Cancer is one of the predominant causes of human
mortality worldwide. Despite several decades of unremitting efforts
towards preventing and curing cancer, the translation of detailed
molecular knowledge into more efficient cancer therapies remains a
significant medical challenge. Cancer cells harbor a considerable
number of genetic and epigenetic alterations; however, only a
limited number of these alterations drive cancer progression. Tumor
formation and metastasis is dependent on intracellular and
intercellular signal transduction (1–5). Emerging
data indicate that the crosstalk of multiple signaling pathways may
account for malignant proliferation and metastasis (6–9). However,
recent research has mainly focused on single pathways, ignoring the
complexity of signaling networks. Exploration of the
cross-regulation of signaling pathways may provide a more
comprehensive understanding of the dissemination of information in
such networks.
The most active field of research is that of the
Hedgehog (Hh) and Wnt signaling pathways, which represent essential
regulators of cell proliferation and differentiation during
embryogenesis and tumorigenicity (10,11).
Convergence of the two pathways involving secreted frizzled-related
protein 1 (SFRP1) has been demonstrated (12,13).
Nevertheless, studies regarding the interactions among signaling
pathways are rare. The current review summarizes the most relevant
literature regarding the cooperative interaction between the Hh and
Wnt signaling pathways, and the role of SFRP1 as an important
mediator of certain oncogenic and pro-metastatic activities that
are associated with the Hh and Wnt signaling pathways. The targeted
inhibition of this key point in the pathways has potential with
regard to the development of therapies for cancer.
The Hh signaling pathway is an important cascade for
cellular growth and differentiation during the embryonic
development. The pathway was first identified in Drosophila
fruit flies, and has been shown to be highly conserved in
vertebrates and invertebrates (14–16). The
Hh signaling pathway is complex and involves numerous regulatory
proteins. In vertebrates, three Hh homologs have been identified:
Sonic Hh (Shh), Indian Hh (Ihh), and desert Hh (Dhh) (17–19).
Notably, the three Hh ligands activate the same signal transduction
pathway, but regulate different organ systems: Shh is most widely
expressed in the central nervous system, lungs, teeth, gut and hair
follicles (20–24), while Ihh is involved in endochondral
bone formation (25), and Dhh is
expressed mostly in the gonads (26).
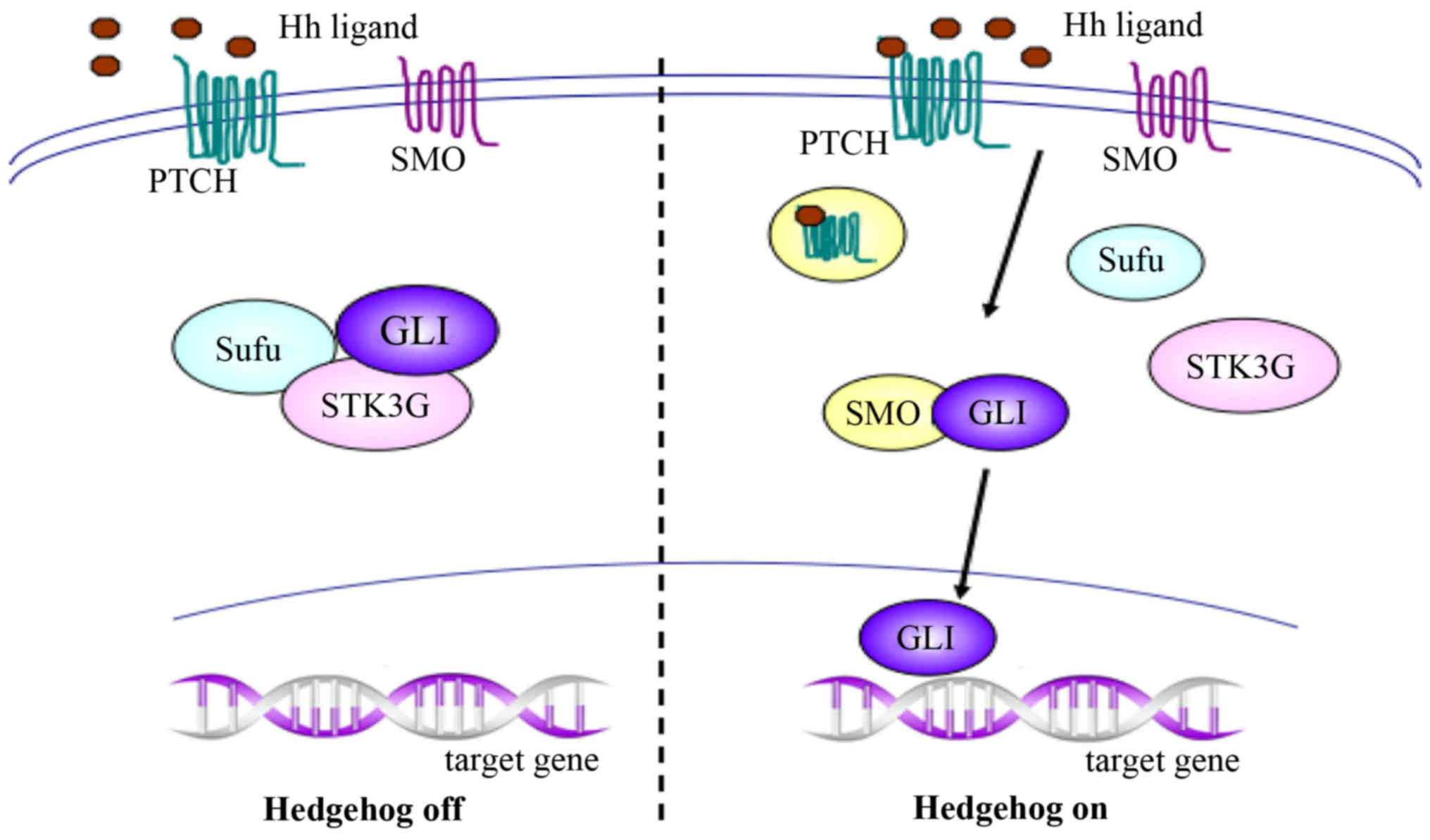
To initiate the signaling pathway, the Hh ligand
binds to its receptor, a 12-transmembrane Patched (PTCH) protein,
which also has two known human homologs, PTCH1 and PTCH2. In the
absence of Hh, PTCH forms an inactive complex with the downstream
protein Smoothened (SMO), and works as a suppressor or inhibitory
protein of SMO. When Hh is activated, binding of the Hh ligand to
PTCH results in endocytosis of the PTCH-ligand complex, followed by
migration of activated SMO to the cytoplasm and association with
glioma-associated oncogene homolog (GLI) proteins. The GLI proteins
subsequently migrate into the nucleus and promote the transcription
of target genes, which are responsible for cellular growth and
differentiation during embryonic development, and are involved in
tissue repair and cancer occurrence and development in adults
(Fig. 1) (7,27).
