Introduction
Gastric cancer (GC) is currently the fourth most
common cancer and the second most common cause of cancer-associated
mortality worldwide (1). In spite of
the continuous development of novel treatment options for GC, the
prognosis of this disease remains generally poor. The
carcinogenesis and progression of GC are complicated processes,
among which the detailed molecular mechanisms have not been
elaborated thus far. The role of long non-coding RNAs (lncRNAs) in
the carcinogenesis and progression of tumors has been receiving
increasing attention. lncRNAs, a type of non-coding RNA of >200
nucleotides, lack an open reading frame and are unable to encode
proteins; however, they are involved in the regulation of cellular
development, proliferation, differentiation and apoptosis (2). The dysregulation of lncRNAs has been
confirmed to be associated with tumor initiation, progression,
invasion and metastasis in various types of cancer (3). Therefore, comprehensive investigation of
the lncRNA function will provide a novel insight into the
diagnosis, prognosis and targeted therapy for cancer.
Colon cancer-associated transcript 2 (CCAT2), a
novel lncRNA first identified by Ling et al (4), encompasses the rs6983267 single
nucleotide polymorphism (SNP) and is highly overexpressed in
microsatellite-stable colorectal cancer. A meta-analysis
demonstrated that high expression of CCAT2 may serve as a novel
biomarker of poor prognosis and metastasis in various cancer types
(5). Two previous experimental
studies examining the association of CCAT2 and GC confirmed that a
high expression of CCAT2 was closely associated with a higher
incidence of lymph node and distant metastases, as well as shorter
overall and progression-free survival rates (6,7). However,
the molecular contributions of CCAT2 to GC progression remain
largely unclear.
In the present study, it was attempted to illustrate
the function of CCAT2 by the construction of an interference short
hairpin RNA (shRNA) plasmid targeting CCAT2. In addition, the
effect of this plasmid on the proliferation, apoptosis and
autophagy of GC cells, as well as the potential underlying
molecular mechanisms, were investigated.
Materials and methods
Construction of shRNA plasmid
targeting CCAT2
An interference sequence was designed according to
the sequence of CCAT2 in GenBank (NC_000008.11), and Basic Local
Alignment Search Tool Analysis (blast.ncbi.nlm.nih.gov/Blast.cgi) indicated no
homology with other genes. shRNA was synthesized by two
complementary oligonucleotide strands, as follows:
5′-CGCGGGATCCCGGTGCAACTCTGCAATTTAATTTTCAAGAGAAATTAAATTGCAGAGTTGCACTTTTTTCCAAAAGCTTAA-3′
(sense) and
5′-AACTTAAGCTTTTGGAAAAAAGTGCAACTCTGCAATTTAATTTCTCTTGAAAATTAAATTGCAGAGTTGCACCGGGATCCC-3′
(antisense). Double-stranded oligonucleotides (ds-oligo) of shRNA
were obtained subsequent to annealing at 95°C for 5 min. Plasmid
pRNAT-U6.1 (GenScript, Jiangsu, China) and shRNA ds-oligo were
digested using the enzymes BamHI and HindIII (Takara
Biotechnology Co., Ltd., Dalian, China) simultaneously in a water
bath of 37°C for 30 min. The reaction system of dual enzyme
digestion included 1 µl BamHI, 1 µl HindIII, 2 µl 10X
K buffer, 5 µl pRNAT-U6.1 plasmid or shRNA ds-oligo, and 11 µl
ddH2O. DNA was purified and recovered according to the
instructions of the DNA gel extraction kit (Tiangen Biotech Co.,
Ltd., Beijing, China). Next, T4 DNA ligase (NEB, Beijing, China)
was used to clone the shRNA ds-oligo into the linear plasmid
pRNAT-U6.1, and the reaction system of DNA directional ligation was
built following the manufacturer's protocol. The products were
transformed into Trans5α competent cells (Tiangen Biotech Co.,
Ltd.), and then monoclonal clones were identified with polymerase
chain reaction (PCR) and enzyme digestion. Briefly, Luria-Bertani
bacterial liquid medium was added to the 96-well plate (200
µl/well). Monoclonal transformants were randomly selected in the
bacterial culture plate and added to the wells of 96-well plate
with sterile toothpicks. Following incubation in a bacterial
incubator for 1 h at 37°C, the bacterial liquid was used for PCR
identification. Reaction system of PCR included 10 µl 2X Taq Master
Mix (Lifefeng Biotechnology Co., Ltd., Shanghai, China), 2 µl 10 µM
primer mixture, 1 µl bacteria liquid, and 7 µl ddH2O.
