Introduction
Bladder cancer is a common urothelial cancer and the
ninth most common type of cancer in the world with 430,000 new
cases and 165,000 associated mortalities reported in 2012 (1–5). Bladder
cancer may be divided into early stage, non-muscle invasive and
higher stage muscle invasive disease (6). Mitomycin C (MMC) is a mitomycin that is
often used as a chemotherapeutic agent for treatment of non-muscle
invasive bladder cancer (7–11). It functions by binding to the DNA of
cancer cells to prevent cell division and thereby inhibit the
growth of the cancer. However, its clinical effectiveness is
limited bythe occurrence of resistance to MMC (12–14). To
overcome this treatment resistance, MMC is often combined with
other agents to increase MMC chemotherapy sensitivity (15).
Aquaporins (AQPs) are small integral membrane
proteins that function as molecular water channels that allow water
to transported rapidly into the cell rather than diffusing slowly
through the cell membrane (16).
Presently, 13 members of AQPs have been reported (17). In addition, previous studies have
demonstrated that AQPs are associated with the development of
cancer (18,19). AQP1, a member of the AQP family, has
been demonstrated to be involved in tumor angiogenesis (20). More specifically, a recent study
revealed that the expression level of AQP1 in bladder uroepithelium
cell carcinoma tissue was significantly increased compared with
that in normal bladder tissue (21).
As combination therapies possess great potential in overcoming
treatment resistance, the present study hypothesized that a
combination of AQP1 inhibition alongside MMC treatment may increase
MMC chemotherapy sensitivity, which has not yet been reported, to
the best of our knowledge. In the present study, AQP1 inhibition
was combined with MMC treatment and it was revealed that inhibition
of AQP-1 enhanced MMC chemotherapy sensitivity in J82 bladder
cancer cells.
Materials and methods
Mammalian cell culture conditions
J82 human bladder cancer cells were purchased from
the American Type Culture Collection (Manassas, VA, USA) and were
cultured in Dulbecco's Modified Eagle Medium (DMEM; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal calf serum (FCS; Invitrogen; Thermo Fisher Scientific,
Inc.) and incubated at 37°C in a cell culture incubator with 5%
CO2.
Construction of recombinant
plasmid
Invitrogen BLOCK-iT RNAi Designer (rnaidesigner.lifetechnologies.com/rnaiexpress)
was used for obtaining the following target sequence from AQP1
gene: 5′-GCTGTACTCATCTACGACTTC-3′ (724–744). The pSuper RNAi System
manual was followed for short hairpin (sh)RNA design (22). The primers designed are as follows:
Sh-AQP1 forward,
5′-GATCCCCGCTGTACTCATCTACGACTTCTTCAAGAGAGAAGTCGTAGATGAGTACAGCTTTTTA-3′;
and reverse,
5′-AGCTTAAAAAGCTGTACTCATCTACGACTTCTCTCTTGAAGAAGTCGTAGATGAGTACAGCGGG-3′;
Sh-AQP1 negative control forward,
5′-GATCCCCGCCAGCTTAGCACTGACTCTTCAAGAGAGAGTCAGTGCTAAGCTGGCTTTTTA-3′;
and reverse,
5′-AGCTTAAAAAGCCAGCTTAGCACTGACTCTCTCTTGAAGAGTCAGTGCTAAGCTGGCGGG-3′.
Primers were synthesized at GenePharma Co., Ltd
(Shanghai, China), dissolved and diluted to 1 µg/µl. Reverse primer
(5 µl) and forward primer (5 µl) were mixed and annealed at 95°C
for 4 min, 70°C for 10 min and then allowed to cool to 4°C. The
resulting double stranded DNA and the plasmid pSUPER-retro-puro
(Cell BioLabs, Inc., San Diego, CA, USA) were then double digested
by Bgl II and Hind III restriction enzymes at 37°C overnight,
ligated together and transferred into E.coli DH5α. Colony PCR was
performed to identify the colonies harboring the recombinant
plasmid. The reaction system included: 23 µl double distilled
H2O, 1 µl reverse primer, 1 µl forward prime rand 25 µl
Taqmix. Pre-denaturation occurred at 95°C for 6 min, denaturation
occurred at 95°C for 20 sec, annealing occurred at 55°C for 30 sec
and extension occurred at 72°C for 30 sec, for 30 cycles. Primers
were then purified, sequenced at GenePharma Co., Ltd and used to
transfect 293FT cells. Prior to transfection, log phase 293FT cells
were collected and seeded (2×105 cells) to 6 well
plates. After 24 h incubation at 37°C, cells were transfected with
liposome Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). At 48 h post-transfection, cells were harvested
and interference analysis was performed using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). The
resulting recombinant plasmid was designated
pSUPER-retro-puro-shAQP1 and was transferred to 293FT cells using
calcium phosphate co-precipitation. At 48 h post-transfection, the
culture supernatant containing recombinant virus was collected and
the virus titrations were determined using multiple proportion
dilutions. J82 cells were then seeded to T25 cell culture flask.
