Introduction
Cervical cancer is one of the three most common
types of malignant cancer of the female reproductive system, with
the highest rates of mortality in gynecology (1). Surgery is the main therapeutic method
for cervical cancer, assisted by chemotherapy, traditional Chinese
medicine and biotherapy. The 5-year survival rate of cervical
cancer is ~20–30% (2). The major
cause of the low survival rates may be due to high recurrence rates
of 80%, poor progression and tolerance to chemotherapy (3). As a first-line agent, paclitaxel serves
an important role in the treatment of cervical cancer. Poor
prognosis, high recurrence and metastasis is closely associated
with resistance to taxol (4).
Toll-like receptors (TLRs) are type 1 transmembrane
proteins, which can detect invasive pathogenic microorganisms and
primarily exist in immune cells, including dendritic cells and
macrophages (5). It was previously
revealed that TLRs are closely associated with the progression of
tumor (5). It has been demonstrated
that tumor cells are able to promote its growth by employing TLR
signal transduction processes (6). By
stimulating inflammatory factors, particularly the activities of
nuclear factor (NF)-kB, TLR ligands are able to facilitate tumor
growth (7). Regarding ovarian tumors,
it has been suggested that TLR2, TLR3, TLR4 and TLR5 have high
levels of expression in normal ovarian epithelium and epithelial
ovarian neoplasms (7). It was also
revealed that TLR4 has a high expression level in ovarian granular
cells (8).
Previous studies demonstrated that the TLR/myeloid
differentiation 88 (MyD88) signaling pathway serves an essential
role in taxol resistance (9,10). Therefore, the TLR/MyD88a signaling
pathway may be a novel direction to investigate how to prevent
postoperative recurrence and chemotherapy tolerance and develop
novel methods and targets.
TLR4, which is composed of an extra-cellular region,
transmembrane domain and intra-cellular region, is a member of the
tumor necrosis factor receptor superfamily (9). The TLR4 extra-cellular region includes a
leucine-rich repeat sequence, which can promote adhesion among
proteins and serves an important role in mediating interleukin
(IL)-1-related protein kinase, MyD88 and tumor necrosis factor
receptor-associated factor (TRAF) (10). The transmembrane domain of TLR4 is a
structural region rich in cysteine. The intracellular region of
TLR4 is similar to the structures of IL-1R (10). The present study aimed to investigate
the effect of TLR4 on the growth of SiHa human cervical cancer
cells and its adjuvant function on cervical cancer.
Materials and methods
Cell lines
SiHa human cervical cancer cells were purchased from
The Cell Bank of Type Culture Collection of Chinese Academy of
Sciences (Shanghai, China) and were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Fumeng Biotechnical
Co., Ltd., Shanghai, China), 100 U/ml penicillin G (Sagon Inc.,
Shanghai, China) and 100 U/ml streptomycin (Sagon Inc.) at 37°C in
5% CO2.
Cell proliferation test
Lipopolysaccharide (LPS; 1, 5, 10, 50 and 100 µg/ml)
was added to SiHa cells (1×103 cell/well) for 4, 6 and 8
h at 37°C. Subsequently, 50 µl MTT (InvivoGen Co., Ltd., Shanghai,
China) was added to the cells for 4 h at 37°C in 5% CO2.
Dimethyl sulfoxide was added to the cells for 20 min at 37°C.
BioTek Elx800™ microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA) was used for detection using a double
wavelength at 490 nm.
Cell apoptosis
LPS (10, 50 and 100 µg/ml) was added to SiHa cells
for 8 h at 37°C. Alexa Fluor1 488 Annexin V/Dead Cell Apoptosis kit
(Thermo Fisher Scientific, Inc.) was added to the cells, and the
cells were incubated in the dark for 30 min at 37°C. A FACSCalibur
flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) was used
to analyze apoptosis and analyzed using FlowJo 7.6.1 (Tree Star,
Inc., Ashland, OR, USA).
