex as previously described (29). Following injection, 1 mg doxycycline
was administered to the mice via food for 28 days. The mice that
demonstrated glioma symptoms were then sacrificed for further
analysis. For Egln3 suppression, the mice that developed glioma
were intraperitoneally injected with dimethyloxalylglycine (DMOG)
in a 50 µg/g of body weight concentration, and samples were
dissected 72 h post-DMOG injection. The experiment was designed so
that all the mice were scarified on day 30.
Western blot analysis
The tissue samples from the normal, glioma-induced
and Egln3-suppressed mice were dissected and cell lysates were
prepared. The cellular proteins were resolved in 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (Sigma-Aldrich) at 60 V
for 5 h, following the previously described protocol (30). Subsequent to membrane blocking, the
mice were probed with the Egln3 antibody (catalog no., MA5-16144;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at a dilution of
1:500 or cell death-inducing DFF-like effector A (CAD) antibody
(catalog no., C7852; Sigma-Aldrich) at a diluted concentration of
1:5,000. The membranes were then incubated with a rabbit anti-mouse
immunoglobulin G (catalog no. ab97043; Abcam, Cambridge, UK)
secondary antibody at a dilution of 1:5,000, and was further
developed with alkaline phosphatase chromogen (BCIP/NBT; Abcam)
obtain the signal.
Immunohistochemistry
For the immunohistochemical analysis of the
dissected brain tissues, the samples were initially fixed in 10%
formalin and processed. The paraffin-embedded samples were sliced
into 6-µm sections and mounted onto glass slides. Following
deparaffinization with xylene, the samples were hydrated and
incubated with freshly prepared 10% H2O2 and
10% methanol in 1X phosphate-buffered saline (PBS) for 30 min.
Following the PBS wash, the tissue sections were incubated with
0.1% trypsin in 0.1% CaCl2 at 37°C for 10 min.
Subsequently, the sections were incubated at 4°C with the primary
anti-Egln3 antibody overnight. Following 3 extensive washes with 1X
PBS, the samples were incubated with the suitable secondary
antibody (dilution, 1:5,000) for 45 min at room temperature.
Finally, subsequent to washing the sections, the primary antibody
was detected using the DAB Substrate kit (Abcam). For the
visualization of individual cells, the samples were counter-stained
with Ehrlich's hematoxylin (Thermo Fisher Scientific, Inc.).
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) assay
Apoptotic cells exhibit characteristic DNA fragments
that are identified using TUNEL assay (31). The end labeling of DNA fragments was
conducted by incorporating 5-bromo-2′-deoxyuridine (Brdu), a
thymidine analogue, to the 3′-ends of the DNA strands. The
incorporated Brdu in the apoptotic cells was then identified using
the monoclonal mouse anti-all Brdu antibody (catalog no., 05–633;
EMD Millipore, Billerica, MA, USA) following staining with the DAB
Substrate kit (Abcam).
Results
Effective initiation of glioma in
mice
The mice that were injected with the rat glioma
cells, as aforementioned, were scarified on day 30, and the glioma
tissue was bisected. The control and the dissected glioma tissue
were then subjected to histological analysis aiming to understand
the nature of the cell arrangement and identify any abnormalities.