Polybrene was added to the culture supernatant collected above to a
final concentration of 4 µg/µl. The culture media for J82 cells
were replaced by 5 ml culture supernatant collected above and the
cells were cultured in a 37°C incubator for 24 h. The transfection
was repeated 3 times at 3 h intervals followed by replacement of
the current media with DMEM supplemented with 10% FCS. After 48 h,
0.5 µg/ml puromycin was added to the culture for screening of
positive clones and culture amplification. This was to establish a
J82 cell line (designated as J82-shAQP1) whose expression of AQP1
gene is inhibited. Another J82 cell line transfected with the empty
plasmid pSUPER-retro-puro was used as the control (designated as
J82-ctrl).
RT-qPCR
The transcription of AQP1 gene was analyzed using
RT-qPCR analysis and quantified using the 2−ΔΔCq method
(23). RT-qPCR was performed
according to the manufacturer's protocol (Bio-Rad Laboratories,
Inc., Hercules USA). Total RNA from J82 cells was extracted using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). GAPDH was used
as an internal control. RT was performed using PrimeScript RT
reagent kit with gDNA Eraser (Takara Biotechnology Co., Ltd.,
Dalian, China). CFX96 Real Time PCR detection system (Bio-Rad
Laboratories, Inc.) with SYBRGreen ІІ (Bio-Rad Laboratories, Inc.)
was used for qPCR. Primers used were as follows: AQP-1 forward,
5′-ACCCGCAACTTCTCAAAC-3; and reverse, 5′-AGGCCAAGCCTCCTCTAT-3′;
GAPDH forward, 5′-ACCACAGTCCATGCCATCAC-3′, and reverse,
5′-TCCACCACCCTGTTGCTGTA-3′. Thermocycling conditions were as
follows: Pre-denaturation at 94°C for 1 min, denaturation at 94°C
for 20 sec, annealing at 55°C for 20 sec, extension at 72°C for 20
sec, for 40 cycles.
MTT assay
Cell viability was assessed using an MTT assay
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). J82-shAQP1 cells
and J82-ctrl cells (2×103 cells/plate) were seeded into
96 well plates and incubated for 24 h in culture media with or
without MMC (1 mg/ml). A total of 200 µl MTT (5 mg/ml) was then
added to each well and cells were incubated for a further 4 hat
37°C, and 150 µl dimethyl sulfoxide was added to each well and
incubated at 37°C for 5 min. A Versamax microplate reader
(Molecular Devices, LLC, Sunnyvale, CA, USA) was used for reading
optical density at 490 nm.
Flow cytometry analysis
Apoptosis was measured using flow cytometry with
propidium iodide (PI) and the annexin V-fluorescein isothiocyanate
(FITC) staining kit (BD Biosciences, Franklin Lakes, NJ, USA).
J82-shAQP1 and J82-ctrl cells (2×103 cells/plate) were
seeded onto 96 well plates and incubated at 37°C for 24 h in DMEM
with or without 1 mg/ml MMC. Then, the cells were detached and
rinsed with DMEM and cold PBS twice. Cells were then centrifuged at
300 × g for 5 min and resuspended in 1× binding buffer at
1×106 cells/ml. In total, 100 µl of the cell buffer
solution (1×105 cells) was transferred to a round bottom
tube and incubated with 5 µl of PI and annexin V-FITC for 15 min in
the dark. Finally, 400 µl of 1× binding buffer was added to each
sample tube and analyzed using a BD FACSCalibur cytometer (BD
Biosciences) according to the manufacturers protocol. Data were
analyzed using FlowJo software (version 7.2.5; FlowJo LLC, Ashland,
OR, USA).