The following primers were used: The following primers for PCR
identification were used: forward, 5′-GGATCCCGGTGCAACTCTG-3′ and
reverse, 5′-AAGGCACAGTCGAGGCTGAT-3′ (Chongqing Western Biological
Medicine Science and Technology Co., Ltd., Chongqing, China). PCR
reaction conditions were as follows: 95°C for 5 min; 94°C for 30
sec, 60°C for 30 sec, 72°C for 10 min, 30 cycles. The PCR products
were analyzed using 1% agarose gel electrophoresis. Positive clones
were considered when there were DNA bands. The steps of enzyme
digestion were the same as those methods mentioned above, and the
products were added to 1% agarose gel electrophoresis. Positive
clones were considered when there were DNA fragments 69 bp long.
The positive clone was further sequenced to identify the
successfully constructed recombinant pRNAT-U6.1-CCAT2.
Cell culture and transfection
The human BGC-823 cell line was purchased from the
Type Culture Collection of the Chinese Academy of Sciences
(Beijing, China). The cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 2 mM glutamine, 100 U/ml penicillin and 100
µg/ml streptomycin, and maintained at 37°C in an atmosphere of 95%
O2 and 5% CO2. The experimental groups were
as follows: i) Blank control (BC) group, untransfected BGC-823
cells; ii) negative control (NC) group, BGC-823 cells transfected
with the empty pRNAT-U6.1 plasmid; and iii) interference group,
BGC-823 cells transfected with the pRNAT-U6.1-CCAT2 plasmid. Prior
to transfection, BGC-823 cells (1×105/ml) were grown in
24-well plates until 70–80% confluence was reached. Cell
transfection was performed using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Briefly, 2 µl/well
Lipofectamine® 2000 or 0.8 µg vector DNA was diluted to
a final volume of 50 µl using Opti-MEM medium (Invitrogen; Thermo
Fisher Scientific, Inc.), respectively. The diluent of vector DNA
was added to Lipofectamine® 2000, and then the mixture
were incubated for 20 min at room temperature. The culture medium
was removed from the plate, and then the transfection complex was
added. Following incubation for 4–6 h at 37°C, fresh medium was
used for further cell culturing.
Cell proliferation assays
BGC-823 cells in the logarithmic growth phase were
counted to adjust the cell concentration to 1×105/ml.
Next, the cells were seeded into 96-well plates with 100 µl/well,
transfected with the plasmid based on the experimental grouping and
then cultured for 12, 24 and 48 h. Triplicate wells were used for
each group. The cell proliferation was assessed using an MTT Cell
Proliferation Reagent kit (Beyotime Institute of Biotechnology,
Jiangsu, China) following the manufacturer's instructions. Briefly,
10 µl MTT (5 g/l) was added to each well subsequent to culture for
12, 24 or 48 h, and the cells were maintained in the culture medium
for 4 h. Next, 150 µl dimethyl sulfoxide per well was added
following the removal of the culture medium. The 96-well plate was
then placed in an oscillator for low speed oscillation for 10 min.
Subsequently, the absorbance of each well at 490 nm was detected
using a microplate reader, and the cell survival was calculated as
follows: Cell survival rate (%) = (absorbance of experimental
group)/(absorbance of untransfected blank control group) ×
100%.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
BGC-823 cells were harvested at 48 h after
transfection. Total RNA was isolated from the cultured cells using
TRIzol® reagent (Shinegene Molecular Biotech,
Inc., Shanghai, China). RT of total RNA into first-strand cDNA and
qPCR analysis were performed using RT kit (Chongqing Western
Biological Medicine Science and Technology Co., Ltd., Chongqing,
China) and Shinegene Real Time PCR Core kit (Shinegene Molecular
Biotech, Inc.), respectively, according to the manufacturer's
instructions.
The reaction system of RT assay included 10 µl 2X RT
buffer, 1 µl 6N random primer (100 pmol/µl), 1 µl RT-Mix, 5 µl RNA
and 3 µl RNase-free ddH2O. The RT reaction was conducted
at 25°C for 10 min, 42°C for 50 min and then 85°C for 5 min.
Subsequently, qPCR was performed in a 50 µl reaction system
containing 25 µl 2X PCR buffer, 1 µl each primer (25 pmol/µl), 0.5
µl SYBR Green I (Shinegene Real Time PCR Core kit; Shinegene), 2 µl
cDNA and 20.5 µl RNase-free ddH2O. The house keeping
gene β-actin mRNA was used to normalize the level of cDNA. The
following primers were used in the qPCR assay: CCAT2 forward,
5′-AAGAGGAAACCACCTTGGACTG-3′ and reverse,
5′-GCAATAAGAGCAGGAAAAGAAGC-3′; POU domain class 5 transcription
factor 1B (POU5F1B) forward, 5′-GCGATCAAGCAGCGACTATG-3′ and
reverse, 5′-CAGGGAAAGGGACTGAGGAG-3′; and β-actin forward,
5′-TGACGTGGACATCCGCAAAG-3′ and reverse, 5′-CTGGAAGGTGGACAGCGAGG-3′.
