Introduction
Chronic myeloid leukemia (CML) is caused by a
reciprocal translocation in a hematopoietic stem cell between
chromosomes 9 and 22, resulting in the bcr-abl1 fusion gene.
The bcr-abl1 fusion gene encodes a constitutively active
tyrosine kinase. Currently, the tyrosine kinase inhibitors (TKIs)
imatinib, dasatinib, and nilotinib are commonly used as the first
line treatment for chronic phase (CP)-CML treatment (1–3). Each TKI
can affect multiple kinases with different activities (4). These TKIs induce a high rate of deep
molecular response, and approximately half of CP-CML patients who
cease TKI therapy can achieve treatment-free remission (TFR)
(5–7).
The lack of relapse in such patients may be brought about by
immunological control of CML. The effectiveness of allogeneic donor
lymphocyte infusion in relapsed CML patients after allogeneic
hematopoietic stem cell transplantation suggests that lymphocytes
can produce an anti-CML effect (8).
An increased number of lymphocytes has been found in patients
treated with dasatinib, and these lymphocytes were reported to be
natural killer (NK) cells and cytotoxic T lymphocytes (CTLs)
(9,10). However, no detailed analysis on
cellular immunity in CP-CML patients who cease TKI therapy has been
performed. Here, we report a case of long-lasting memory of
cellular immunity against CML.
Materials and methods
bcr-abl1 expression analyses
mRNA extracted from bone marrow aspirates or
peripheral blood samples was subjected to bcr-abl1