Dysregulation of the Hh signaling pathway has now
been implicated in various types of human malignancy, including
gastrointestinal, bladder and ovarian carcinomas, lung cancer, and
hematological malignancies (28–35).
Aberrant activation of the Hh signaling pathway in human cancers
can occur in three ways. In the first, mutated component proteins
can be secreted from cells and constantly activate Hh signaling
pathway. An example of this is the inactivation of PTCH or
oncogenic activation of SMO, which have been demonstrated to be
common features in a high proportion of tumors. To date, this mode
of Hh signaling is considered the most important for tumor
development (36–40). The second mode of aberrant activation
is autocrine: The Hh ligand is secreted by tumor cells and also
affects the tumor cells themselves (41,42). In
the third mode, which is paracrine activation, tumor cells secrete
Hh ligands to act on peripheral stroma cells, which activates
vascular endothelial growth factor, insulin-like growth factor and
Wnt signaling pathways to promote self-proliferation (43,44). A
paracrine pattern in which stromal cells secret Hh ligands, thus
contributing to the activation of Hh signaling in the tumor cells,
has also been described (45).
Based on the etiological study of the Hh signaling
pathway, molecular targeted therapy is considered a promising
therapeutic strategy for cancer. For example, methods for
increasing the inhibitory action of PTCH or suppressing the
activation of SMO may be utilized the treatment of tumors with a
hyper-activated Hh pathway. A number of small molecule SMO
antagonists have been evaluated in clinical trials and demonstrated
promising therapeutic benefits (46,47).
Vismodegib, a small 2-pyridyl amide molecule, blocks Hh signaling
by selectively inhibiting SMO, and thus prevents the consequent
induction of target genes (48). The
therapeutic success of Hh inhibitors also depends on their
appropriate combination with other drugs that target cooperative
signaling pathways (49–51); therefore, the points of interaction
between Hh and other signaling pathways in malignancies may be
potential therapeutic targets.
The Wnt signaling pathway participates in the
physiological processes of embryonic development, cellular
proliferation and differentiation, and also plays an important role
in the occurrence and development of various malignancies (52–55). Wnt
signaling is conducted via three pathways, as follows. The
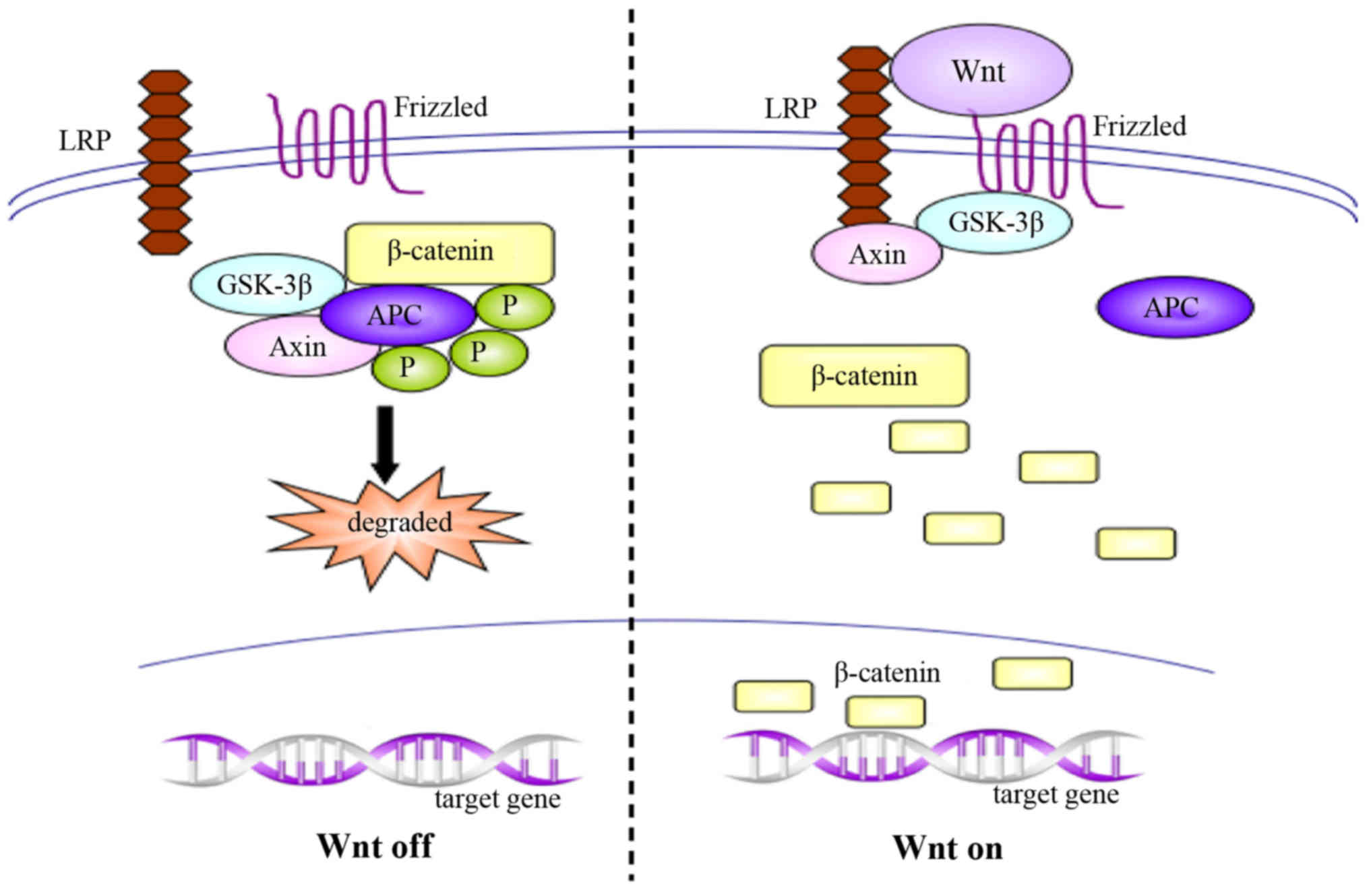
canonical Wnt/β-catenin signaling pathway, which is considered the
most important pathway, results in the accumulation of β-catenin in
the nucleus and initiates the expression of target genes. In normal
organisms, Wnt pathway is inactivated, and unconjugated β-catenin
is scarce; the majority of the β-catenin molecules are combined
with glycogen synthase kinase 3β (GSK-3β), adenomatous polyposis
coli (APC) and Axin, which lead to the phosphorylation and
degradation of β-catenin via the ubiquitin pathway. Conversely,
activation of the Wnt signaling pathway inhibits the
GSK-3β/APC/Axin complex, inducing the abnormal accumulation and
translocation to the nucleus of β-catenin, and resulting in gene
transcription (Fig. 2) (56–58). In
another pathway, Wnt5a and Wnt11 activate cyclin-dependent kinase 2
and protein kinase C to increase cellular Ca2+
concentration, and promote nuclear factor of activated
T-cells-induced gene transcription; this pathway is designated the