Caspase-3 activity
LPS (10, 50 and 100 µg/ml) was added to SiHa cells
for 8 h at 37°C. SiHa cells were washed once in ice-cold PBS and
lysed in ice-cold lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) for 30 min on ice. Protein
concentration was determined using Enhanced BCA Protein Assay kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocol, following centrifugation at 10,000 × g at
4°C. A total of 30 µg protein and Ac-DEVD-pNA were incubated for 2
h at 37°C. A microplate reader (BioTek Elx800™; BioTek
Instruments, Inc.) was used for detection using a double wavelength
of 405 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from SiHa cells induced with
LPS using the NucleoSpin1 RNA II kit supplied by Machery-Nagel GmbH
(Düren, Germany). The RevertAid™ First Strand cDNA
Synthesis kit (VWR International, Darmstadt, Germany) was used to
transcribe into cDNA according to the manufacturer's protocol (37°C
for 1 h and 62°C for 10 min). SYBR Green Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used for RT-qPCR to
determine the relative levels of target mRNA for IL-6, tumor
necrosis factor-α (TNF-α) and monocyte chemoattractant protein-1
(MCP-1). PCR was performed in a three-step program as follows: 95°C
for 20 sec, 60°C for 35 sec and 72°C for 30 sec for 40 cycles.
Analysis of relative gene expression data was quantified using the
2−ΔΔCq method (11). The
primers of target genes are presented in Table I.
 | Table I.The primers of target genes. |
Table I.
The primers of target genes.
| Gene | Primers (5′-3′) |
|---|
| GAPDH |
|
|
Forward |
ACGGATTTGGTCGTATTG |
|
Reverse |
GGAAGATGGTGATGGGATT |
| TAK1 |
|
|
Forward |
AGCAAGTTCCTGCCACAAATGATA |
|
Reverse |
GCGGCGATCCTAGCTTCTATTTC |
| TRAF6 |
|
|
Forward |
TGATAGTGTGGGTGGAACTGC |
|
Reverse |
CAGATGGGGCATTCATACTTG |
| IL-6 |
|
|
Forward |
GTGGAGATTGTTGCCATCAACG |
|
Reverse |
CAGTGGATGCAGGGATGATGTTCTG |
| TNF-α |
|
|
Forward |
AACTTCTCCACAACCCTCTGC |
|
Reverse |
AGATCCATGCCGTTGGCCAG |
| MCP-1 |
|
|
Forward |
GACATACTCCAAACCTTTCCAC |
|
Reverse |
AACTTCTCCACAACCCTCTGC |
Western blot analysis
LPS (10, 50 and 100 µg/ml) was added to SiHa cells
for 8 h at 37°C. SiHa cells were washed once in ice-cold PBS and
lysed in ice-cold lysis buffer (Beyotime Institute of
Biotechnology) for 30 min on ice. Protein concentration was
determined using Enhanced BCA Protein Assay kit (Beyotime Institute
of Biotechnology) following centrifugation at 10,000 × g at 4°C. A
total of 50 µg protein was subjected to 8–12% SDS-PAGE and
transferred to polyvinylidene fluoride membranes (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The membranes were blocked
with 5% bovine serum albumin (Beyotime Institute of Biotechnology)
supplemented with TBST (0.1% Tween-20; Beyotime Institute of
Biotechnology) for 1 at 37°C and incubated with antibodies against
MyD88 (sc-11356, 1:500, Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), NF-κB (cat. no. sc-7151; 1:500, Santa Cruz Biotechnology,
Inc.), cyclin D1 (cat. no., sc-717; 1:500, Santa Cruz
Biotechnology, Inc.), phosphorylated (p)-signal transducer and
activator of transcription 3 (STAT3; cat. no., sc-8001-R; 1:500,
Santa Cruz Biotechnology, Inc.) and GAPDH (cat. no., AF1186;
Beyotime Institute of Biotechnology) at 4°C overnight followed by
incubation with horseradish peroxidase-labeled Goat Anti-Rabbit IgG
(H+L) secondary antibody (cat. no., A0208; dilution, 1:2,000;
Beyotime Institute of Biotechnology) for 1 h at 37°C. The protein
blots were visualized using the enhanced chemiluminescence method
(EMD Millipore, Billerica, MA, USA) and analyzed using
Image_Lab_3.0 (Bio-Rad Laboratories, Inc.).