The qPCR reaction conditions were as follows: 94°C for 4 min,
followed by 35 cycles of amplification at 94°C for 20 sec, 60°C for
15 sec and 72°C for 30 sec. All samples were amplified in
triplicates. The results were evaluated with the Bio-Rad CFX
Manager software (version 3.1; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The quantification cycle (Cq) value was read,
and the relative expression of target gene was calculated according
to the 2−ΔΔCq method (8).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
BGC-823 cells were seeded in the 6-well plates
containing sterile coverslips, and cultured 48 h after plasmid
transfection. Subsequent to fixing using 4% paraformaldehyde for 30
min, apoptotic cells were detected with an In Situ Cell Apoptosis
Detection kit (Boster Biological Technology, Pleasanton, CA, USA)
according to the method described by Zhao et al (9). Apoptotic cells exhibited positive
staining (pale brown) in cell nucleus. Five high power fields of
each slide at a magnification of ×400 were randomly examined under
a light microscope to determine the percentage of apoptotic cells
(expressed as the apoptotic index) among at least 500 cells.
Western blot analysis
The culture medium of BGC-823 cells was discarded 48
h after transfection. Cells were then lysed and total proteins were
extracted using a radioimmunoprecipitation assay protein extraction
reagent (Beyotime Institute of Biotechnology). Next, the protein
concentration was quantified using a bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology). Equal amounts (20
µg) of protein extracts were separated by 10% SDS-PAGE, and then
transferred to polyvinylidene fluoride membranes (Bio-Rad
Laboratories, Inc.). Subsequent to blocking with 5% skim-milk for 2
h at room temperature, the membranes were incubated with rabbit
polyclonal antibodies against beclin-1 (1:500; cat. no. ab62557),
mammalian target of rapamycin (mTOR; 1:50; cat. no. ab2732),
phosphoinositide 3-kinase (PI3K; 1:500; cat. no. ab151549) and
β-actin (1:1,000; cat. no. ab8227; all from Abcam, Cambridge, UK)
at 4°C overnight. The membranes were then washed in Tris-buffered
saline/Tween-20 and incubated with goat anti-rabbit immunoglobulin
G (1:1,000; cat. no. A0545; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) for 2 h at 37°C. Protein bands were visualized using the
BeyoECL Plus reagent (Beyotime Institute of Biotechnology), and the
fluorescence intensity was quantified using the ImageJ v.2.1.4.7
software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation. Differences among groups were tested by one-way analysis
of variance, followed by Student-Newman-Keuls test. Statistical
analyses were performed using SPSS v.22.0 software (IBM Corp.,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference. Graphs were constructed with
GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA,
USA).
Results
Construction of pRNAT-U6.1-CCAT2
interference plasmid
In the present study, a recombinant shRNA
interference plasmid was constructed to investigate the function of
CCAT2 gene in GC cells. The positive clones were sequenced and
compared with target DNA sequencing, and the results demonstrated
that the inserted DNA fragment was identical to the expected CCAT2
interference sequence, suggesting that the interference plasmid
pRNAT-U6.1-CCAT2 was successfully constructed.
MTT assay for determination of the
cell viability
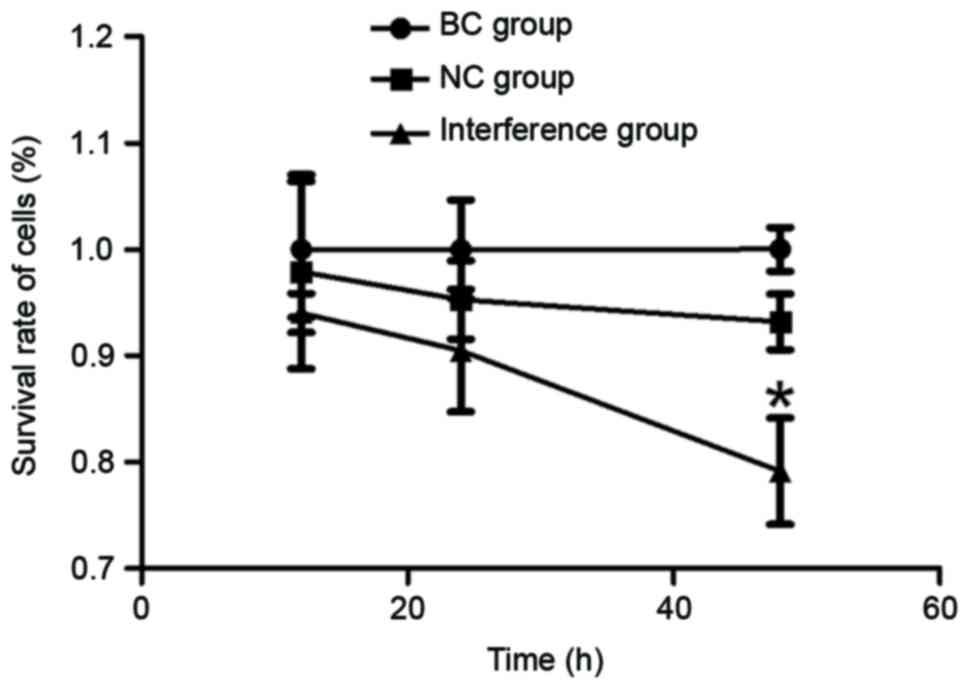
The viability of the GC cells was determined at
three time-points, namely 12, 24 and 48 h, using an MTT assay. As
observed in Fig. 1, the survival rate
of BGC-823 cells gradually declined with the increase in culture
time following transfection with the pRNAT-U6.1-CCAT2 plasmid. The
lowest cell viability was observed at 48 h, with a statistically
significant difference observed at this time-point when compared
with the BC and NC groups (P<0.05).