Western blot analysis
For Western blot analysis, J82-shAQP1 and J82-ctrl
cells (2×103 cells/plate) were seeded into 96 well
plates and incubated at 37°C for 24 h in DMEM with or without 1
mg/ml MMC. Total cellular proteins were extracted in EBC lysis
buffer (50 mM Tris-HCl, pH 8.0, 300 mM NaCl, 0.5% NP40;
Sigma-Aldrich; Merck KGaA) containing a proteinase inhibitor
cocktail (100 µg/ml lysozyme, 10 µg/ml aprotinin and 10 µg/ml
lepupetin; Sigma-Aldrich; Merck KGaA), as described previously
(24). Protein concentrations were
measured using Bradford protein assay. A total of 30 µg protein
samples were then subjected to SDS-PAGE (12% gel). Following
electrophoresis, proteins were transferred to polyvinylidene
difluoride membranes, where they were subsequently blocked by TBST
and 5% non-fat dry milk at 4°C for 2 h, followed by incubation with
primary antibodies at 4°C for 12 h. The primary antibodies used
were: Anti-AQP-1 (1:100; cat. no. BM5035, Wuhan Boster Biological
Technology, Ltd., Wuhan, China), anti-proliferating cell nuclear
antigen (PCNA; 1:5,000; cat. no. sc7907; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), anti-Bcl-2 associated X protein (Bax;
1:2,000; cat. no. ab32503; Abcam, Cambridge UK), anti-B cell
lymphoma 2 (Bcl-2; 1:500; cat. no. ab692; Abcam), anti-caspase-3
(1:5,000; cat. no. sc-98758; Santa Cruz Biotechnology, Inc.) and
anti-β-actin (1:1,000; cat. no. sc-130656; Santa Cruz
Biotechnology, Inc.). Following incubation with corresponding
primary antibody at 4°C overnight, the membranes were washed and
incubated with either horseradish peroxidase-conjugated-goat
anti-rabbit immunoglobulin G secondary antibody (1:2,000; cat. no.
ab6721; Abcam) for 1 h at 4°C and developed with
electrochemiluminescence (ECL) Western Blotting Substrate (BD
Biosciences). Data was analyzed using Quantity One 1D image
analysis software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Statistical analysis
Each experiment was performed ≥3 times and analyzed
using SPSS (v19; IBM Corp., Armonk, NY, USA) was used for
statistical analysis of the data collected. A two-tailed Student's
t-test was adopted for comparison of the expressions of AQP-1 mRNA
between J82-ctrl and J82-shAQP1 cells, whereas comparisons among
groups were assessed using one-way analysis of variance with a
Student-Newman-Keuls post-hoc test. Data were presented as the mean
± standard deviation. P<0.05 was considered to indicate as
statistically significant difference.
Results
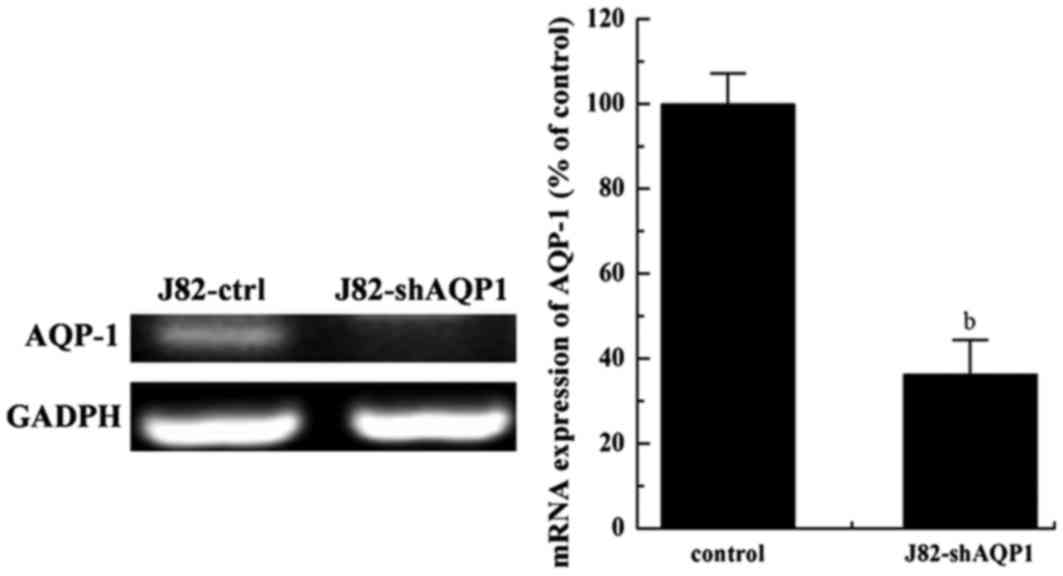
AQP-1mRNA expression was inhibited in
J82-shAQP1 cells
The expression of AQP-1 mRNA was detected by
RT-qPCR. As presented in Fig. 1, the
expression of AQP-1 mRNA in J82-shAQP1 cells decreased
significantly and was only 36% of that in J82-ctrl cells. In
addition, the expression of GAPDH mRNA in the 2 cell types remained
consistent. GAPDH is a commonly used loading control for RT-PCR as
the GAPDH gene is a housekeeping gene often stably and
constitutively expressed in the majority of tissues and cells.