Statistical analysis
All data are presented as the mean ± standard
deviation using SPSS 19.0 (IBM Corp., Armonk, NY, USA). All data
comparisons were performed using one-way analysis of variance
followed by Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Proliferation analysis of
LPS-stimulated SiHa cervical cancer cells
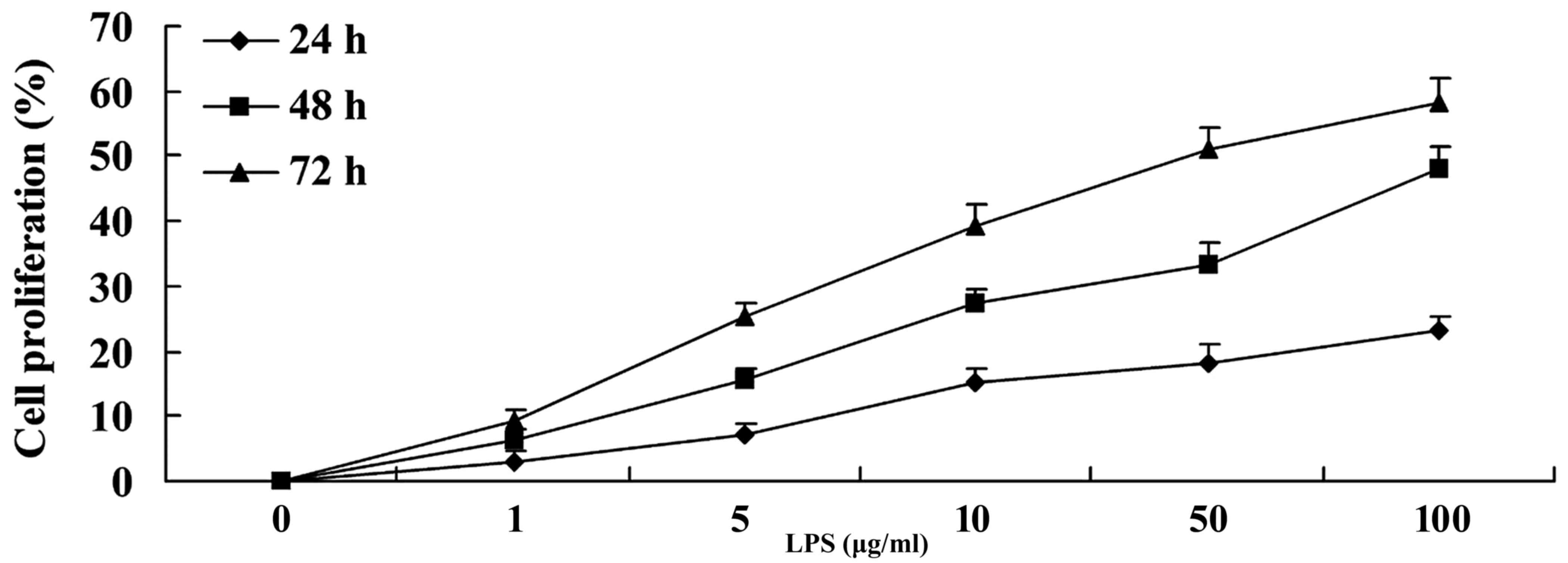
To investigate whether LPS has an effect on the
proliferation of SiHa cervical cancer cells, cell proliferation was
evaluated by MTT assay. It was revealed that LPS was able to
increase the proliferation of SiHa cervical cancer cells in a time-
and dose-dependent manner compared with the control group (0 µg/ml
LPS; Fig. 1).