Wnt/Ca2+ pathway (59).
The third pathway, Wnt/planar cell polarity signaling pathway
mainly participates in the regulation of cytoskeletal rearrangement
during embryonic development (60).
The present review primarily focuses on the functional interaction
between the canonical Wnt/β-catenin signaling pathway and the Hh
signaling pathway.
Inappropriate activation of the Wnt signaling
pathway is associated with a variety of malignant diseases, such as
gallbladder, lung and breast cancers (61–63);
therefore, the development of drugs targeting this pathway is an
area of interest with regard to cancer therapy research. Several
Wnt inhibitors have been investigated in preclinical studies; for
example, Lu et al(64)
demonstrated that salinomycin is a potent inhibitor of the Wnt
signaling pathway and acts by interfering with lipoprotein-related
receptor 6 phosphorylation, and an anti-Frizzled antibody is
currently being tested as potential cancer therapy (65,66).
Although therapies targeting the Wnt signaling pathway are
attractive in theory, in practice it has been difficult to create
specific therapeutic agents, as numerous components of the Wnt
signaling pathways are also involved in other cellular processes.
The Wnt/β-catenin signaling pathway has been demonstrated to have
crosstalk with other signaling pathways, such as the Hh, NOTCH,
Hippo and mammalian target of rapamycin pathways (12,13,67–69).
Elucidating the regulatory mechanisms and biological functions of
these pathways may reveal potential therapeutic targets for the
treatment of tumors.
The network of signaling pathways, including Hh,
Wnt, signal transducer and transcription activator (STAT) and
NOTCH, contributes to cellular proliferation and differentiation,
and to maintaining the stability of the internal environment
(14–16,52–55). In
invertebrates and lower vertebrates, activation of Wnt and Hh
signaling pathways is crucial in embryogenesis and cellular
differentiation (70,71). Previous evidence has indicated that
the expression of myogenic basic helix-loop-helix genes in
embryonic somites can be induced by the Wnt and Shh signaling
pathways (65). Despite complete
truncation, the limbs of amphibians show remarkable regeneration
through wound healing, blastema formation and tissue
differentiation (72–74). Pharmacological research has revealed
the integration between Hh and Wnt signaling via active and
inhibitory drugs. The Hh pathway acts upstream of Wnt to inhibit
the activation of Wnt signaling; however, Wnt activation may rescue
the suppressive signaling of Hh in regulating amphibian limb
regeneration (75). The synergetic
interaction was postulated by Day et al(76), who reported that Ihh signaling is
activated at an early stage of osteoblast maturation during
fracture repair, and that Wnt signaling is subsequently upregulated
in differentiated osteoblasts. Further research has indicated that
the deletion of the motor protein kinesin family member 3A in
dental mesenchyme results in the suppression of Hh and activation
of Wnt, affecting incisor and molar development (77). These findings may reveal as
association between the two signaling pathways at the gene level.
In addition, Oberhofer et al(78) investigated Hh and Wnt signaling in the
head anlagen and growth zone of early insect embryos, and indicated
that Wnt/β-catenin signaling acts upstream of Hh in the growth
zone, yet downstream of Hh in the head, anlagen, suggesting the
different roles of Hh and Wnt in these two regions (78).