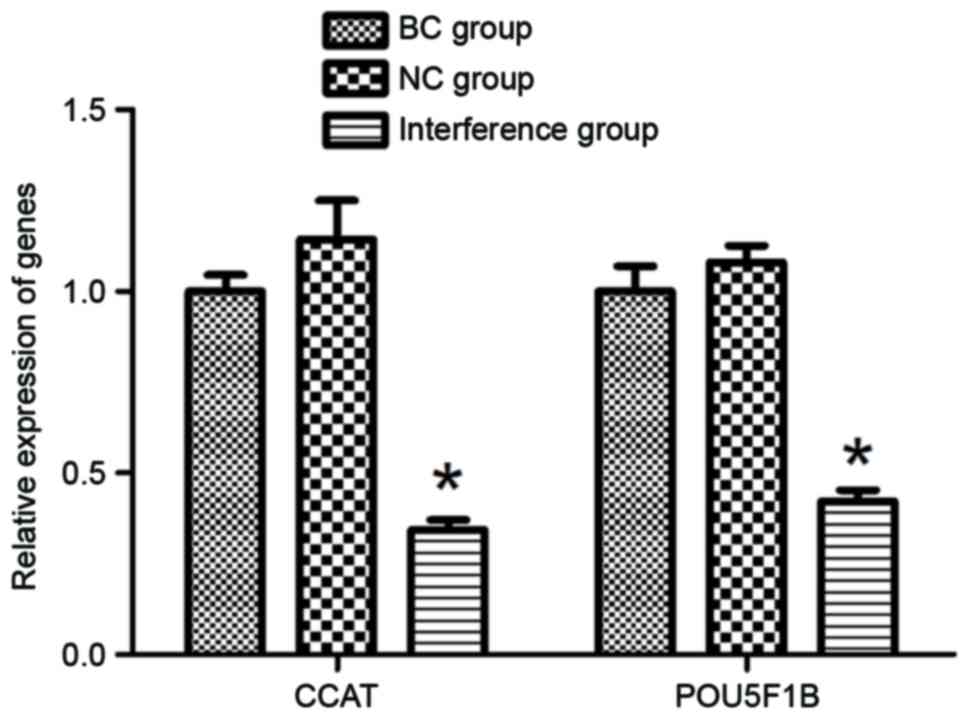
RT-qPCR detection of CCAT2 and POU5F1B
gene expression levels
To confirm the effect of pRNAT-U6.1-CCAT2 plasmid on
the gene expression levels of CCAT2 and POU5F1B in BGC-823 cells,
RT-qPCR detection was performed. As presented in Fig. 2, the RT-qPCR results demonstrated that
the relative expression of CCAT2 in the interference group
decreased significantly as compared with that of the BC and NC
groups (P<0.05), whereas no significant difference in CCAT2
expression was observed between the BC and NC groups, indicating
that the pRNAT-U6.1-CCAT2 plasmid was able to silence the function
of the CCAT2 gene in BGC-823 cells. Along with the decrease in the
expression of CCAT2 in the plasmid-transfected BGC-823 cells, the
relative expression of POU5F1B gene was also downregulated in the
interference group, with a significant difference detected compared
with the BC and NC groups (P<0.05; Fig. 2).