Analysis of apoptosis of
LPS-stimulated SiHa cervical cancer cells
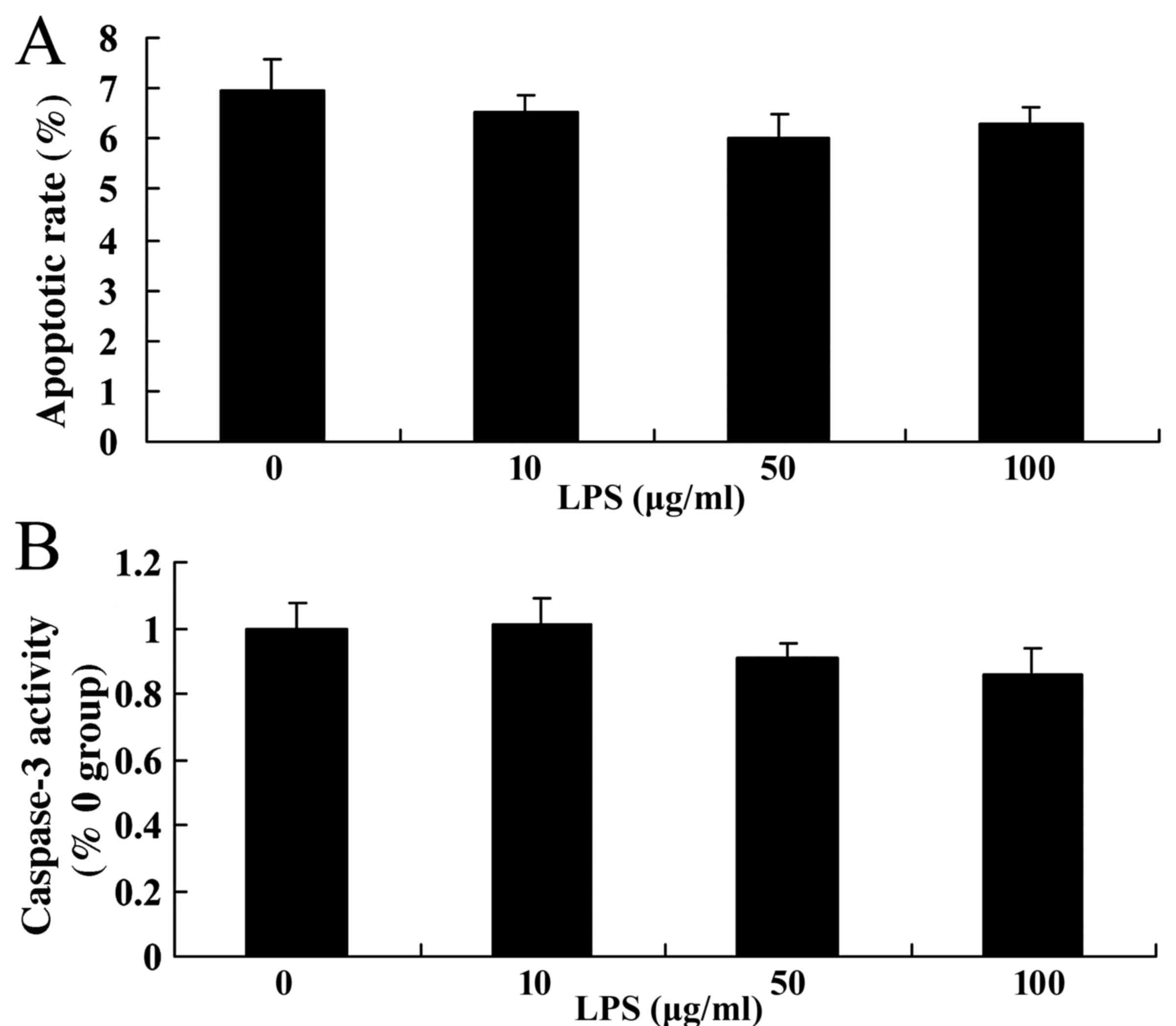
The rate of apoptosis was determined following the
stimulation of the cells with various doses of LPS by performing
flow cytometry, and the activity of caspase-3 was analyzed. It was
indicated that LPS at various doses did not have an effect on
apoptotic rate and caspase-3 activity in SiHa cells (Fig. 2), compared with the control group (0
µg/ml).