Although a number of studies have demonstrated that
synergy between the two pathways promotes embryogenesis and
cellular differentiation, conflicting data has also been reported
that the Hh and Wnt signaling pathways are functionally
antagonistic in vertebrates and invertebrates (80–84). The
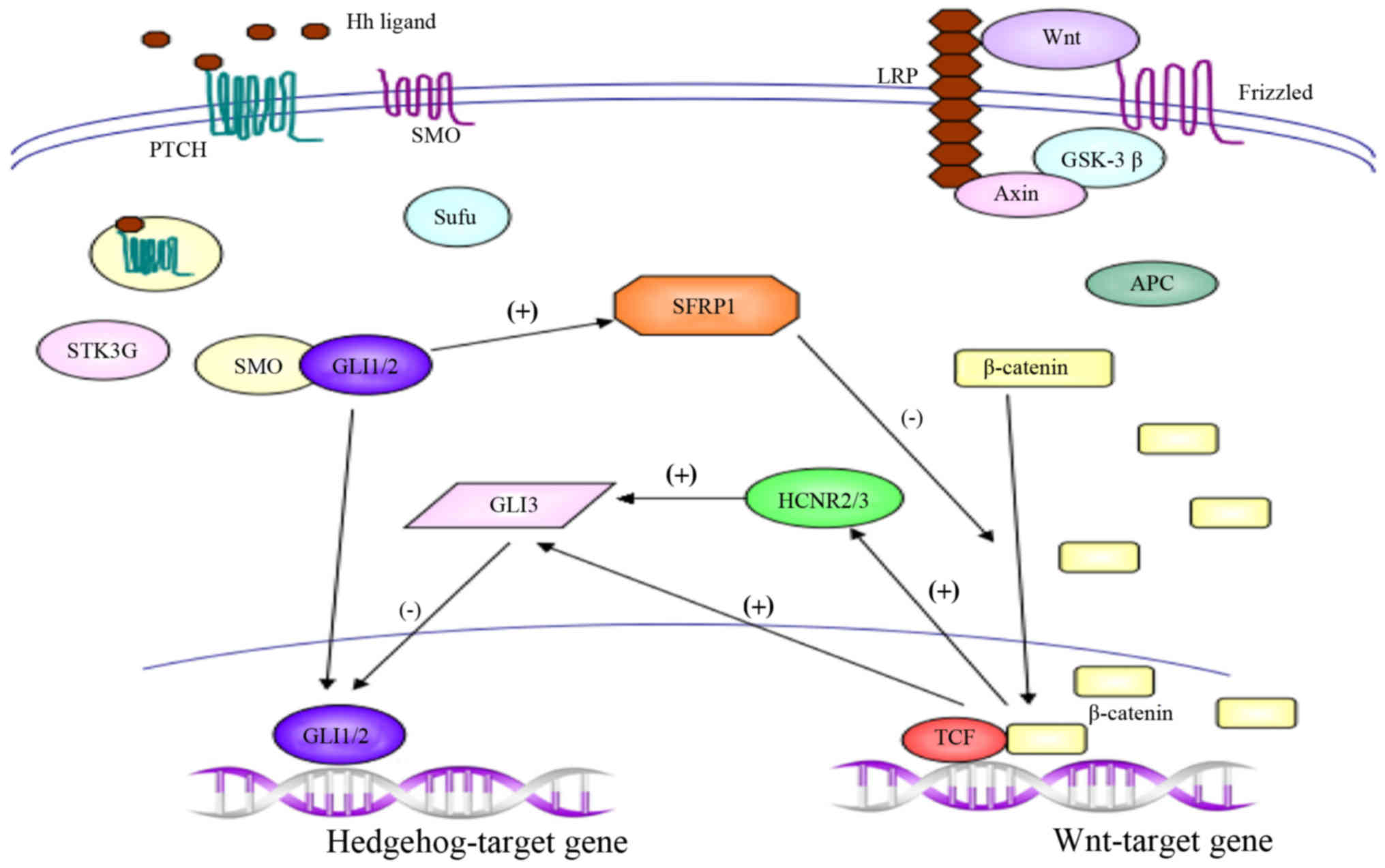
crosstalk protein SFRP1, which acts as an antagonist of Wnt
signaling, was initially identified in 1998, and has subsequently
been shown to be regulated by Hh in the developing spinal cord and
gastric cancer cells (12,80–82).
Borday et al(83) investigated
the potential cross-regulation between the Wnt and Hh signaling
pathways in neural stem/progenitor cells in the ciliary marginal
zones using pharmacological tools. They detected Wnt activity and
subsequently Hedgehog inhibition by 6-bromoindirubin-3′-oxime
treatment, which worked as a selective activator of the canonical
Wnt pathway. By contrast, Hedgehog signaling restricts Wnt
activity, using Smoothened agonist purmorphamine, which was
sufficient for activation of Hedgehog signaling. Furthermore, Hh
signaling pathway negatively regulates Wnt activity via
transcriptional regulation of SFRP1, and the Wnt/β-catenin pathway
downregulates Hedgehog activity through et al
transcriptional regulation. The reciprocal inhibition between Hh
and Wnt signaling pathways regulates a delicate balance between
proliferation and differentiation of neural stem cells (83).
Although the Wnt and Hh signaling pathways
participate in the physiological processes of cellular
proliferation and differentiation, recent research has indicated
that the two pathways also serve a crucial role in the pathological
processes of various diseases, particularly malignancies. For
example, enhanced Shh signaling restricts canonical Wnt signaling
in the lambdoidal region by promoting the expression of genes
encoding Wnt inhibitors, which is involved in the development of
cleft lip (85). In addition,
downregulation of Ihh expression may contribute to the activation
of Wnt signaling via APC mutation, and subsequently lead to the
development of colorectal tumors (86). Further studies have indicated that Wnt
signaling may be a downstream pathway of Hh signaling, and that
SFRP1 acts as an important cross-point to repress the canonical Wnt
signaling pathway and restrict the expression of Wnt target genes.
A gene chip assay of squamous cell carcinoma of the uterine cervix
revealed that the expression of Hh signaling molecules was
significantly increased in cervical intraepithelial neoplasia
II/III and carcinoma, while SFRP1 gene expression was silent or
low, which strongly suggests that the differential activation of
the Wnt and Hh pathways may be involved in the development of
uterine cervical carcinoma (87).