expression analyses. Quantitative analyses of major bcr-abl1
mRNA were performed using mRNA extracted from bone
marrow aspirates or peripheral blood samples until June 2015, and
the International Scale (IS) method of major bcr-abl1 mRNA
analyses using peripheral blood samples has been applied since July
2015 (11). All analyses were
performed by the SRL Corporation (Tokyo, Japan). We defined
molecular response (MR) 3 as ≤0.1%, MR4 as ≤0.01%, and MR5 as
≤0.001% of major bcr-abl1 expression, respectively.
Cellular immunity analyses
Cellular immunity analyses were conducted using flow
cytometry. Antibodies for assays of NK cells and CTLs were
purchased from DACO Japan (Tokyo, Japan). IOTest Beta Mark T cell
receptor (TCR) Vβ (Vb) repertoire kit (Beckman Coulter, Tokyo,
Japan) was used for TCR Vb gene repertoire assays in
CD8+ T cells. We performed flow cytometric analyses
according to the manufacturers' instructions.
Informed consent
Informed consent was obtained from the patient's son
in Case 1, because the patient's consent could not be obtained due
to dementia. Informed consent was obtained from the patients
described in Cases 2 and 3.
Case reports
Case 1
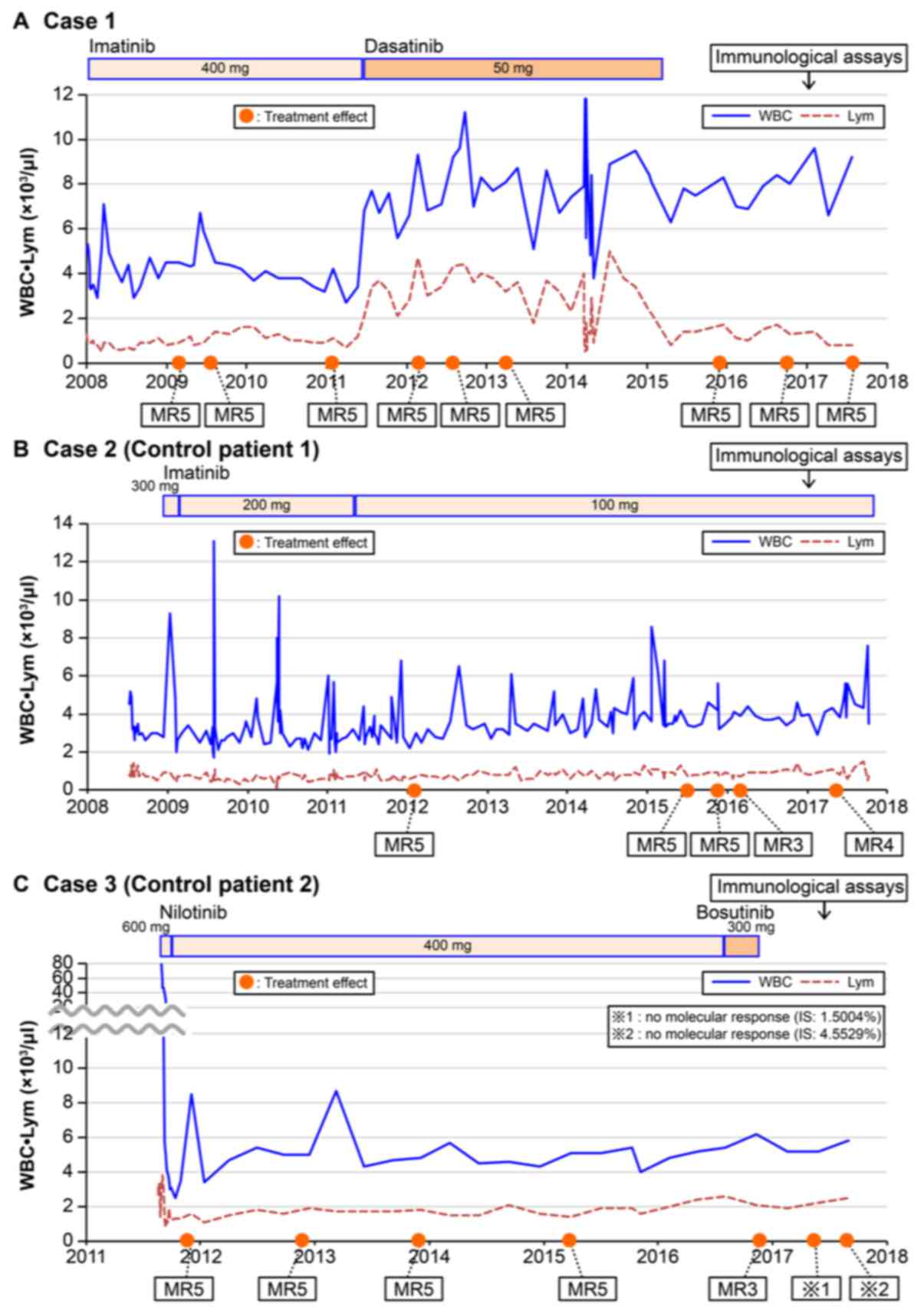
A 75-year-old woman was diagnosed with CP-CML, and
400 mg/day of imatinib, the first generation TKI, was started in
January 2008. The results of gene expression analyses using
mRNA extracted from bone marrow aspirates in April 2009 and
February 2011 revealed absence of bcr-abl1, indicating MR5.