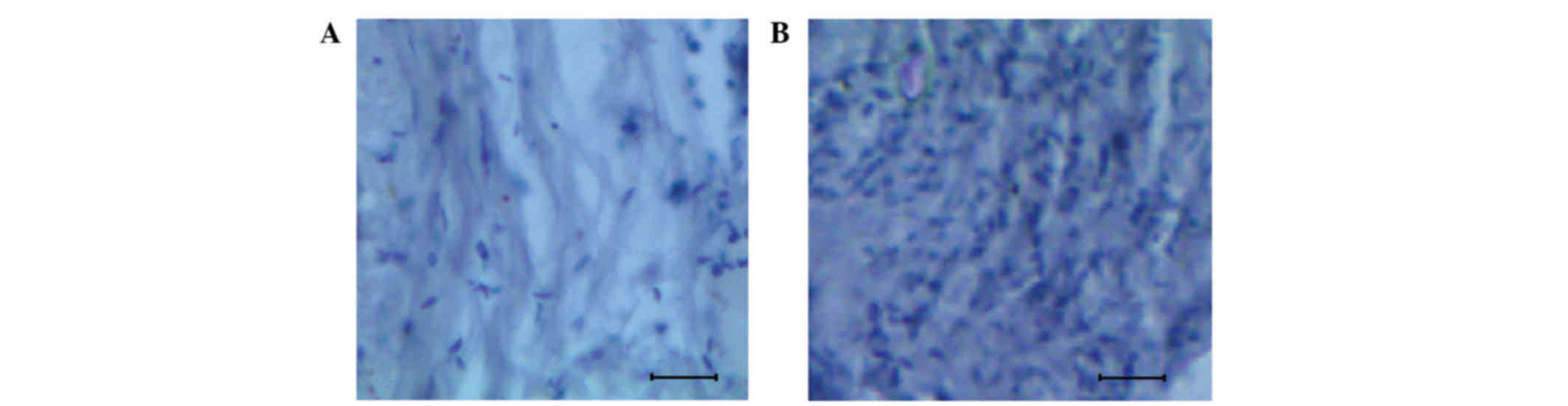
The histological brain sectioning of the control and glioma tissue
are presented in Fig. 1A and B. By
comparing the normal and glioma-induced brain tissue,
well-distinguishable features were observed. The normal brain was
characterized by a uniform cellular pattern (Fig. 1A), whereas a proliferative mass of
cells with wide variation among samples was observed in glioma
tissue (Fig. 1B).
Role of Egln3 in glioma tissue
In order to investigate the function of Egln3 in the
regulation of glioma growth, a mouse model system was successfully
developed with early-stage glioma (Fig.
1B). The normal and glioma tissues were subjected to
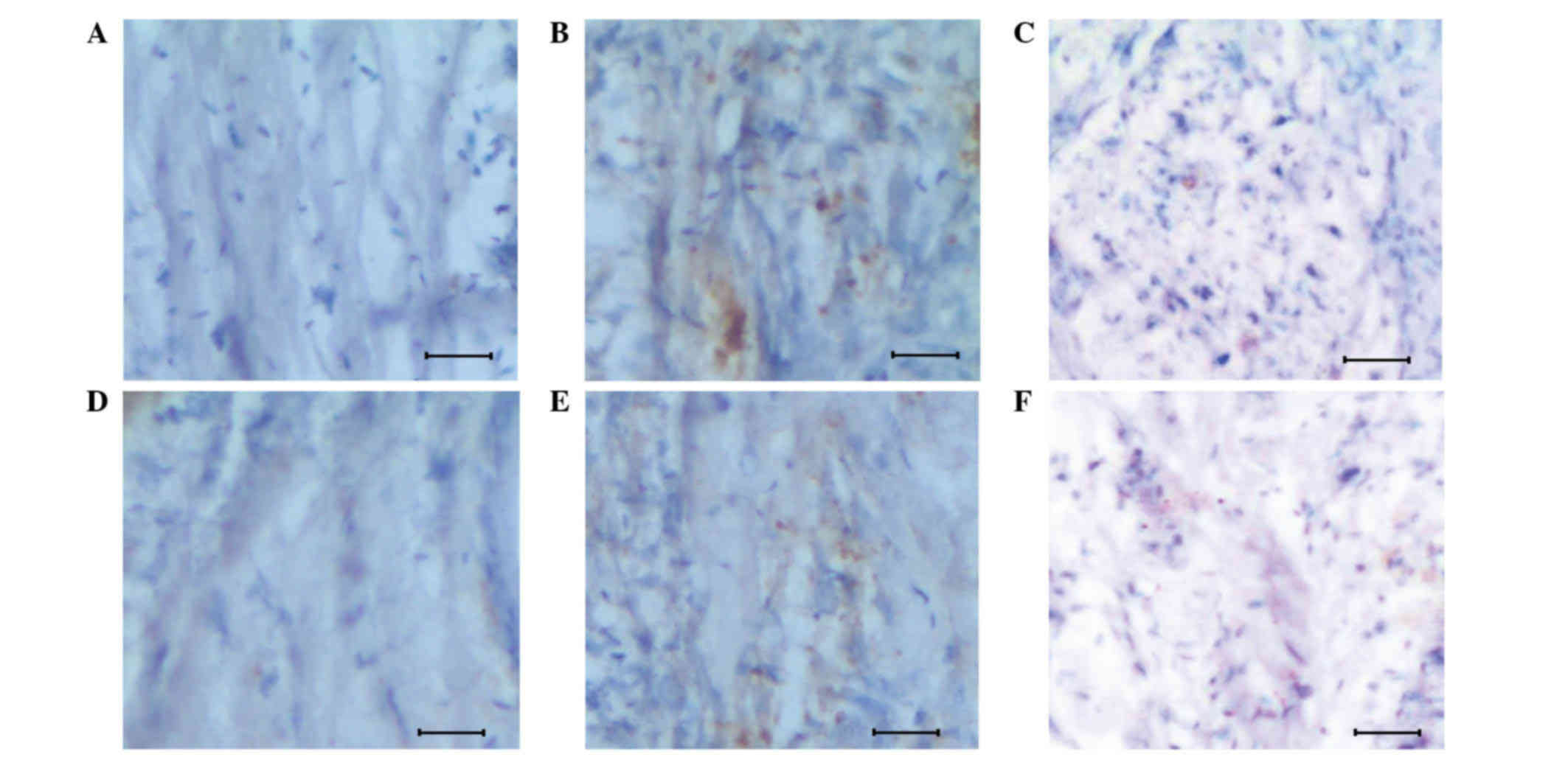
immunohistochemical analysis to study the apoptosis signal
(determined by the brown color produced by the DAB Substrate kit),
in addition to assess the association between Egln3 and apoptosis
signals (Fig. 2). It was observed,
during the initial stages of glioma, that the host exhibited
increased expression of Egln3 (Fig.