GLI3 is known to be a transcriptional repressor of
the Hh signaling pathway in the absence of ligand stimulation
(89). Alvarez-Medina et
al(90) investigated dorsoventral
neuron development, which is achieved by the combined activity of
signaling pathways. In their study, the canonical Wnt signaling
pathway was demonstrated to be important in dorsoventral patterning
of the spinal cord, and this role was largely dependent on GLI
activity. Furthermore, the study revealed that the expression of
GLI3 within the dorsal neural tube is directly controlled by Wnt
activity, as mice with mutated Wnt1 and Wnt3a exhibited diminished
GLI3 expression, and gain and loss of β-catenin/T cell factor
(β-catenin/Tcf) function in chick embryos also directly regulated
GLI3 expression (90). Furthermore,
previous studies characterized four highly conserved non-coding DNA
regions (HCNRs) within the human GLI3 locus that work as potential
enhancer modules; it was demonstrated that HCNR2 and HCNR3 contain
sufficient information to direct the expression of GLI3 in the
dorsal spinal cord, and that the activity of these two modules is
dependent on β-catenin/Tcf transcriptional activity (90–92).
Collectively, these data demonstrate that the Wnt/β-catenin pathway
downregulates GLI3 expression, indicating an indirect mechanism
initiated by Wnt signaling to repress Shh activity in the dorsal
neural tube (90–92).
As a whole, the network of signaling pathways, such
as Hh, Wnt, STAT and NOTCH, is crucial in cellular differentiation
and tissue homeostasis, and its dysregulation may result in tumor
occurrence and metastasis. Studies from numerous laboratories have
made great efforts in exploring the complexity of the regulatory
networks and the interaction between the Hh and Wnt signaling
pathways. Inappropriate activation of Hh and Wnt signaling has been
demonstrated in certain types of cancer. However, the mechanism of
interaction between the two signaling pathways remains unclear, and
several key questions remain to be addressed. Firstly, research has
mainly been limited to cell and animal experiments, and the
findings reviewed in the present study must be demonstrated in
appropriate preclinical investigations. Secondly, some of the
apparently conflicting reports regarding the interactions between
the different signaling pathways must be studied and discussed in
greater depth.
More than 20 years after the discovery of the Hh and
Wnt pathways, we have entered an exciting era of research into
these signaling pathways. A single pathway is susceptible to be
affected by other pathways, and does not fully represent the entire
signaling network. Therefore, further investigation of the
crosstalk between different transcriptional signals could overcome
this limitation of single pathways, and provide a more
comprehensive understanding of the importance of these signaling
pathways in the development of cancer. The therapeutic benefits of
pathway antagonists are gradually being revealed in clinical
studies, and the outcomes may have a far-reaching impact on the
design of novel cancer therapies. In summary, the interactions
between the Hh and Wnt signaling pathways in malignancies may
provide a theoretical basis for potential cancer therapies.
This review article was supported by the National
Natural Science Foundation of China (grant nos. 81270598 and
81473486), the National Public Health Grand Research Foundation
(grant no. 201202017), the Natural Science Foundation of Shandong
Province (grant nos. 2009ZRB14176 and ZR2012HZ003), the Technology
Development Projects of Shandong Province (grant nos. 2010GSF10250
and 2014GSF118021), the Program of Shandong Medical Leading Talent,
and the Taishan Scholar Foundation of Shandong Province.
|
1
|
Parmigiani G, Boca S, Lin J, Kinzler KW,
Velculescu V and Vogelstein B: Design and analysis issues in
genome-wide somatic mutation studies of cancer. Genomics. 93:17–21.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sjöblom T, Jones S, Wood LD, Parsons DW,
Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, et al:
The consensus coding sequences of human breast and colorectal
cancers. Science. 314:268–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Greenman CP, Stephens P, Smith R,
Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A,
Stevens C, et al: Patterns of somatic mutation in human cancer
genomes. Nature. 446:153–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomas RK, Baker AC, Debiasi RM, Winckler
W, Laframboise T, Lin WM, Wang M, Feng W, Zander T, MacConaill L,
et al: High-throughput oncogene mutation profiling in human cancer.
Nat Genet. 39:347–351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mangelberger D, Kern D, Loipetzberger A,
Eberl M and Aberger F: Cooperative Hedgehog-EGFR signaling. Front
Biosci (Landmark Ed). 17:90–99. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsushita S, Onishi H, Nakano K,
Nagamatsu I, Imaizumi A, Hattori M, Oda Y, Tanaka M and Katano M:
Hedgehog signaling pathway is a potential therapeutic target for
gallbladder cancer. Cancer Sci. 105:272–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rubin LL and de Sauvage FJ: Targeting the
Hedgehog pathway in cancer. Nat Rev Drug Discov. 5:1026–1033. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog
pathways. Nat Rev Clin Oncol. 8:1–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karamboulas C and Ailles L: Developmental
signaling pathways in cancer stem cells of sol-id tumors. Biochim
Biophys Acta. 1830:2481–2495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dodge ME and Lum L: Drugging the cancer
stem cell compartment: Lessons learned from the hedgehog and Wnt
signal transduction pathways. Annu Rev Pharmacol Toxicol.
51:289–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
JP IV Morris, Wang SC and Hebrok M: KRAS,
Hedgehog, Wnt and the twisted developmental biology of pancreatic
ductal adenocarcinoma. Nat Rev Cancer. 10:683–695. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Katoh Y and Katoh M: WNT antagonist,
SFRP1, is Hedgehog signaling target. Int J Mol Med. 17:171–175.
2006.PubMed/NCBI
|
|
13
|
Bovolenta P, Esteve P, Ruiz JM, Cisneros E
and Lopez-Rios J: Beyond Wnt inhibition: New functions of secreted
Frizzled-related proteins in development and disease. J Cell Sci.
121:737–746. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nüsslein-Volhard C and Wieschaus E:
Mutations affecting segment number and polarity in Drosophila.
Nature. 287:795–801. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Varjosalo M and Taipale J: Hedgehog
signaling. J Cell Sci. 120:3–6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wilson CW and Chuang PT: Mechanism and
evolution of cytosolic Hedgehog signal transduction. Development.