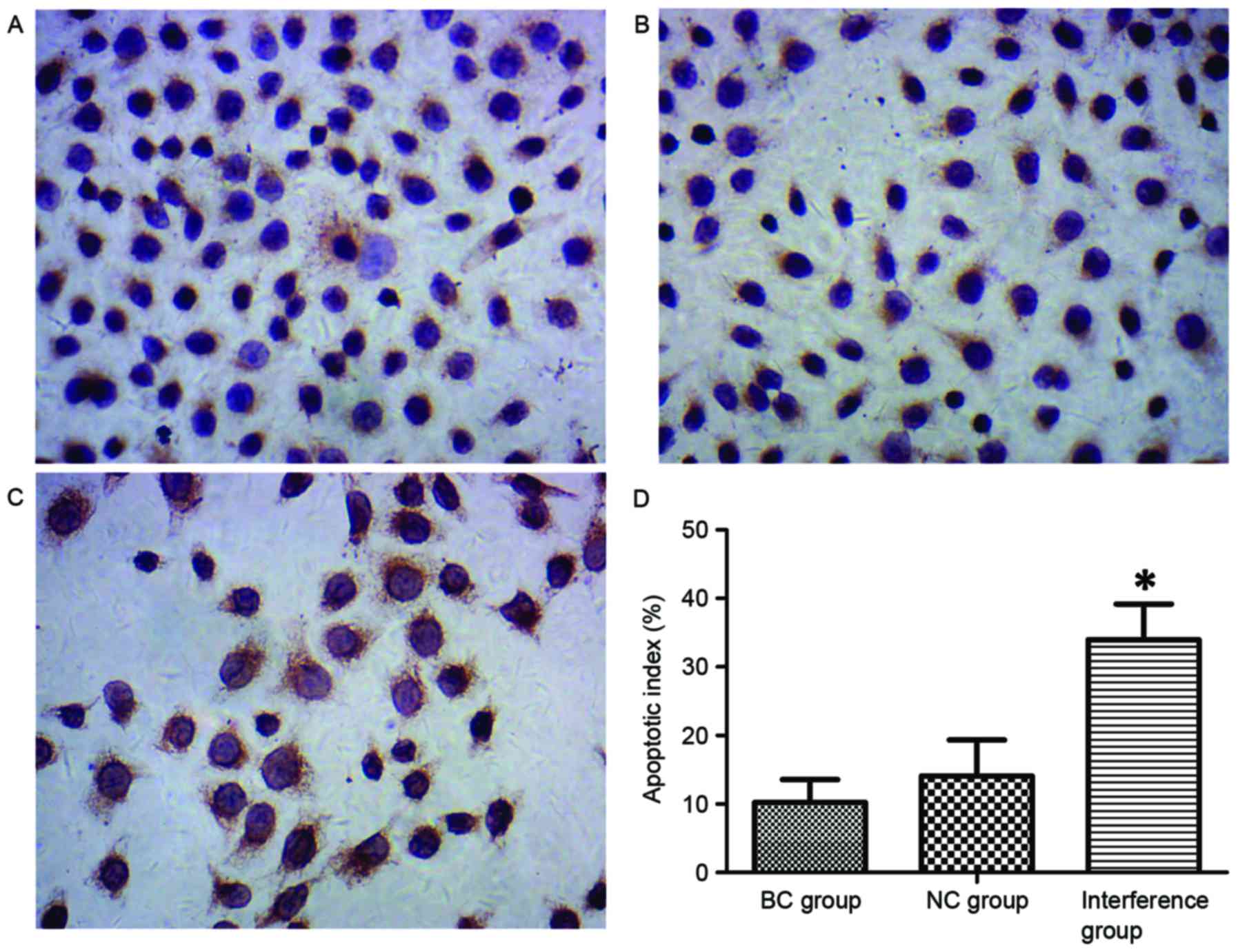
TUNEL detection of cell apoptosis
TUNEL staining identified that typical apoptotic
features were observed in the BGC-823 cells, with numerous brown
granules products in the nucleus of apoptotic cells (Fig. 3A-C). The TUNEL results revealed that
the apoptotic index of BGC-823 cells in the interference group
(33.98±5.22%) was significantly higher as compared with that in the
BC and NC groups (10.23±3.34 and 14.12±5.23%, respectively;
P<0.05; Fig. 3D).