Inflammation in LPS-stimulated SiHa
cervical cancer cells
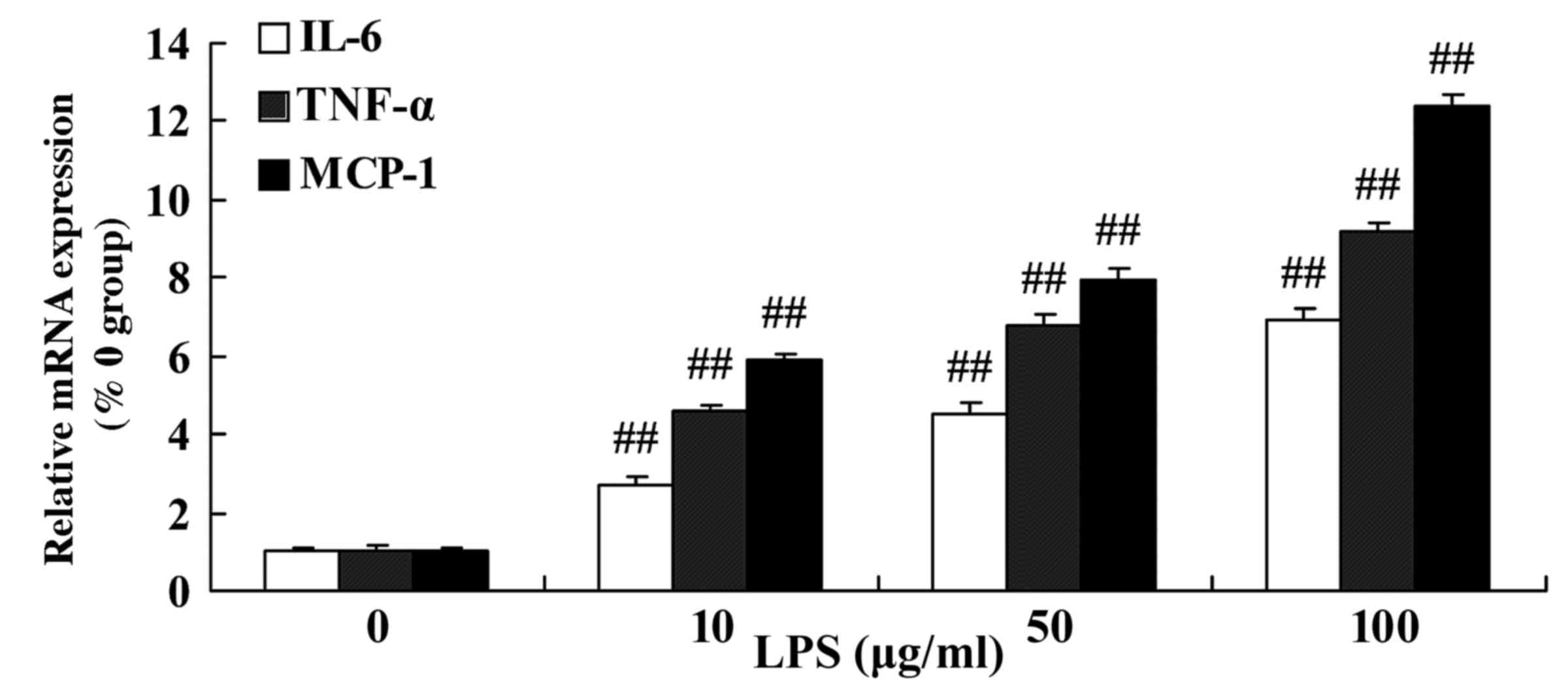
The levels of mRNA expression of IL-6, TNF-α and
MCP-1 were detected in SiHa cervical cancer cells following
stimulation by LPS. As presented in Fig.
3, treatment with 10–100 µg/ml LPS was able to significantly
increase the level of IL-6, TNF-α and MCP-1 mRNA in SiHa cells
compared with the control group (0 µg/ml LPS).