137:2079–2094. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rohatgi R, Milenkovic L, Corcoran RB and
Scott MP: Hedgehog signal transduction by Smoothened: Pharmacologic
evidence for a 2-step activation process. Proc Natl Acad Sci USA.
106:pp. 3196–3201. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Varjosalo M and Taipale J: Hedgehog:
Functions and mechanisms. Genes Dev. 22:2454–2472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rohatgi R, Milenkovic L and Scott MP:
Patched1 regulate hedgehog signaling at the primary cilium.
Science. 317:372–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marigo V and Tabin CJ: Regulation of
patched by sonic hedgehog in the developing neural tube. Proc Natl
Acad Sci USA. 93:pp. 9346–9351. 1996; View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bellusci S, Furuta Y, Rush MG, Henderson
R, Winnier G and Hogan BL: Involvement of Sonic hedgehog (Shh) in
mouse embryonic lung growth and morphogenesis. Development.
124:53–63. 1997.PubMed/NCBI
|
|
22
|
Hardcastle Z, Mo R, Hui CC and Sharpe PT:
The Shh signalling pathway in tooth development: Defects in Gli2
and Gli3 mutants. Development. 125:2803–2811. 1998.PubMed/NCBI
|
|
23
|
Litingtung Y, Lei L, Westphal H and Chiang
C: Sonic hedgehog is essential to foregut development. Nat Genet.
20:58–61. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
St-Jacques B, Dassule HR, Karavanova I,
Botchkarev VA, Li J, Danielian PS, McMahon JA, Lewis PM, Paus R and
McMahon AP: Sonic hedgehog signaling is essential for hair
development. Curr Biol. 8:1058–1068. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vortkamp A, Lee K, Lanske B, Segre GV,
Kronenberg HM and Tabin CJ: Regulation of rate of cartilage
differentiation by Indian hedgehog and PTH-related protein.
Science. 273:613–622. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bitgood MJ, Shen L and McMahon AP: Sertoli
cell signaling by Desert hedgehog regulates the male germline. Curr
Biol. 6:298–304. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y and Jiang J: Decoding the
phosphorylation code in Hedgehog signal transduction. Cell Res.
23:186–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Merchant JL: Hedgehog signaling in gut
development, physiology and cancer. J Physiol. 590:421–432. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bertrand FE, Angus CW, Partis WJ and
Sigounas G: Developmental pathways in colon cancer: Crosstalk
between WNT, BMP, Hedgehog and Notch. Cell Cycle. 11:4344–4351.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Niu Y, Li F, Tang B, Shi Y, Hao Y and Yu
P: Clinicopathological correlation and prognostic significance of
sonic hedgehog protein overexpression in human gastric cancer. Int
J Clin Exp Pathol. 7:5144–5153. 2014.PubMed/NCBI
|
|
31
|
Kai K, Aishima S and Miyazaki K:
Gallbladder cancer: Clinical and pathological approach. World J
Clin Cases. 2:515–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nigam A: Breast cancer stem cells,
pathways and therapeutic perspectives 2011. Indian J Surg.
75:170–180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hwang J, Kang MH, Yoo YA, Quan YH, Kim HK,
Oh SC and Choi YH: The effects of sonic hedgehog signaling pathway
components on non-small-cell lung cancer progression and clinical
outcome. World J Surg Oncol. 12:2682014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ok CY, Singh RR and Vega F: Aberrant
activation of the hedgehog signaling pathway in malignant
hematological neoplasms. Am J Pathol. 180:2–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Irvine DA and Copland M: Targeting
hedgehog in hematologic malignancy. Blood. 119:2196–2204. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Harwood CA, Attard NR, O'Donovan P,
Chambers P, Perrett CM, Proby CM, McGregor JM and Karran P: PTCH
mutations in basal cell carcinomas from azathioprine-treated organ
transplant recipients. Br J Cancer. 99:1276–1284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Soufir N, Gerard B, Portela M, Brice A,
Liboutet M, Saiag P, Descamps V, Kerob D, Wolkenstein P, Gorin I,
et al: PTCH mutations and deletions in patients with typical nevoid
basal cell carcinoma syndrome and in patients with a suspected
genetic predisposition to basal cell carcinoma: A French study. Br
J Cancer. 95:548–553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nitzki F, Tolosa EJ, Cuvelier N, Frommhold
A, Salinas-Riester G, Johnsen SA, Fernandez-Zapico ME and Hahn H:
Overexpression of mutant Ptch in rhabdomyosarcomas is associated
with promoter hypomethylation and increased Gli1 and H3K4me3
occupancy. Oncotarget. 6:9113–9124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lim CB, Prêle CM, Cheah HM, Cheng YY,
Klebe S, Reid G, Watkins DN, Baltic S, Thompson PJ and Mutsaers SE:
Mutational analysis of hedgehog signaling pathway genes in human
malignant mesothelioma. PLoS One. 8:e666852013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Y, Li S, Tong C, Zhao Y, Wang B, Liu
Y, Jia J and Jiang J: G protein-coupled receptor kinase 2 promotes
high-level Hedgehog signaling by regulating the active state of Smo
through kinase-dependent and kinase-independent mechanisms in
Drosophila. Genes Dev. 24:2054–2067. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou X, Liu Z, Jang F, Xiang C, Li Y and
He Y: Autocrine Sonic hedgehog attenuates inflammation in
cerulein-induced acute pancreatitis in mice via upregulation of
IL-10. PLoS One. 7:e441212012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ertao Z, Jianhui C, Chuangqi C, Changjiang
Q, Sile C, Yulong H, Hui W and Shirong C: Autocrine Sonic hedgehog
signaling promotes gastric cancer proliferation through induction
of phospholipase Cy1 and the ERK1/2 pathway. J Exp Clin Cancer Res.