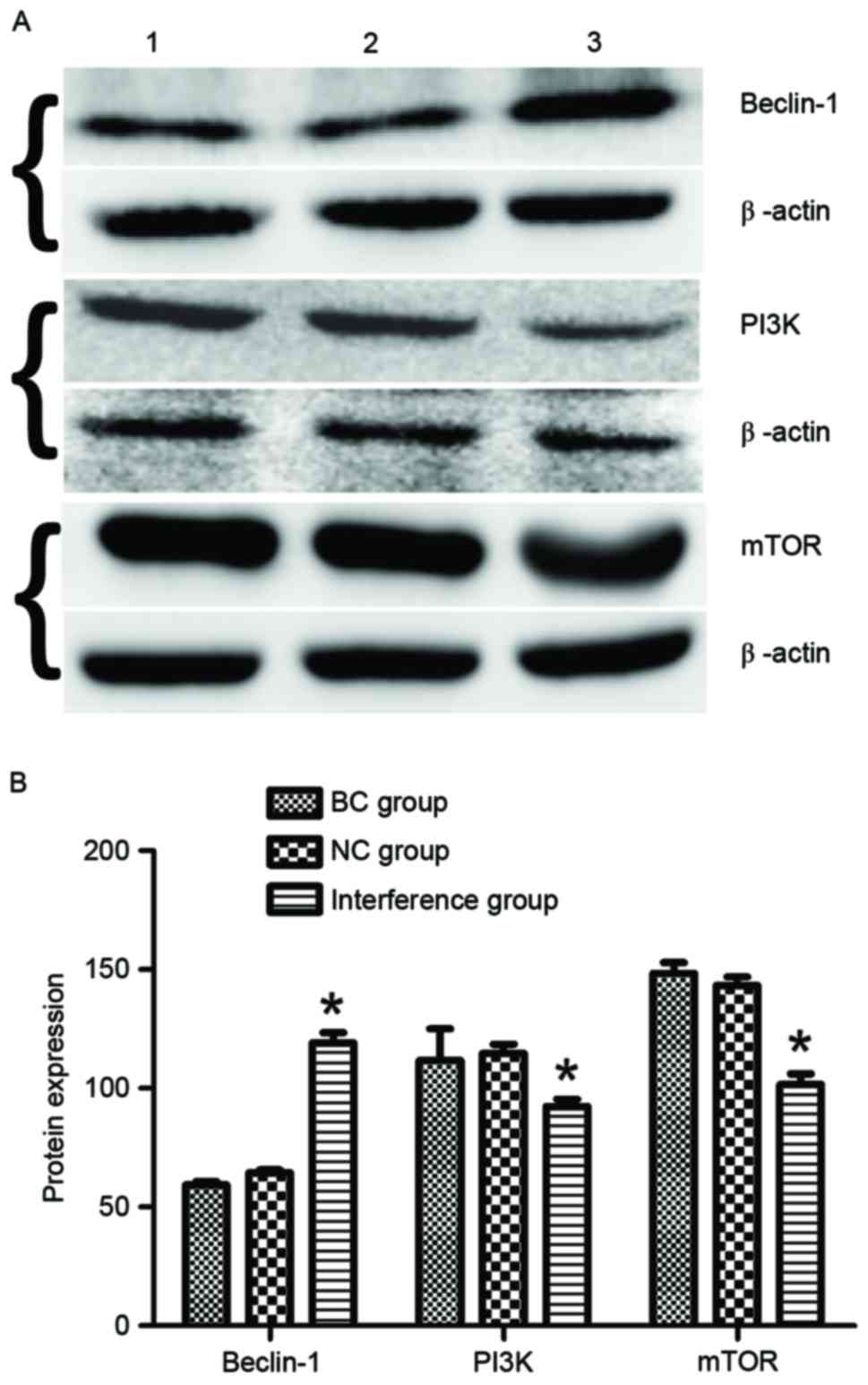
Western blot detection of protein
expression
The autophagy-associated protein beclin-1 and the
PI3K/mTOR signaling pathway proteins in BGC-823 cells at 48 h after
transfection with the plasmids were detected using western blot
assay. The results demonstrated that BGC-823 cells in the
interference group exhibited a significantly increased expression
of beclin-1 protein (1.99- and 1.81-fold increase vs. the BC and NC
groups, respectively; P<0.05; Fig.
4). Furthermore, the expression levels of PI3K and mTOR
proteins in the interference group were downregulated by 0.78- and
0.69-fold compared with those in the BC and NC groups
(P<0.05).
Discussion
lncRNAs function as oncogenes or tumor suppressors
during the occurrence and development of GC. Several lncRNAs,
including LINC00152, GHET1, HULC, HOTAIR, GACAT3, MALAT2 and H19,
have been demonstrated to serve as oncogenes, whereas other
lncRNAs, including LEIGC, GAS5 and FER1L4, have been considered to
be tumor suppressors (10). Emerging
evidence indicated that CCAT2 functions as a cancer-promoting
molecule in several types of solid cancer, such as in non-small
cell lung cancer (11), oral squamous
cell carcinoma (12), ovarian cancer
(13), and hepatocellular carcinoma
(14). The present study investigated
the effects of the lncRNA CCAT2 on the biological behavior of
BGC-823 cells, and revealed that knockdown of CCAT2 expression
effectively inhibited the proliferation, as well as induced the
apoptosis and autophagy of GC cells in vitro. These effects
may be derived from the downregulation of the expression of POU5F1B
gene as a result of silencing CCAT2.
Recent studies confirmed that the lncRNA CCAT2,
located at the 8q24 amplicon of the cancer risk-associated
rs6983267 SNP, regulated the cancer metabolism in vitro and
in vivo by directly interacting in an allele-specific manner
with a protein complex (15,16). The chromosomal region 8q24 emerged as
an important region for genetic susceptibility in various cancer
types, thus DNA methylation or SNPs at this locus may contribute to
cancer risk (17,18). The retrogene POU5F1B has been observed
to be located adjacent to the MYC gene within this risk locus at
chromosome 8q24 (19). POU5F1B has a
preserved open reading frame encoding a homolog of the master
embryonic stem cell transcription factor POU5F1, also known as
octamer-binding transcription factor 4. A study revealed that the
expression of POU5F1B was upregulated in GC cell lines and tissues,
while POU5F1B also exhibited mitogenic, angiogenic and
antiapoptotic effects in vivo (20). In the present study, it was observed
that the expression of POU5F1B was downregulated along with the
decreased expression of CCAT2 in BGC-823 cells transfected with the
CCAT2-silencing plasmid, which suggested that CCAT2 positively
regulated the expression of POU5F1B gene to a certain extent.