Fifty milligrams of dasatinib, a second generation TKI, was
administered instead of imatinib starting in May 2011 due to
cramping in the calf that was thought to be an adverse effect of
imatinib. Absence of bcr-abl1 was reconfirmed using
mRNA extracted from bone marrow aspirates in February 2012
and April 2013. She suffered from subdural hematoma due to a fall
in January 2015, and dasatinib was stopped in March 2015. Absence
of bcr-abl1 was reconfirmed using mRNA extracted from
peripheral blood in July 2017, more than 2.4 years after stopping
dasatinib. Interestingly, elevation of lymphocyte count was
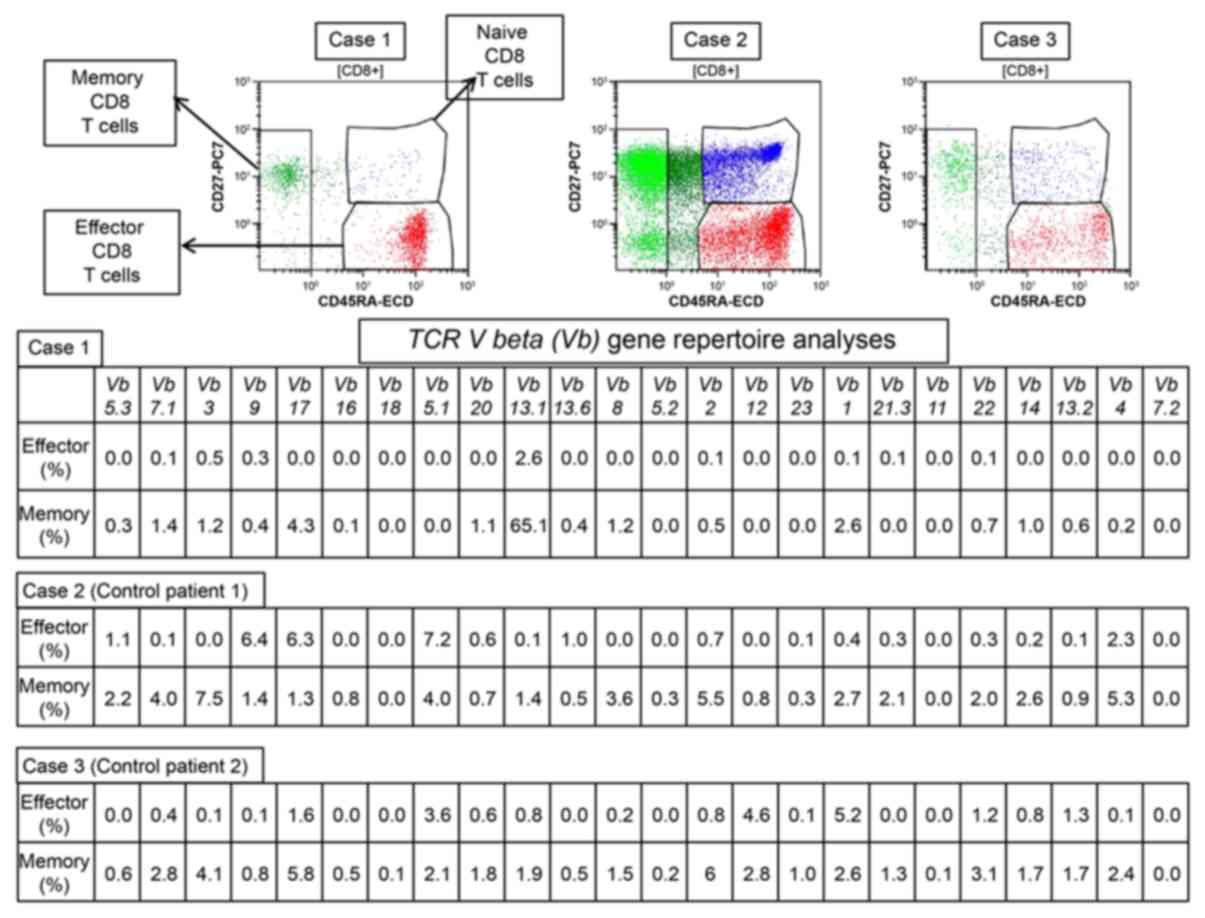
observed only when the patient was receiving dasatinib (Fig. 1). Cellular immunity, represented by
the NK cell, CTL, and regulatory T (Treg) cell populations, was
investigated in July and August 2017. The percentage of NK cells,
defined as CD3−CD16+CD56+, was
54.5%. The percentages of effector memory CTLs, defined as
CD8+CD27+CD57−, and terminal
effector CTLs, defined as
CD8+CD27−CD57+, were 14.3 and
24.2%, respectively (Table I). A
TCR Vb gene repertoire assay showed that Vb13.1 was
the most common gene variant in both effector and memory
CD8+ T cells (Fig. 2). The
rate of Vb13.1 in memory CD8+ T cells was
extremely high (65.1%). The percentages of effector and naïve Treg
were 1.1 and 0.9%, respectively, but we could not evaluate the
changes in these populations because Tregs were not measured during
TKI treatment.
 | Table I.Percentage of NK cells, effector
memory CTLs and terminal effector CTLs in the patient and
controls. |
Table I.