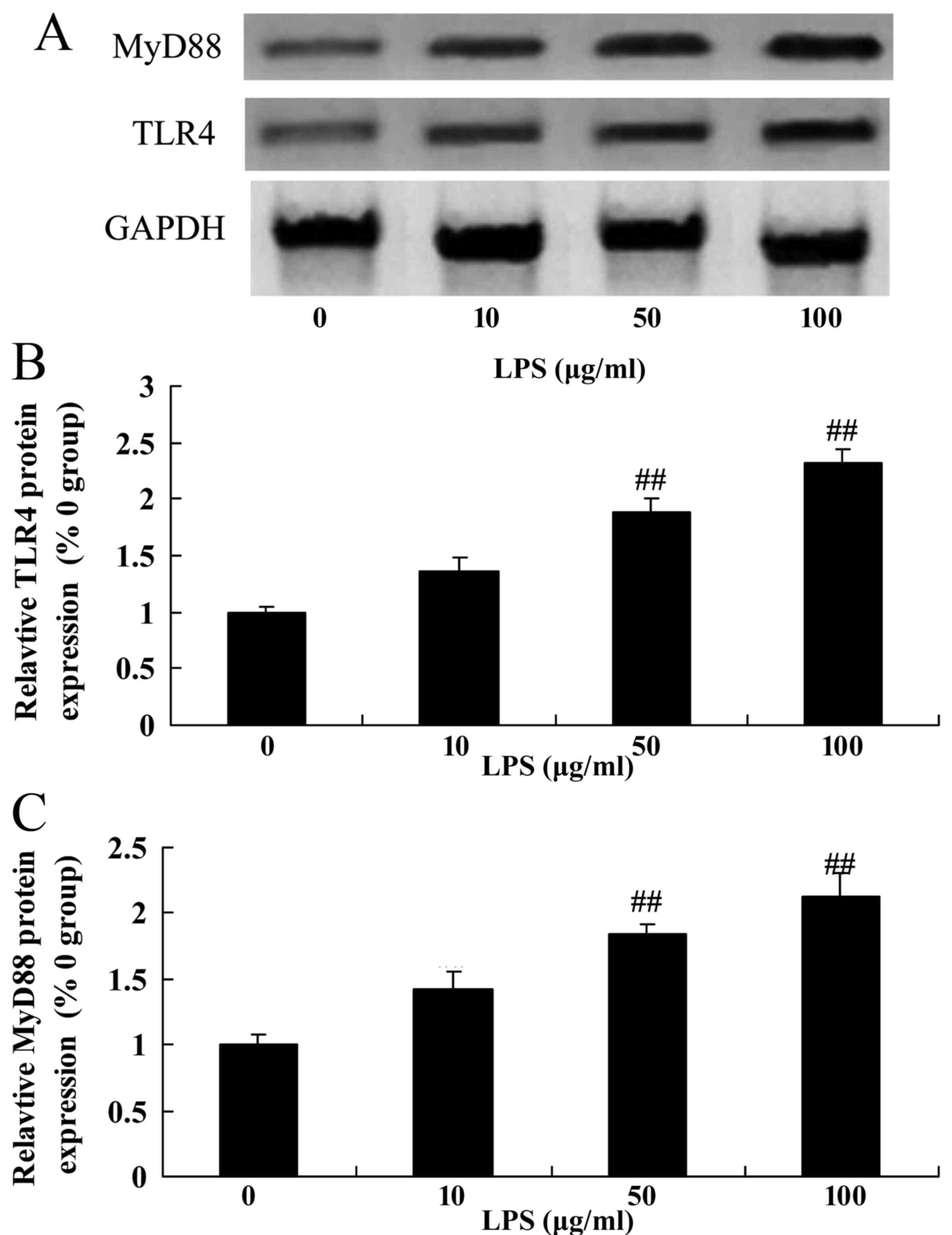
LPS may upregulate the levels of TLR4
and MyD88 expression in SiHa cervical cancer cells
The TLR4 signaling pathway, an important
inflammatory signaling pathway, was evaluated by western blotting.