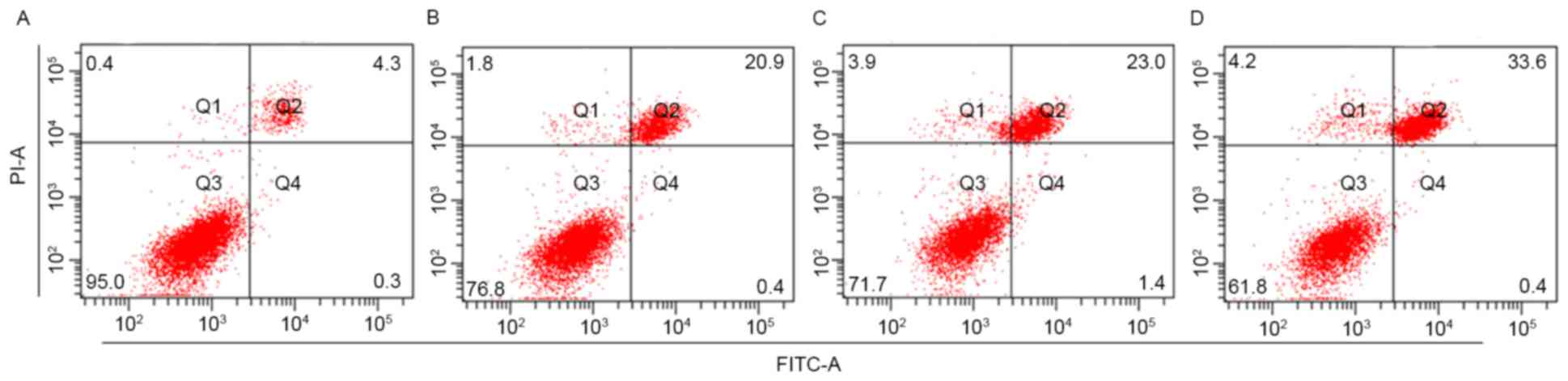
Combined effect of recombinant virus
transfection and MMC chemotherapy on J82 cell proliferation and
apoptosis
As presented in Table
I, there was a significant decrease in cell viability for
J82-ctrl cells following treatment with MMC (P<0.05). The cell
viability was also significantly decreased in J82-shAQP1 cells
compared with that in J82-ctrl cells (P<0.05). Treatment of
J82-shAQP1 cells with MMC further decreased cell viability, which
was significantly decreased compared with that in J82-ctrl cells
treated by MMC (P<0.01). A similar association was also observed
in cell apoptosis. As presented in Fig.
2, when cells were treated with MMC, J82-shAQP1 cells exhibited
a significantly increased level of cell apoptosis compared with
J82-ctrl cells (P<0.05).
 | Table I.Cell viability of J82-shAQP1 and
J82-ctrl cells. |
Table I.
Cell viability of J82-shAQP1 and
J82-ctrl cells.
|
| Cell viability (%
of the control), mean ± standard deviation |
|---|
|
|
|
|---|
| MMC (mg/ml) | J82-ctrl | J82-shAQP1 |
|---|
| 0 |
100±8.39 |
77.42±9.26a |
| 2 |
81.58±6.30a |
60.05±6.77b,c |
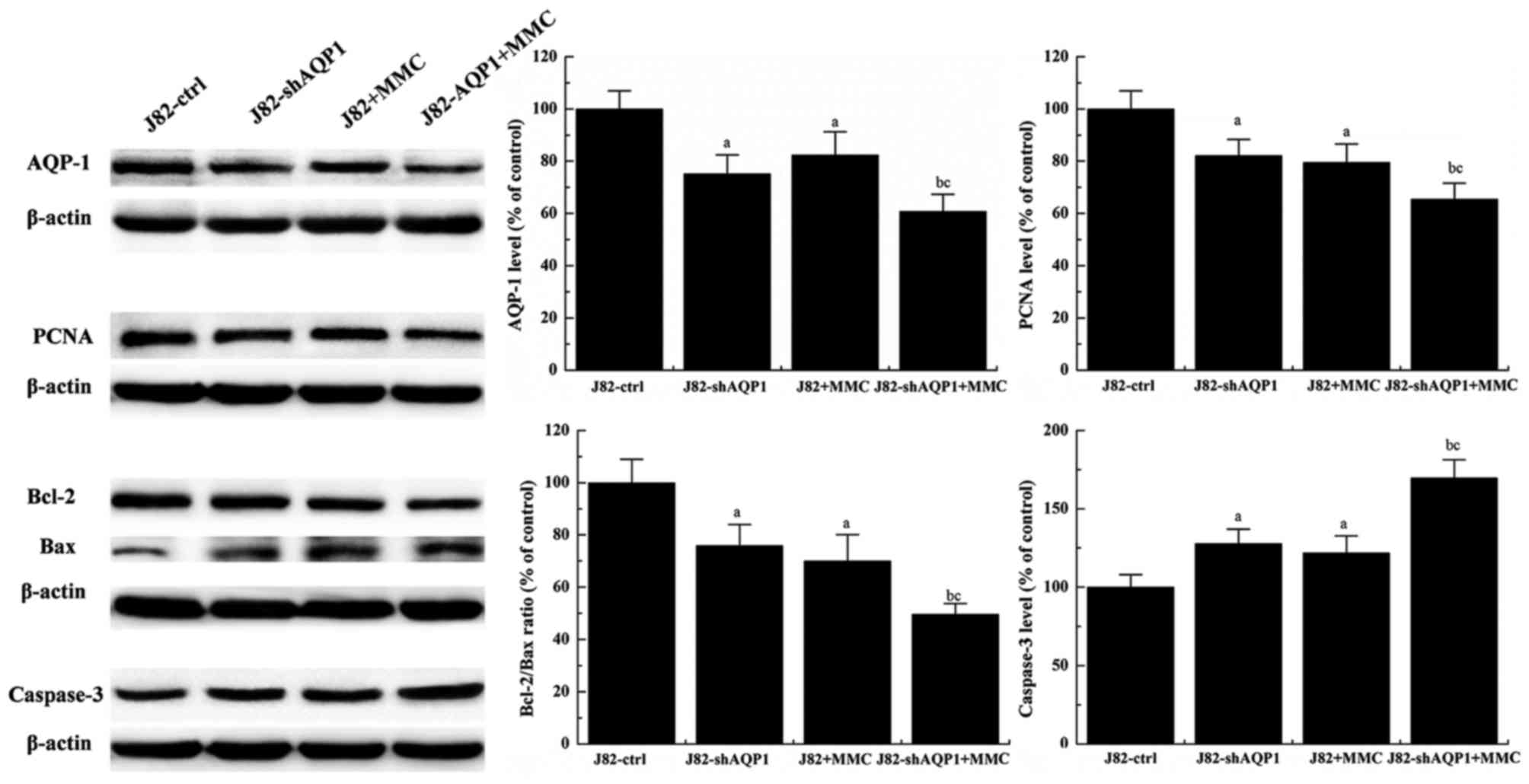
The combined effect of recombinant
virus transfection and MMC treatment on expressions of AQP-1, Bax,
Bcl-2, caspase-3 and PCNA
As presented in Fig.
3, significantly decreased expression of AQP-1 was observed in
J82-shAQP1 cells compared with J82-ctrl cells (P<0.05).
Treatment of J82 cells with MMC also significantly decreased AQP-1
expression (P<0.05). Additionally, AQP-1 expression was
decreased in J82-shAQP1 cells treated with MMC compared with
J82-ctrl cells treated by MMC and was decreased more as compared
with J82 cells without MMC treatment.
To further assess how MMC treatment and inhibition
of AQP-1 expression affected J82 cell viability and apoptosis, the
expressions of PCNA, Bcl-2, Bax and Caspase-3 in J82-shAQP1 cells