Percentage of NK cells, effector
memory CTLs and terminal effector CTLs in the patient and
controls.
| Cell type | Case 1 | Case 2 (control
patient 1) | Case 3 (control
patient 2) |
|---|
| NK cells (%) | 54.5 | 24.1 | 16.6 |
| Effector memory CTLs
(%) | 14.3 | 6.4 | 21.8 |
| Terminal effector
CTLs (%) | 24.2 | 11.6 | 7.4 |
Case-2 (control patient 1)
A 72-year-old woman was diagnosed with CP-CML, and
600 mg/day of imatinib was started in June 2004. Three months
later, imatinib was stopped due to skin rash. Three hundred mg/day
of imatinib was started again due to disease progression in July
2008 with no further evidence of skin rash. The dose of imatinib
was reduced gradually to 100 mg/day according to the patient's
will. Absence of bcr-abl1 mRNA was confirmed through
December 2015. However, the molecular response level decreased from
MR5 to MR3 (March 2016) and MR4 (September 2017). The patient's
clinical course beginning in July 2008 is illustrated in Fig. 1B.
Case 3 (control patient 2)
A 50-year-old man was diagnosed with CP-CML, and 600
mg/day of nilotinib, a second generation TKI, was started in August
2011. Three weeks later, the dose of nilotinib was reduced to 400
mg/day due to slight leukocytopenia and the dose was maintained
until August 2016. Nilotinib was changed to 300 mg/day of
bosutinib, a second generation TKI, due to nilotinib-induced skin
rash in August 2016. However, bosutinib was stopped due to
bosutinib-induced diarrhea. After that, no TKIs have been
administered, according to the patient's will. Although absence of
bcr-abl1 mRNA was confirmed through March 2015, the
molecular response levels decreased from MR5 to MR3 (November
2016), and later, the patient had no molecular response (May and
September 2017) (Fig. 1C).
Discussion
TKIs such as imatinib, dasatinib, and nilotinib have
made a drastic impact on survival in patients with CP-CML (1–3). These
patients now have life expectancies comparable to patients with
lifestyle-related diseases such as hypertension and diabetes
mellitus (12). However, TKIs cannot
completely eliminate CML stem cells, and patients must continue
treatment, which can be expensive. Some previous reports regarding
cessation of TKIs showed that approximately half of patients could
safely stop treatment under stringent treatment conditions
(5–7);
however, the parameters for safely stopping TKI treatment are still
unclear. In contrast, patients who obtained TFR after interferon
alpha treatment showed upregulation of cellular immunity, including
CD8+ T cells and NK cells (13–15).
Furthermore, allogeneic donor lymphocyte infusion has been reported
to be effective in relapsed CML patients (8). These data suggest that upregulation of
cellular immunity is critical to eliminate CML stem cells and
obtain TFR. All three TKIs (imatinib, dasatinib, and nilotinib) can
inhibit not only BCR-ABL but also other tyrosine kinases, such as
KIT, PDGFR, and SRC (4). However, the
increase in large granular lymphocytes (LGLs) has only been
reported in patients treated with dasatinib (16). Activation of NK cells and CTLs also
was observed in those patients (9,10). By
contrast, clonal expansion of TCR Vb genes in patients who
receive multiple TKI therapies has been reported using polymerase
chain reaction (PCR)-based clonality testing (44% in
dasatinib-treated patients, 46% in imatinib-treated patients, and
33% in nolitinib-treated patients) (17).