Following treatment with various doses of LPS, it was revealed that
treatment with 10–100 µg/ml LPS was able to markedly induce the
level of TLR4 and MyD88 protein expression in SiHa cells compared
with the control group (0 µg/m LPS; Fig.
4).
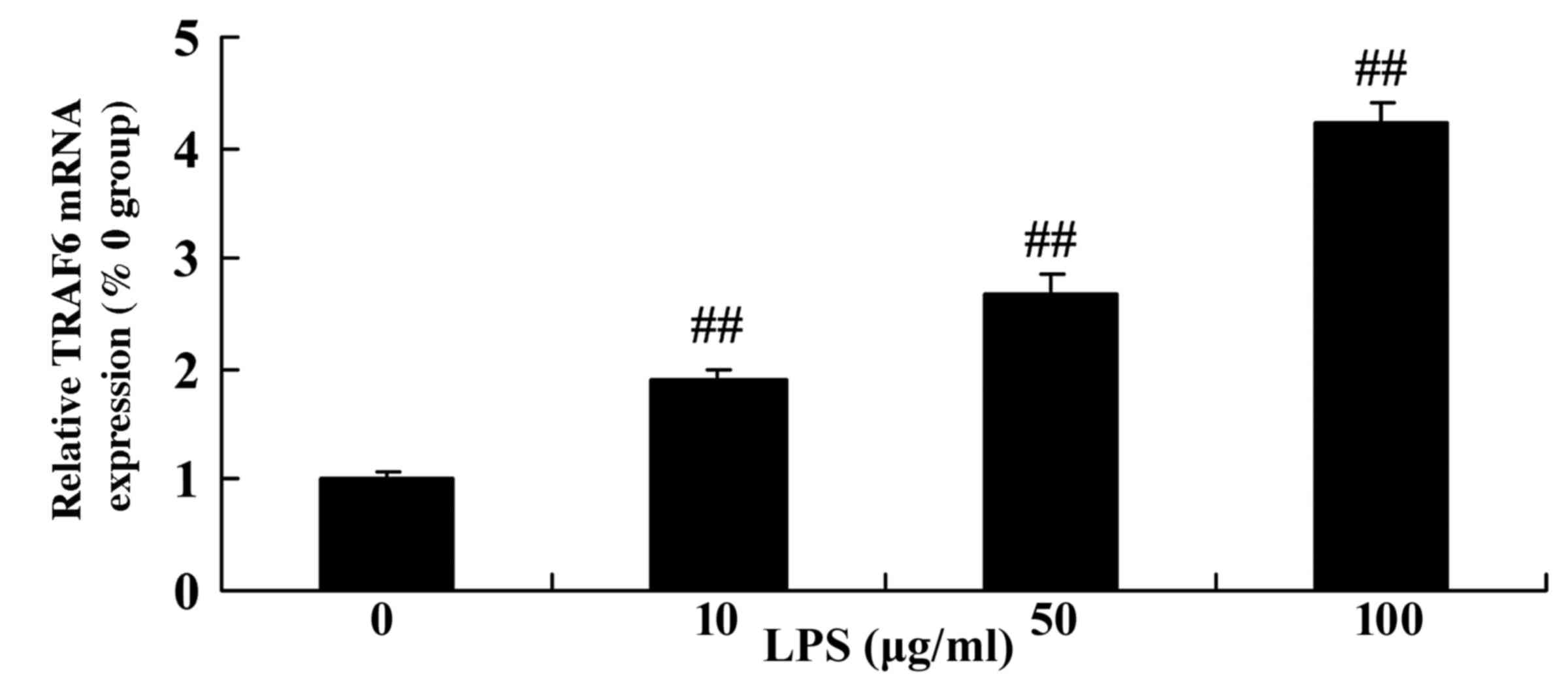
Level of TRAF6 expression in
LPS-stimulated SiHa cervical cancer cells
The levels of TRAF6 mRNA were detected following
treatment of SiHa cells with various concentrations of LPS.
Following the stimulation of SiHa cells with LPS, it was
demonstrated that the level of TRAF6 mRNA expression was markedly
upregulated compared with the control group (0 µg/ml LPS; Fig. 5).