and J82-ctrl cells were measured. PCNA, Bcl-2, Bax and Caspase-3
are cell proliferation- and apoptosis-associated proteins. As
presented in Fig. 3, PCNA was most
highly expressed in J82-ctrl cells and was decreased significantly
by MMC treatment. Significantly decreased expression of PCNA was
also observed in J82-shAQP1 cells compared with J82 cells and was
further decreased by MMC treatment (P<0.05). Significantly
decreased expression of PCNA was observed between J82-shAQP1 cells
treated with MMC (P<0.01) and J82-ctrl cells treated with MMC
(P<0.05), compared with the untreated control.
In contrast with PCNA expression, an opposite
expression pattern was observed for caspase-3. As presented in
Fig. 3, the expression of caspase-3
in J82-shAQP1 cells was significantly increased compared with that
in J82-ctrl cells (P<0.05), suggesting recombinant virus
transfection of J82 cells increased caspase-3 expression. Treatment
of J82 cells with MMC also increased caspase-3 expression. Of all
the conditions tested, the highest expression level of caspase-3
was observed in J82-shAQP1 cells treated with MMC.
In addition, the Bcl-2/Bax ratio was also measured.
As presented in Fig. 3, the highest
Bcl-2/Bax ratio was observed in J82 cells and was significantly
decreased following MMC treatment and recombinant virus
transfection (P<0.05). The Bcl-2/Bax ratio in J82-shAQP1 cells
was further decreased by MMC treatment. A significantly decreased
Bcl-2/Bax ratio was observed in J82-shAQP1 cells treated with MMC
compared with that in J82 cells treated with MMC (P<0.05).
Discussion
Bladder cancer is associated with an increased
morbidity rate and common relapses and metastases. It may be
treated either by removal of the bladder or by radiation therapy
and chemotherapy (25). MMC,
anaziridine-containing natural product (26), is often applied as a chemotherapeutic
agent for the treatment of bladder cancer (7–11).