Cessation of dasatinib treatments induces a rapid
decline in the LGL counts, almost to the baseline levels (18). Similarly, in Case 1, we observed an
elevation of lymphocytes immediately after changing to dasatinib
treatment, and a decrease in lymphocyte levels was observed soon
after cessation of dasatinib treatment (Fig. 1A), while there was no obvious
elevation of lymphocytes observed in Cases 2 and 3 (Fig. 1B and C). The immunological analyses of
Case 1 showed not only maintenance of memory and effector CTL
activation and NK cells, but also TCR clonality more than 2.4 years
from cessation of dasatinib, despite the absence of lymphocyte
elevation (Figs. 1A and 2, Table I). NK
cells and CTLs were also analyzed in Cases 2 and 3 (Table I and Fig.
2); the percentages of NK cells and terminal effector CTLs were
higher in Case 1 compared to Case 2 and 3, while there were no
differences between effector memory T cells among the three cases
(Table I). TCR Vb gene
analysis revealed that there was marked elevation of Vb13.1
in memory CD8+ T cells in Case 1 (65.1%), but no such
elevation of TCR Vb genes was observed in the two control
patients (Fig. 2). These data suggest
that the maintenance of cellular immunity against CML protected the
patient from CML relapse and provided a cure. Although LGLs were
not observed in the patients treated with imatinib and nilotinib,
TCR clonality was observed in approximately 45% of these patients
by PCR-based TCR repertoire analyses (17). Both control patients could not retain
long-term MR5 status, which may be related to the absence of
significant clonality in the TCR repertoire of memory
CD8+ T cells. These data suggest that the activation of
CTLs against CML plays an important role in obtaining TFR in
patients treated with TKIs. If this speculation is correct, testing
the TCR Vb repertoire may be a useful tool to consider
whether TKI treatment can be safely stopped under stringent
treatment conditions.
In summary, we report a case of MR5 more than 2.4
years after cessation of dasatinib. Maintenance of memory and
effector CTLs with TCR clonality as well as NK cells was observed
in this case. These data suggest that clonal expansion of CTLs
against CML cells may be a meaningful marker for cessation of
TKIs.
Acknowledgements
The authors would like to thank Ms. Kana Okabe for
her editorial work.
References
|
1
|
O'Brien SG, Guilhot F, Larson RA, Gathmann
I, Baccarani M, Cervantes F, Cornelissen JJ, Fischer T, Hochhaus A,
Hughes T, et al: Imatinib compared with interferon and low-dose
cytarabine for newly diagnosed chronic-phase chronic myeloid
leukemia. N Engl J Med. 348:994–1004. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kantarjian H, Shah NP, Hochhaus A, Cortes
J, Shah S, Ayala M, Moiraghi B, Shen Z, Mayer J, Pasquini R, et al:
Dasatinib versus imatinib in newly diagnosed chronic-phase chronic
myeloid leukemia. N Engl J Med. 362:2260–2270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saglio G, Kim DW, Issaragrisil S, le
Coutre P, Etienne G, Lobo C, Pasquini R, Clark RE, Hochhaus A,
Hughes TP, et al: Nilotinib versus imatinib for newly diagnosed
chronic myeloid leukemia. N Engl J Med. 362:2251–2259. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hantschel O, Rix U and Superti-Furga G:
Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and
dasatinib. Leuk Lymphoma. 49:615–619. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mahon FX, Réa D, Guilhot J, Guilhot F,
Huguet F, Nicolini F, Legros L, Charbonnier A, Guerci A, Varet B,
et al: Discontinuation of imatinib in patients with chronic myeloid
leukaemia who have maintained complete molecular remission for at
least 2 years: The prospective, multicentre Stop Imatinib (STIM)
trial. Lancet Oncol. 11:1029–1035. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ross DM, Branford S, Seymour JF, Schwarer
AP, Arthur C, Yeung DT, Dang P, Goyne JM, Slader C, Filshie RJ, et
al: Safety and efficacy of imatinib cessation for CML patients with
stable undetectable minimal residual disease: Results from the
TWISTER study. Blood. 122:515–522. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Imagawa J, Tanaka H, Okada M, Nakamae H,
Hino M, Murai K, Ishida Y, Kumagai T, Sato S, Ohashi K, et al:
Discontinuation of dasatinib in patients with chronic myeloid
leukaemia who have maintained deep molecular response for longer
than 1 year (DADI trial): A multicentre phase 2 trial. Lancet
Haematol. 2:e528–e535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kolb HJ, Schattenberg A, Goldman JM,
Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A,
Verdonck L, Niederwieser D, et al: Graft-versus-leukemia effect of
donor lymphocyte transfusions in marrow grafted patients. Blood.