2E), which acted as a resistance mechanism to regulate glioma,
when compared with the normal tissue, which demonstrated lower
expression (Fig. 2D). Additional
evidence of Egln3 expression came from the increased apoptotic
signals observed in the glioma tissue (Fig. 2B) when compared with the controls
(Fig. 2A and D).
To analyze the regulative role of Egln3 in
early-stage glioma, the Egln3 inhibitor DMOG was used to
investigate the apoptosis signal response. The apoptosis signal was
measured by the intensity of the brown staining. As presented in
Fig. 2F, the inhibitor effectively
suppressed Egln3 expression, and was associated with a poor
apoptotic signal and a higher proliferative mass of cells (Fig. 2C). The results confirm that Egln3
positively regulates early-stage glioma by triggering apoptotic
signals in abnormal cells.
Validation of the results using
western blotting
Western blot analysis was used to evaluate the
expression of Egln3 and the apoptotic signals (assessed in the
normal brain and glioma tissue) based on the observed intensity of
anti-CAD bands, and also to cross-check the results obtained from
immunohistochemistry. The results from western blotting using the
anti-Egln3 and anti-CAD antibodies are presented in Fig. 3. Upregulation of the apoptotic signal
with increasing expression of Egln3 was observed, and in the
presence of the Egln3 inhibitor, there was a marked difference in
the expression pattern of the apoptotic signal and Egln3.
Discussion
The Egln3 protein is unregulated in numerous types
of human cancer, including pancreatic cancer (32) and glioblastoma (27). The function of Egln3 is dependent on
prolyl hydroxylase activity through which it hydroxylates a number
of targets, including Hif-2α (19)
and other α subunits of Hif, larger subunits of RNA polymerase II,
pyruvate kinase M2 and β2-adrenergic receptors (11,14,33–36).
In addition to the prolyl hydroxylase-dependent activity of Egln3,
studies have reported the hydroxylase-independent activity of Egln3
in regulating the nuclear factor-κB pathway (37). However, the function of Egln3 in
carcinogenesis and its biological function have yet to be fully
elucidated. At present, the role of Egln3 in cancer is under
debate, with one theory suggesting that it may function as a tumor
suppressor, whilst another proposes that it may promote tumor
aggressiveness (23–28).
In the present study, an association was observed
between Egln3 expression and apoptosis. A glioma mouse model was
successfully developed through the injection of rat glioma cells,
which are easily identifiable during histological analysis, into a
sample of mice. The dose of rat glioma cells that were injected
into the mice was reduced from the standard dose of 50,000 to
30,000 glioma cells/µl so that slow-growing glioma could form prior
to day 30 (29). Reliable data was
obtained from immunohistochemical analysis and demonstrated that
Egln3 expression in glioma is associated with apoptotic signals.
Furthermore, it was also observed that the Egln3 inhibitor caused a
marked decrease in the apoptotic signaling of the glioma tissue.
Analysis of the results indicated that the Egln3 suppression
contributed to a poor apoptotic signal and reduced regulation of
the glioma, therefore resulting in a higher proliferative cell
mass. The data obtained was cross-checked by western blot analysis,
which demonstrated consistent results.