Numerous individuals with heart disease in China also take MMC
(27,28). However, occurrence of resistance to
MMC often limits its clinical effectiveness (12–14). To
increase MMC chemotherapy sensitivity, MMC is often combined with
other agents (17). Previous studies
have demonstrated that multiple AQPs, particularly AQP1, are
associated with cancer development and progression, including
bladder cancer (29–31). In the present study, a novel J82
bladder cancer cell line, J82-shAQP1 cell line, was established.
RT-qPCR analysis demonstrated that AQP1expression in J82-shAQP1
cells was inhibited. Investigating cell viability and apoptosis
demonstrated that, when treated with MMC, J82-shAQP1 cells
exhibited decreased cell viability and increased apoptosis compared
with that exhibited by J82 cells. In addition, it was observed that
J82-shAQP1 cells exhibited decreased expression of PCNA,
decreasedBcl-2/Bax ratio and increased expression of caspase-3
compared with that exhibited by the J82 cells.
The association of AQPs with cancer development and
progression has been previously reported (29–31). For
example, AQP1 has been revealed to be overexpressed in glioblastoma
multiforme, adenoid cystic carcinoma, renal cell carcinoma and
breast cancer (32–37). There have also been previous studies
using AQP inhibitor or RNA interference to inhibit AQP1 expression
in cancer cells (38). Therefore,
AQP1 may represent an important therapeutic target for cancer
treatment.
As demonstrated in the present study, a combination
of AQP1 inhibition with MMC treatment decreased cell viability and
increased the apoptosis rate in human J82 cells. Similar changes
were observed in the expression of PCNA, Bcl-2, Bax and caspase-3,
which are proteins associated with cell proliferation and apoptosis
(39). PCNA has sustained activity
and functions as a processivity factor for eukaryotic DNA
polymerase, serving an essential function in DNA replication, cell
proliferation and apoptosis (40).
Inhibition of PCNA as a cancer treatment strategy has been reported
previously (41). Caspases
(cysteine-dependent aspartate-directed proteases) are an
evolutionarily conserved family of cysteine proteases that serve
central functions in cell apoptosis and inflammation (42). It has been reported that activated
caspase 3 stimulates tumor growth, while inactive caspase 3 caused
substantial tumor sensitivity to radiotherapy (43). Bcl-2is a member of the Bcl-2 family
proteins that regulate cell apoptosis (44,45). Bcl-2
is an anti-apoptotic protein; Bax is a pro-apoptotic protein in the
Bcl-2 family (46,47).
Taken together, the results of the present study
demonstrated that inhibiting AQP1 expression decreased cell
viability and increased cell apoptosis in human J82 bladder cancer
cells, suggesting that a combination of MMC1 treatment with AQP1
inhibition may be a promising treatment strategy to enhance MMC1
chemotherapy sensitivity for bladder cancer treatment.
References
|
1
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. EurUrol. 71:96–108. 2017.
|
|
2
|
NCI, . SEER stat fact sheets: Bladder
cancer. 18–20142014.
|
|
3
|
World Health Organization, . World Cancer
Report 2014 Chapter 1. 1 ISBN 9283204298. 2014.
|
|
4
|
Cancer Research UK: Bladder cancer key
stats. 29–2014
|
|
5
|
Shen Z, Shen T, Wientjes MG, O'Donnell MA
and Au JL: Intravesical treatments of bladder cancer: Review. Pharm
Res. 25:1500–1510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dinney CP, McConkey DJ, Millikan RE, Wu X,
Bar-Eli M, Adam L, Kamat AM, Siefker-Radtke AO, Tuziak T, Sabichi
AL, et al: Focus on bladder cancer. Cancer Cell. 6:111–116. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Allona Moncada A, García Vaquero S,
Zuloaga Gómez A, Martínez Torres JL, López-Pardo R, Molina J,
Camacho JE, Rodríguez Rubio F and Varo C: Preliminary results of a
multicenter study with mitomycin C in superficial bladder tumors
(Ta, T1). Actas Urol Esp. 12:424–429. 1988.(In Spanish). PubMed/NCBI
|
|
8
|
van der Meijden AP and DeBruyne FM:
Treatment schedule of intravesical chemotherapy with mitomycin C in
superficial bladder cancer: Short-term courses or maintenance
therapy. Urology. 31(3 Suppl): S26–S29. 1988.
|
|
9
|
Colombo R, Lev A, Da Pozzo LF, Freschi M,
Gallus G and Rigatti P: A new approach using local combined
microwave hyperthermia and chemotherapy in superficial transitional
bladder carcinoma treatment. J Urol. 153:959–963. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kondás J, Kiss L, Határ A, Kiss A, Lukács
T, Szeldeli P, Törzsök F and Bodrogi I: The effect of intravesical
mitomycin C on the recurrence of superficial (Ta-T1) bladder
cancer. A hungarian multicenter study. Int Urol Nephrol.