35:632016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Levi B, James AW, Nelson ER, Li S, Peng M,
Commons GW, Lee M, Wu B and Longaker MT: Human adipose-derived
stromal cells stimulate autogenous skeletal repair via paracrine
Hedgehog signaling with calvarial osteoblasts. Stem Cells Dev.
20:243–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chan IS, Guy CD, Chen Y, Lu J,
Swiderska-Syn M, Michelotti GA, Karaca G, Xie G, Krüger L, Syn WK,
et al: Paracrine Hedgehog signaling drives metabolic changes in
hepatocellular carcinoma. Cancer Res. 72:6344–6350. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Scales SJ and de Sauvage FJ: Mechanisms of
Hedgehog pathway activation in cancer and implications for therapy.
Trends Pharmacol Sci. 30:303–312. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rudin CM, Hann CL, Laterra J, Yauch RL,
Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, et
al: Treatment of medulloblastoma with hedgehog pathway inhibitor
GDC-0449. N Engl J Med. 361:1173–1178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Von Hoff DD, LoRusso PM, Rudin CM, Reddy
JC, Yauch RL, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, et
al: Inhibition of the hedgehog pathway in advanced basal-cell
carcinoma. N Engl J Med. 361:1164–1172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dubey AK, Dubey S, Handu SS and Qazi MA:
Vismodegib: The first drug approved for advanced and metastatic
basal cell carcinoma. J Postgrad Med. 59:48–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Stecca B, Ruiz I and Altaba A:
Context-dependent regulation of the GLI code in cancer by HEDGEHOG
and non-HEDGEHOG signals. J Mol Cell Biol. 2:84–95. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lauth M and Toftgård R: Non-canonical
activation of GLI transcription factors: Implications for targeted
anti-cancer therapy. Cell Cycle. 6:2458–2463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Riobo NA, Lu K and Emerson CP Jr: Hedgehog
signal transduction: Signal integration and cross talk in
development and cancer. Cell Cycle. 5:1612–1615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Muñoz-Descalzo S, Hadjantonakis AK and
Arias AM: Wnt/ß-catenin signalling and the dynamics of fate
decisions in early mouse embryos and embryonic stem (ES) cells.
Semin Cell Dev Biol. 47-48:1–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sokol SY: Maintaining embryonic stem cell
pluripotency with Wnt signaling. Development. 138:4341–4350. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang K, Wang X, Zhang H, Wang Z, Nan G, Li
Y, Zhang F, Mohammed MK, Haydon RC, Luu HH, et al: The evolving
roles of canonical WNT signaling in stem cells and tumorigenesis:
Implications in targeted cancer therapies. Lab Invest. 96:116–136.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mohammed MK, Shao C, Wang J, Wei Q, Wang
X, Collier Z, Tang S, Liu H, Zhang F, Huang J, et al: Wnt/β-catenin
signaling plays an ever-expanding role in stem cell self-renewal,
tumorigenesis and cancer chemoresistance. Genes Dis. 3:11–40. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kalderon D: Similarities between the
Hedgehog and Wnt signaling pathways. Trends Cell Biol. 12:523–531.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
He X, Semenov M, Tamai K and Zeng X: LDL
receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling:
Arrows point the way. Development. 131:1663–1677. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Peifer M and McEwen DG: The ballet of
morphogenesis: Unveiling the hidden choreographers. Cell.
109:271–274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shigemitsu K, Sekido Y, Usami N, Mori S,
Sato M, Horio Y, Hasegawa Y, Bader SA, Gazdar AF, Minna JD, et al:
Genetic alteration of the beta-catenin gene (CTNNBI) in human lung
cancer and malignant mesothelioma and identification of a new
3p21.3 homozygous deletion. Oncogene. 20:4249–4257. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kimura Y, Furuhata T, Mukaiya M, Kihara C,
Kawakami M, Okita K, Yanai Y, Zenbutsu H, Satoh M, Ichimiya S and
Hirata K: Frequent beta-catenin alteration in gallbladder
carcinomas. J Exp Clin Cancer Res. 22:321–328. 2003.PubMed/NCBI
|
|
62
|
Coscio A, Chang DW, Roth JA, Ye Y, Gu J,
Yang P and Wu X: Genetic variants of the Wnt signaling pathway as
predictors of recurrence and survival in early-stage non-small cell
lung cancer patients. Carcinogenesis. 35:1284–1291. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li S, Li S, Sun Y and Li L: The expression
of β-catenin in different subtypes of breast cancer and its
clinical significance. Tumour Biol. 35:7693–7698. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lu D, Choi MY, Yu J, Castro JE, Kipps TJ
and Carson DA: Salinomycin inhibits Wnt signaling and selectively
induces apoptosis in chronic lymphocytic leukemia cells. Proc Matl
Acad Sci USA. 108:pp. 13253–13257. 2011; View Article : Google Scholar
|
|
65
|
Price MA: CKI, there's more than one:
casein kinase I family members in Wnt and Hedgehog signaling. Genes
Dev. 20:399–410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rhee CS, Sen M, Lu D, Wu C, Leoni L, Rubin
J, Corr M and Carson DA: Wnt and frizzled receptors as potential
targets for immunotherapy in head and neck squamous cell
carcinomas. Oncogene. 21:6598–6605. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Collu GM, Hidalgo-Sastre A and Brennan K:
Wnt-Notch signaling crosstalk in development and disease. Cell Mol
Life Sci. 71:3553–3567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xu F, Zhang J and Ma D: Crosstalk of
Hippo/YAP and Wnt/β-catenin pathways. Yi Chuan. 36:95–102. 2014.(In
Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shimobayashi M and Hall MN: Making new
contacts: The mTOR network in metabolism and signalling crosstalk.