In conclusion, the present study successfully
developed a mouse model of the initial stage of low-grade glioma at
day 30 post-injection. Using this model system, the role of the
Egln3 protein in glioma tissue was studied, and it was observed
that Egln3 expression in glioma is associated with positive
regulation through the induction of apoptosis. The continued
investigation into this pathological condition may improve our
understanding of the mechanisms underlying glioma and may assist
with the development of novel therapeutics required to treat
patients with this disease.
Acknowledgements
The authors would like to thank the Institutional
Review Board Approval Committee and Ethical Committee (West China
Hospital of Sichuan University, Chengdu, China) for the successful
completion of this project.
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: European Organisation for Research and Treatment of
Cancer Brain Tumor and Radiotherapy Groups; National Cancer
Institute of Canada Clinical Trials Group: Radiotherapy plus
concomitant and adjuvant temozolomide for glioblastoma. N Engl J
Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cuddapah VA, Robel S, Watkins S and
Sontheimer H: A neurocentric perspective on glioma invasion. Nat
Rev Neurosci. 15:455–465. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nieder C, Grosu AL, Astner S and Molls M:
Treatment of unresectable glioblastoma multiforme. Anticancer Res.
25(6C): 4605–4610. 2005.PubMed/NCBI
|
|
6
|
Stupp R, Pavlidis N and Jelic S: ESMO
Guidelines Task Force: ESMO Minimum Clinical Recommendations for
diagnosis, treatment and follow-up of malignant glioma. Ann Oncol.
16:(Suppl 1). i64–i65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huse JT and Holland EC: Targeting brain
cancer: Advances in the molecular pathology of malignant glioma and
medulloblastoma. Nat Rev Cancer. 10:319–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Epstein AC, Gleadle JM, McNeill LA,
Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI,
Dhanda A, et al: C. elegans EGL-9 and mammalian homologs define a
family of dioxygenases that regulate HIF by prolyl hydroxylation.
Cell. 107:43–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ivan M, Haberberger T, Gervasi DC,
Michelson KS, Günzler V, Kondo K, Yang H, Sorokina I, Conaway RC,
Conaway JW and Kaelin WG Jr: Biochemical purification and
pharmacological inhibition of a mammalian prolyl hydroxylase acting
on hypoxia-inducible factor. Proc Natl Acad Sci USA.
99:13459–13464. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kivirikko KI and Myllyharju J: Prolyl
4-hydroxylases and their protein disulfide isomerase subunit.
Matrix Biol. 16:357–368. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bruick RK and McKnight SL: A conserved
family of prolyl-4-hydroxylases that modify HIF. Science.
294:1337–1340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McNeill LA, Hewitson KS, Gleadle JM,
Horsfall LE, Oldham NJ, Maxwell PH, Pugh CW, Ratcliffe PJ and
Schofield CJ: The use of dioxygen by HIF prolyl hydroxylase (PHD1).
Bioorg Med Chem Lett. 12:1547–1550. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maxwell PH, Wiesener MS, Chang G-W,
Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER and
Ratcliffe PJ: The tumour suppressor protein VHL targets
hypoxia-inducible factors for oxygen-dependent proteolysis. Nature.
399:271–275. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ivan M, Kondo K, Yang H, Kim W, Valiando
J, Ohh M, Salic A, Asara JM, Lane WS and Kaelin WG Jr: HIFalpha
targeted for VHL-mediated destruction by proline hydroxylation:
Implications for O2 sensing. Science. 292:464–468. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gordan JD and Simon MC: Hypoxia-inducible
factors: Central regulators of the tumor phenotype. Curr Opin Genet
Dev. 17:71–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Keith B and Simon MC: Hypoxia-inducible
factors, stem cells, and cancer. Cell. 129:465–472. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kaelin WG Jr: The von Hippel-Lindau tumour
suppressor protein: O2 sensing and cancer. Nat Rev
Cancer. 8:865–873. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Semenza GL: Angiogenesis in ischemic and
neoplastic disorders. Annu Rev Med. 54:17–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Appelhoff RJ, Tian Y-M, Raval RR, Turley
H, Harris AL, Pugh CW, Ratcliffe PJ and Gleadle JM: Differential
function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the
regulation of hypoxia-inducible factor. J Biol Chem.
279:38458–38465. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rankin EB, Rha J, Unger TL, Wu CH, Shutt
HP, Johnson RS, Simon MC, Keith B and Haase VH: Hypoxia-inducible
factor-2 regulates vascular tumorigenesis in mice. Oncogene.