31:451–456. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Stasi SM, Giannantoni A, Stephen RL,
Capelli G, Navarra P, Massoud R and Vespasiani G: Intravesical
electromotive mitomycin C versus passive transport mitomycin C for
high risk superficial bladder cancer: A prospective randomized
study. J Urol. 170:777–782. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moertel CG, Reitemeier RJ and Hahn RG:
Mitomycin C therapy in advanced gastrointestinal cancer. JAMA.
204:1045–1048. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lenaz L: Mitomycin C in advanced breast
cancer. Cancer Treat Rev. 12:235–249. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu BH, Gupta V and Singh SV: Mechanism of
differential sensitivity of human bladder cancer cells to mitomycin
C and its analogue. Br J Cancer. 69:242–246. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zargar H, Aning J, Ischia J, So A and
Black P: Optimizing intravesical mitomycin C therapy in
non-muscle-invasive bladder cancer. Nat Rev Urol. 11:220–230. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cooper GM and Hausman RE: The cell: A
molecular approach. Washington, DC: ASM PRESS; pp. 5442009
|
|
17
|
Oberg F, Ekvall M, Nyblom M, Backmark A,
Neutze R and Hedfalk K: Insight into factors directing high
production of eukaryotic membrane proteins; production of 13 human
AQPs in Pichia pastoris. Mol Membr Biol. 26:215–227. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi YH, Chen R, Talafu T, Nijiati R and
Lalai S: Significance and expression of aquaporin 1, 3, 8 in
cervical carcinoma in xinjianguygur women of China. Asian Pac J
Cancer Prev. 13:1971–1975. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Verkman AS, Hara-Chikuma M and
Papadopoulos MC: Aquaporins-new players in cancer biology. J Mol
Med (Berl). 86:523–529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deb P, Pal S, Dutta V, Boruah D, Chandran
VM and Bhatoe HS: Correlation of expression pattern of aquaporin-1
in primary central nervous system tumors with tumor type, grade,
proliferation, microvessel density, contrast-enhancement and
perilesional edema. J Cancer Res Ther. 8:571–577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Zhang WY and Ding DG: Expression of
aquaporin 1 in bladder uroepithelial cell carcinoma and its
relevance to recurrence. Asian Pac J Cancer Prev. 16:3973–3976.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shrestha YB, Wickwire K and Giraudo S:
Effect of reducing hypothalamic ghrelin receptor gene expression on
energy balance. Peptides. 30:1336–1341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang M, Jeong C, Ku JH, Kwak C and Kim HH:
Inhibition of autophagy potentiates atorvastatin-induced apoptotic
cell death in human bladder cancer cells in vitro. Int J Mol Sci.
15:8106–8121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gong YQ, Peng D, Ning XH, Yang XY, Li XS,
Zhou LQ and Guo YL: UBE2T silencing suppresses proliferation and
induces cell cycle arrest and apoptosis in bladder cancer cells.
Oncol Lett. 12:4485–4492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Danshiitsoodol N, de Pinho CA, Matoba Y,
Kumagai T and Sugiyama M: The mitomycin C (MMC)-binding protein
from MMC-producing microorganisms protects from the lethal effect
of bleomycin: Crystallographic analysis to elucidate the binding
mode of the antibiotic to the protein. J Mol Biol. 360:398–408.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang Y and Zhu D: Subjective well-being
of Chinese landless peasants in relatively developed regions:
Measurement using PANAS and SWLS. Social Indicat Res.
123:pp817–835. 2015. View Article : Google Scholar
|
|
28
|
Liang Y: Trust in Chinese government and
quality of life (QOL) of sichuan earthquake survivors: Does trust
in government help to promote QOL? Social Indicat Res. 127:541–564.