86:2041–2050. 1995.PubMed/NCBI
|
|
9
|
Mustjoki S, Ekblom M, Arstila TP, Dybedal
I, Epling-Burnette PK, Guilhot F, Hjorth-Hansen H, Höglund M,
Kovanen P, Laurinolli T, et al: Clonal expansion of T/NK-cells
during tyrosine kinase inhibitor dasatinib therapy. Leukemia.
23:1398–1405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim DH, Kamel-Reid S, Chang H, Sutherland
R, Jung CW, Kim HJ, Lee JJ and Lipton JH: Natural killer or natural
killer/T cell lineage large granular lymphocytosis associated with
dasatinib therapy for Philadelphia chromosome positive leukemia.
Haematologica. 94:135–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
White HE, Matejtschuk P, Rigsby P, Gabert
J, Lin F, Lynn Wang Y, Branford S, Müller MC, Beaufils N, Beillard
E, et al: Establishment of the first World Health Organization
International Genetic Reference Panel for quantitation of BCR-ABL
mRNA. Blood. 116:e111–e117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sasaki K, Strom SS, O'Brien S, Jabbour E,
Ravandi F, Konopleva M, Borthakur G, Pemmaraju N, Daver N, Jain P,
et al: Relative survival in patients with chronic-phase chronic
myeloid leukaemia in the tyrosine-kinase inhibitor era: Analysis of
patient data from six prospective clinical trials. Lancet Haematol.
2:e186–e193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rohon P, Porkka K and Mustjoki S:
Immunoprofiling of patients with chronic myeloid leukemia at
diagnosis and during tyrosine kinase inhibitor therapy. Eur J
Haematol. 85:387–398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kreutzman A, Rohon P, Faber E, Indrak K,
Juvonen V, Kairisto V, Voglová J, Sinisalo M, Flochová E, Vakkila
J, et al: Chronic myeloid leukemia patients in prolonged remission
following interferon-α monotherapy have distinct cytokine and
oligoclonal lymphocyte profile. PLoS One. 6:e230222011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Castro FA, Palma PV, Morais FR, Simões
BP, Carvalho PV, Ismael SJ, Lima CP and Voltarelli JC:
Immunological effects of interferon-alpha on chronic myelogenous
leukemia. Leuk Lymphoma. 44:2061–2067. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schiffer CA, Cortes JE, Hochhaus A, Saglio
G, le Coutre P, Porkka K, Mustjoki S, Mohamed H and Shah NP:
Lymphocytosis after treatment with dasatinib in chronic myeloid
leukemia: Effects on response and toxicity. Cancer. 122:1398–1407.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Powers JJ, Dubovsky JA, Epling-Burnette
PK, Moscinski L, Zhang L, Mustjoki S, Sotomayor EM and
Pinilla-Ibarz JA: A molecular and functional analysis of large
granular lymphocyte expansions in patients with chronic myelogenous
leukemia treated with tyrosine kinase inhibitors. Leuk Lymphoma.
52:668–679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kreutzman A, Juvonen V, Kairisto V, Ekblom
M, Stenke L, Seggewiss R, Porkka K and Mustjoki S: Mono/oligoclonal
T and NK cells are common in chronic myeloid leukemia patients at
diagnosis and expand during dasatinib therapy. Blood. 116:772–782.
2010. View Article : Google Scholar : PubMed/NCBI
|