Nat Rev Mol Cell Biol. 15:155–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Moussaif M and Sze JY: Intraflagellar
transport/Hedgehog-related signaling components couple sensory
cilium morphology and serotonin biosynthesis in Caenorhabditis
elegans. J Neurosci. 29:4065–4075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Brás-Pereira C, Potier D, Jacobs J, Aerts
S, Casares F and Janody F: dachshund Potentiates Hedgehog Signaling
during Drosophila Retinogenesis. PLoS Genet. 12:e10062042016.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Poss KD, Keating MT and Nechiporuk A:
Tales of regeneration in zebrafish. Dev Dyn:. 226:202–210. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Akimenko MA, Mari-Beffa M, Becerra J and
Géraudie J: Old questions, new tools, and some answers to the
mystery of fin regeneration. Dev Dyn. 226:190–201. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Stoick-Cooper CL, Weidinger G, Riehle KJ,
Hubbert C, Major MB, Fausto N and Moon RT: Distinct Wnt signaling
pathways have opposing roles in appendage regeneration.
Development. 134:479–489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Singh BN, Doyle MJ, Weaver CV,
Koyano-Nakagawa N and Garry DJ: Hedgehog and Wnt coordinate
signaling in myogenic progenitors and regulate limb regeneration.
Dev Biol. 371:23–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Day TF and Yang Y: Wnt and hedgehog
signaling pathways in bone development. J Bone Joint Surg Am. 90
Suppl 1:S19–S24. 2008. View Article : Google Scholar
|
|
77
|
Liu B, Chen S, Cheng D, Jing W and Helms
JA: Primary cilia integrate hedgehog and Wnt signaling during tooth
development. J Dent Res. 93:475–482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Oberhofer G, Grossmann D, Siemanowski JL,
Beissbarth T and Bucher G: Wnt/β-catenin signaling integrates
patterning and metabolism of the insect growth zone. Development.
141:4740–4750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Shin K, Lee J, Guo N, Kim J, Lim A, Qu L,
Mysorekar IU and Beachy PA: Hedgehog/Wnt feedback supports
regenerative proliferation of epithelial stem cells in bladder.
Nature. 472:110–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xu Q, D'Amore PA and Sokol SY: Functional
and biochemical interactions of Wnts with FrzA, a secreted Wnt
antagonist. Development. 125:4767–4776. 1998.PubMed/NCBI
|
|
81
|
He J, Sheng T, Stelter AA, Li C, Zhang X,
Sinha M, Luxon BA and Xie J: Suppressing Wnt signalling by the
hedgehog pathway through sFRP-1. J Biol Chem. 281:35598–35602.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Alvarez-Medina R, Le Dreau G, Ros M and
Marti E: Hedgehog activation is required upstream of Wnt signalling
to control neural progenitor proliferation. Development.
136:3301–3309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Borday C, Cabochette P, Parain K, Mazurier
N, Janssens S, Tran HT, Sekkali B, Bronchain O, Vleminckx K, Locker
M and Perron M: Antagonistic cross-regulation between Wnt and
Hedgehog signalling pathways controls post-embryonic retinal
proliferation. Development. 139:3499–3509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kim JH, Shin HS, Lee SH, Lee I, Lee YS,
Park JC, Kim YJ, Chung JB and Lee YC: Contrasting activity of
Hedgehog and Wnt pathways according to gastric cancer cell
differentiation: Relevance of crosstalk mechanisms. Cancer Sci.
101:328–335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kurosaka H, Lulianella A, Williams T and
Trainor PA: Disrupting hedgehog and WNT signaling interactions
promotes cleft lip pathogenesis. J Clin Invest. 124:1660–1671.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fu X, Shi L, Zhang W, Zhang X, Peng Y,
Chen X, Tang C, Li X and Zhou X: Expression of Indian hedgehog is
negatively correlated with APC gene mutation in colorectal tumors.
Int J Clin Exp Med. 7:2150–2155. 2014.PubMed/NCBI
|
|
87
|
Xuan YH, Jung HS, Choi YL, Shin YK, Kim
HJ, Kim KH, Kim WJ, Lee YJ and Kim SH: Enhanced expression of
hedgehog signaling molecules in squamous cell carcinoma of uterine
cervix and its precursor lesions. Mod Patho. 19:1139–1147.
2006.
|
|
88
|
Yanai K, Nakamura M, Akiyoshi T, Nagai S,
Wada J, Koga K, Noshiro H, Nagai E, Tsuneyoshi M, Tanaka M and
Katano M: Crosstalk of hedgehog and Wnt pathways in gastric cancer.
Cancer Lett. 263:145–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Jacob J and Briscoe J: Gli proteins and
the control of spinal-cord patterning. EMBO Rep. 4:761–765. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Alvarez-Medina R, Cayuso J, Okubo T,
Takada S and Marti E: Wnt canonical pathway restricts graded
Shh/Gli patterning activity through the regulation of Gli3
expression. Development. 135:237–247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Muroyama Y, Fujihara M, Ikeya M, Kondoh H
and Takada S: Wnt signalling plays an essential role in neuronal
specification of the dorsal spinal cord. Genes Dev. 16:548–553.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Abbasi AA, Paparidis Z, Malik S, Goode DK,
Callaway H, Elgar G and Grzeschick KH: Human GLI3 intragenic
conserved non-coding sequences are tissue-specific enhancers. PLoS
One. 2:e3662007. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Meijer L, Skaltsounis AL, Magiatis P,
Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA,
Brivanlou A, Dajani R, et al: GSK-3-selective inhibitors derived
from Tyrian purple indirubins. Chem Biol. 10:1255–1266. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan
CW, Wei S, Hao W, Kilgore J, Williams NS, et al: Small
molecule-mediated disruption of Wnt-dependent signalling in tissue
regeneration and cancer. Nat Chem Biol. 5:100–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Pöschl J, Bartels M, Ohli J, Bianchi E,
Kuteykin-Teplyakov K, Grammel D, Ahlfeld J and Schüller U:
Wnt/β-catenin signaling inhibits the Shh pathway and impairs tumor
growth in Shh-dependent medulloblastoma. Acta Neuropathol.
127:605–607. 2014. View Article : Google Scholar : PubMed/NCBI
|