27:5354–5358. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ryan HE, Lo J and Johnson RS: HIF-1 alpha
is required for solid tumor formation and embryonic
vascularization. EMBO J. 17:3005–3015. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lipscomb EA, Sarmiere PD, Crowder RJ and
Freeman RS: Expression of the SM-20 gene promotes death in nerve
growth factor-dependent sympathetic neurons. J Neurochem.
73:429–432. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chan DA, Kawahara TL, Sutphin PD, Chang
HY, Chi JT and Giaccia AJ: Tumor vasculature is regulated by
PHD2-mediated angiogenesis and bone marrow-derived cell
recruitment. Cancer Cell. 15:527–538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chan DA and Giaccia AJ: PHD2 in tumour
angiogenesis. Br J Cancer. 103:1–5. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Erez N, Milyavsky M, Eilam R, Shats I,
Goldfinger N and Rotter V: Expression of prolyl-hydroxylase-1
(PHD1/EGLN2) suppresses hypoxia inducible factor-1alpha activation
and inhibits tumor growth. Cancer Res. 63:8777–8783.
2003.PubMed/NCBI
|
|
26
|
Hatzimichael E, Dasoula A, Shah R, Syed N,
PapoudouBai A, Coley HM, Dranitsaris G, Bourantas KL, Stebbing J
and Crook T: The prolyl-hydroxylase EGLN3 and not EGLN1 is
inactivated by methylation in plasma cell neoplasia. Eur J
Haematol. 84:47–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Henze AT, Riedel J, Diem T, Wenner J,
Flamme I, Pouyseggur J, Plate KH and Acker T: Prolyl hydroxylases 2
and 3 act in gliomas as protective negative feedback regulators of
hypoxia-inducible factors. Cancer Res. 70:357–366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mazzone M, Dettori D, de Oliveira R Leite,
Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, de
Almodovar C Ruiz, et al: Heterozygous deficiency of PHD2 restores
tumor oxygenation and inhibits metastasis via endothelial
normalization. Cell. 136:839–851. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z, Bao S, Wu Q, Wang H, Eyler C,
Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, et al:
Hypoxia-inducible factors regulate tumorigenic capacity of glioma
stem cells. Cancer Cell. 15:501–513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hamidouche Z, Haÿ E, Vaudin P, Charbord P,
Schüle R, Marie PJ and Fromigué O: FHL2 mediates
dexamethasone-induced mesenchymal cell differentiation into
osteoblasts by activating Wnt/β-catenin signaling-dependent Runx2
expression. FASEB J. 22:3813–3822. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fulda S and Pervaiz S: Apoptosis signaling
in cancer stem cells. Int J Biochem Cell Biol. 42:31–38. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Su Y, Loos M, Giese N, Hines OJ, Diebold
I, Görlach A, Metzen E, Pastorekova S, Friess H and Büchler P: PHD3
regulates differentiation, tumour growth and angiogenesis in
pancreatic cancer. Br J Cancer. 103:1571–1579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kovalenko A, ChableBessia C, Cantarella G,
Israël A, Wallach D and Courtois G: The tumour suppressor CYLD
negatively regulates NF-kappaB signalling by deubiquitination.
Nature. 424:801–805. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jaakkola P, Mole DR, Tian Y-M, Wilson MI,
Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji
M, Schofield CJ, et al: Targeting of HIF-alpha to the von
Hippel-Lindau ubiquitylation complex by O2-regulated
prolyl hydroxylation. Science. 292:468–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie L, Xiao K, Whalen EJ, Forrester MT,
Freeman RS, Fong G, Gygi SP, Lefkowitz RJ and Stamler JS:
Oxygen-regulated β(2)-adrenergic receptor hydroxylation by EGLN3
and ubiquitylation by pVHL. Sci Signal. 2:ra332009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luo W, Hu H, Chang R, Zhong J, Knabel M,
O'Meally R, Cole RN, Pandey A and Semenza GL: Pyruvate kinase M2 is
a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell.
145:732–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xue J, Li X, Jiao S, Wei Y, Wu G and Fang
J: Prolyl hydroxylase-3 is down-regulated in colorectal cancer
cells and inhibits IKKbeta independent of hydroxylase activity.
Gastroenterology. 138:606–615. 2010. View Article : Google Scholar : PubMed/NCBI
|