2016. View Article : Google Scholar
|
|
29
|
Shi YH, Rehemu N, Ma H, Tuokan T, Chen R
and Suzuke L: Increased migration and local invasion potential of
SiHa cervical cancer cells expressing Aquaporin 8. Asian Pac J
Cancer Prev. 14:1825–1828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi YH, Tuokan T, Lin C and Chang H:
Aquaporin 8 involvement in human cervical cancer SiHa migration via
the EGFR-Erk1/2 pathway. Asian Pac J Cancer Prev. 15:6391–6395.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Warth A, Muley T, Meister M, Herpel E,
Pathil A, Hoffmann H, Schnabel PA, Bender C, Buness A, Schirmacher
P and Kuner R: Loss of aquaporin-4 expression and putative function
in non-small cell lung cancer. BMC Cancer. 11:1612011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zou LB, Shi S, Zhang RJ, Wang TT, Tan YJ,
Zhang D, Fei XY, Ding GL, Gao Q, Chen C, et al: Aquaporin-1 plays a
crucial role in estrogen-induced tubulogenesis of vascular
endothelial cells. J Clin Endocrinol Metab. 98:E672–E682. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi YH, Chen R, Talafu T, Nijiati R and
Lalai S: Significance and expression of aquaporin 1, 3, 8 in
cervical carcinoma in Xinjiang Uygur women of China. Asian Pac J
Cancer Prev. 13:1971–1975. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
El Hindy N, Rump K, Lambertz N, Zhu Y,
Frey UH, Bankfalvi A, Siffert W, Sure U, Peters J, Adamzik M and
Sandalcioglu IE: The functional aquaporin 1-783G/C-polymorphism is
associated with survival in patients with glioblastoma multiforme.
J Surg Oncol. 108:492–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Morrissey JJ, Mobley J, Song J, Vetter J,
Luo J, Bhayani S, Figenshau RS and Kharasch ED: Urinary
concentrations of aquaporin-1 and perilipin-2 in patients with
renal cell carcinoma correlate with tumor size and stage but not
grade. Urol. 83:256.e9–e14. 2014. View Article : Google Scholar
|
|
36
|
Morrissey JJ, Mobley J, Figenshau RS,
Vetter J, Bhayani S and Kharasch ED: Urine aquaporin 1 and
perilipin 2 differentiate renal carcinomas from other imaged renal
masses and bladder and prostate cancer. Mayo Clin Proc. 90:pp.
35–42. 2015; View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tan M, Shao C, Bishop JA, Feng Z, Trock
BJ, Westra WH and Ha PK: Aquaporin-1 promoter hypermethylation is
associated with improved prognosis in salivary gland adenoid cystic
carcinoma. Otolaryngol Head Neck Surg. 150:801–807. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stigliano C, Aryal S, de Tullio MD,
Nicchia GP, Pascazio G, Svelto M and Decuzzi P: siRNA-chitosan
complexes in poly(lactic-co-glycolic acid) nanoparticles for the
silencing of aquaporin-1 in cancer cells. Mol Pharm. 10:3186–3194.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu B, Hua J, Zhang Y, Jiang X, Zhang H, Ma
T, Zheng W, Sun R, Shen W, Sha J, et al: Proliferating cell nuclear
antigen (PCNA) regulates primordial follicle assembly by promoting
apoptosis of oocytes in fetal and neonatal mouse ovaries. PLoS One.
6:e160462011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Paunesku T, Mittal S, Protić M, Oryhon J,
Korolev SV, Joachimiak A and Woloschak GE: Proliferating cell
nuclear antigen (PCNA): Ringmaster of the genome. Int J Radiat
Biol. 77:1007–1021. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang X, Barile G, Chang S, Hays A,
Pachydaki S, Schiff W and Sparrow J: Apoptosis and cell
proliferation in proliferative retinal disorders: PCNA, Ki-67,
caspase-3, and PARP expression. Curr Eye Res. 30:395–403. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lamkanfi M, Festjens N, Declercq W, Vanden
Berghe T and Vandenabeele P: Caspases in cell survival,
proliferation and differentiation. Cell Death Differ. 14:44–55.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang Q, Li F, Liu X, Li W, Shi W, Liu FF,
O'Sullivan B, He Z, Peng Y, Tan AC, et al: Caspase 3-mediated
stimulation of tumor cell repopulation during cancer radiotherapy.
Nat Med. 17:860–866. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tsujimoto Y, Finger LR, Yunis J, Nowell PC
and Croce CM: Cloning of the chromosome breakpoint of neoplastic B
cells with the t(14;18) chromosome translocation. Science.
226:1097–1099. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cleary ML, Smith SD and Sklar J: Cloning
and structural analysis of cDNAs for bcl-2 and a hybrid
bcl-2/immunoglobulin transcript resulting from the t(14;18)
translocation. Cell. 47:19–28. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
EI-Emshaty HM, Saad EA, Toson EA, Abdel
Malak CA and Gadelhak NA: Apoptosis and cell proliferation:
Correlation with BCL-2 and P53 oncoprotein expression in human
hepatocellular carcinoma. Hepatogastroenterology. 61:1393–1401.
2014.PubMed/NCBI
|
|
47
|
Koshida Y, Saegusa M and Okayasu I:
Apoptosis, cell proliferation and expression of Bcl-2 and Bax in
gastric carcinomas: Immunohistochemical and clinicopathological
study. Br J Cancer. 75:367–373. 1997. View Article : Google Scholar : PubMed/NCBI
|