Therefore, the POU5F1B gene may be one of the downstream target
genes of CCAT2.
As the two main forms of programmed cell death,
autophagy and apoptosis induce the degradation of proteins and
organelles, or cell death upon cellular stress (21). Autophagy is associated with both the
tumorigenic and protective effects in cancer; however, the role of
autophagy in GC remains unclear (22). During the autophagic process, the
autophagy-associated markers beclin-1 (which is the mammalian
homologue of yeast Atg6) serves not only as a key autophagy
regulator with its specific interactors, but also as a potential
therapeutic target in cancer (23). A
meta-analysis revealed that the expression of beclin-1 in GC
tissues was significantly reduced when compared with that in non-GC
tissues, and the upregulated expression of beclin-1 was associated
with marked differentiation of tumor cells, no distant metastasis
and a favorable overall survival of GC (24–26).
Furthermore, there are close interactions between autophagy and
apoptosis through shared signaling pathways, among which the
PI3K/protein kinase B (AKT)/mTOR is a crucial intracellular
signaling pathway in tumorigenesis (27). Liu et al (28) demonstrated that celecoxib may impact
apoptosis and autophagy via the PI3K/AKT signaling pathway in the
SGC-7901 GC cells. Therefore, the PI3K/AKT/mTOR signaling pathway
serves a critical role in the regulation of apoptosis and
autophagy. In the present study, the data revealed that silencing
of the CCAT2 gene induced upregulation of the apoptosis and
autophagy of GC cells by downregulating the expression levels of
PI3K and mTOR proteins.
In conclusion, the present study constructed a CCAT2
interference plasmid, and further examined the effect of CCAT2 on
the biological behavior of GC cells and its possible underlying
molecular mechanism. However, the specific regulatory mechanisms
require further investigation.
Acknowledgements
This study was supported by grants from the Guizhou
Provincial Fund Project of Science and Technology [grant no.
(2015)2095], the Science and Technology Fund Projects of Guizhou
Provincial Health and Family Planning Commission (grant no.
gzwjkj2015-1-019), and the Joint Fund Project of Guizhou Provincial
Science and Technology Agency [grant no. (2015)7170].
References
|
1
|
Brenner H, Rothenbacher D and Arndt V:
Epidemiology of stomach cancer. Methods Mol Biol. 472:467–477.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang M and Du X: Noncoding RNAs in
gastric cancer: Research progress and prospects. World J
Gastroenterol. 22:6610–6618. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang R, Xia LQ, Lu WW, Zhang J and Zhu
JS: LncRNAs and cancer. Oncol Lett. 12:1233–1239. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ling H, Spizzo R, Atlasi Y, Nicoloso M,
Shimizu M, Redis RS, Nishida N, Gafà R, Song J, Guo Z, et al:
CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic
progression and chromosomal instability in colon cancer. Genome
Res. 23:1446–1461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan YH, Fang H, Ji CX, Xie H, Xiao B and
Zhu XG: Long noncoding RNA CCAT2 can predict metastasis and poor
prognosis: A meta-analysis. Clin Chim Acta. 466:120–126. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang CY, Hua L, Yao KH, Chen JT, Zhang JJ
and Hu JH: Long non-coding RNA CCAT2 is up-regulated in gastric
cancer and associated with poor prognosis. Int J Clin Exp Pathol.
8:779–785. 2015.PubMed/NCBI
|
|
7
|
Wang YJ, Liu JZ, Lv P, Dang Y, Gao JY and
Wang Y: Long non-coding RNA CCAT2 promotes gastric cancer
proliferation and invasion by regulating the E-cadherin and LATS2.
Am J Cancer Res. 6:2651–2660. 2016.PubMed/NCBI
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao X, Ma C, Cai X, Lei D, Liu D, Xu F,
Jin T, Liu J and Pan X: RNA interference of caveolin-1 via
lentiviral vector inhibits growth of hypopharyngeal squamous cell
carcinoma FaDu cells in vitro and in vivo. Asian Pac J Cancer Prev.
12:397–401. 2011.PubMed/NCBI
|
|
10
|
Sun W, Yang Y, Xu C, Xie Y and Guo J:
Roles of long noncoding RNAs in gastric cancer and their clinical
applications. J Cancer Res ClinOncol. 142:2231–2237. 2016.
View Article : Google Scholar
|
|
11
|
Chen S, Wu H, Lv N, Wang H, Wang Y, Tang
Q, Shao H and Sun C: LncRNA CCAT2 predicts poor prognosis and
regulates growth and metastasis in small cell lung cancer. Biomed
Pharmacother. 82:583–588. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ouyang S, Zhang P, Wang J, Huang Z and
Liao L: Expression of long non-coding RNA colon cancer associated
transcript 2 and its clinicopathologic significance in oral
squamous cell carcinoma. Zhonghua Kou Qiang Yi XueZaZhi.
51:286–291. 2016.(In Chinese).
|
|
13
|
Huang S, Qing C, Huang Z and Zhu Y: The
long non-coding RNA CCAT2 is up-regulated in ovarian cancer and
associated with poor prognosis. Diagn Pathol. 11:492016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou N, Si Z, Li T, Chen G, Zhang Z and Qi
H: Long non-coding RNA CCAT2 functions as an oncogene in
hepatocellular carcinoma, regulating cellular proliferation,
migration and apoptosis. Oncol Lett. 12:132–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Redis RS, Vela LE, Lu W, Ferreira de
Oliveira J, Ivan C, Rodriguez-Aguayo C, Adamoski D, Pasculli B,
Taguchi A, Chen Y, et al: Allele-specific reprogramming of cancer
metabolism by the long non-coding RNA CCAT2. Mol Cell. 61:520–534.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Redis RS and Calin GA: The interplay
between lnRNAs, SNPs, and protein complexes-what does it mean for
cancer metabolism? Mol Cell Oncol. 3:e11663082016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barry KH, Moore LE, Sampson J, Yan L,
Meyer A, Oler AJ, Chung CC, Wang Z, Yeager M, Amundadottir L and
Berndt SI: DNA methylation levels at chromosome 8q24 in peripheral
blood are associated with 8q24 cancer susceptibility loci. Cancer
Prev Res (Phila). 7:1282–1292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brisbin AG, Asmann YW, Song H, Tsai YY,
Aakre JA, Yang P, Jenkins RB, Pharoah P, Schumacher F, Conti DV, et
al: Meta-analysis of 8q24 for seven cancers reveals a locus between
NOV and ENPP2 associated with cancer development. BMC Med Genet.
12:1562011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Breyer JP, Dorset DC, Clark TA, Bradley
KM, Wahlfors TA, McReynolds KM, Maynard WH, Chang SS, Cookson MS,
Smith JA, et al: An expressed retrogene of the master embryonic
stem cell gene POU5F1 is associated with prostate cancer
susceptibility. Am J Hum Genet. 94:395–404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hayashi H, Arao T, Togashi Y, Kato H,
Fujita Y, De Velasco MA, Kimura H, Matsumoto K, Tanaka K, Okamoto
I, et al: The OCT4 pseudogene POU5F1B is amplified and promotes an
aggressive phenotype in gastric cancer. Oncogene. 34:199–208. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu G, Pei F, Yang F, Li L, Amin AD, Liu
S, Buchan JR and Cho WC: Role of autophagy and apoptosis in
non-small-cell lung cancer. Int J Mol Sci. 18:E3672017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou H, Yuan M, Yu Q, Zhou X, Min W and
Gao D: Autophagy regulation and its role in gastric cancer and
colorectal cancer. Cancer Biomark. 17:1–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu LL, Cheng Y and Liu B: Beclin-1:
Autophagic regulator and therapeutic target in cancer. Int J
Biochem Cell Biol. 45:921–924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu ZY, Ding J, Yang XF and Zhang ZM:
Significance of autophagy-related protein beclin-1 expression in
gastric cancer: A Meta-analysis. Chin J Gen Surg. 24:1389–1395.
2015.
|
|
25
|
He Y, Zhao X, Subahan NR, Fan L, Gao J and
Chen H: The prognostic value of autophagy-related markers beclin-1
and microtubule-associated protein light chain 3B in cancers: A
systematic review and meta-analysis. Tumour Biol. 35:7317–7326.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao QH, Liu F, Yang ZL, Fu XH, Yang ZH,
Liu Q, Wang L, Wan XB and Fan XJ: Prognostic value of autophagy
related proteins ULK1, Beclin 1, ATG3, ATG5, ATG7, ATG9, ATG10,
ATG12, LC3B and p62/SQSTM1 in gastric cancer. Am J Transl Res.
8:3831–3847. 2016.PubMed/NCBI
|
|
27
|
Polivka J Jr and Janku F: Molecular
targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol
Ther. 142:164–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu M, Li CM, Chen ZF, Ji R, Guo QH, Li Q,
Zhang HL and Zhou YN: Celecoxib regulates apoptosis and autophagy
via the PI3K/Akt signaling pathway in SGC-7901 gastric cancer
cells. Int J Mol Med. 33:1451–1458. 2014. View Article : Google Scholar : PubMed/NCBI
|