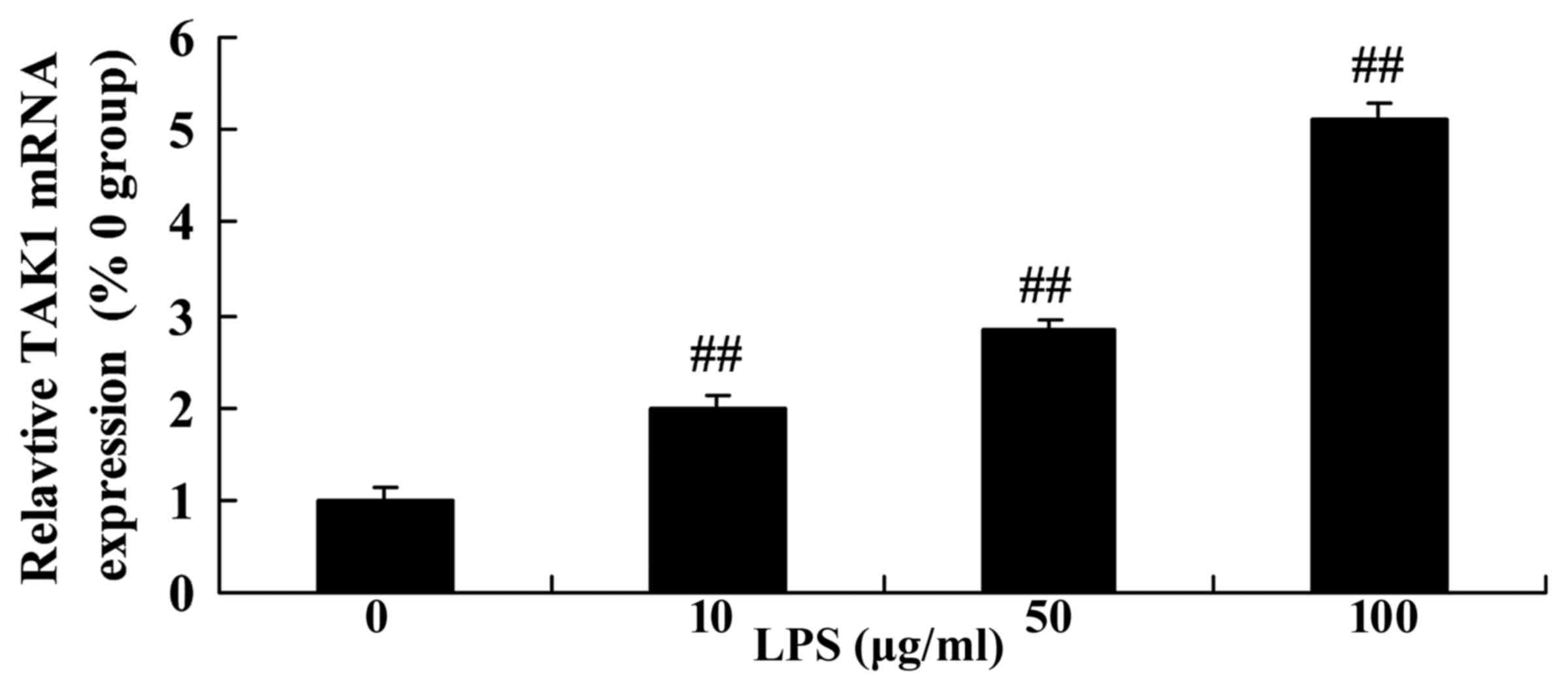
Level of transforming growth
factor-activated kinase 1 (TAK1) expression in LPS-stimulated SiHa
cervical cancer cells
In the present study, the levels of TAK1 expression
were detected by RT-qPCR in SiHa cervical cancer cells following
treatment with various concentrations of LPS. Fig. 6 revealed that the level of TAK1 mRNA
expression was significantly upregulated by treatment with 10–100
µg/ml LPS in SiHa cells compared with the control group (0 µg/ml
LPS).
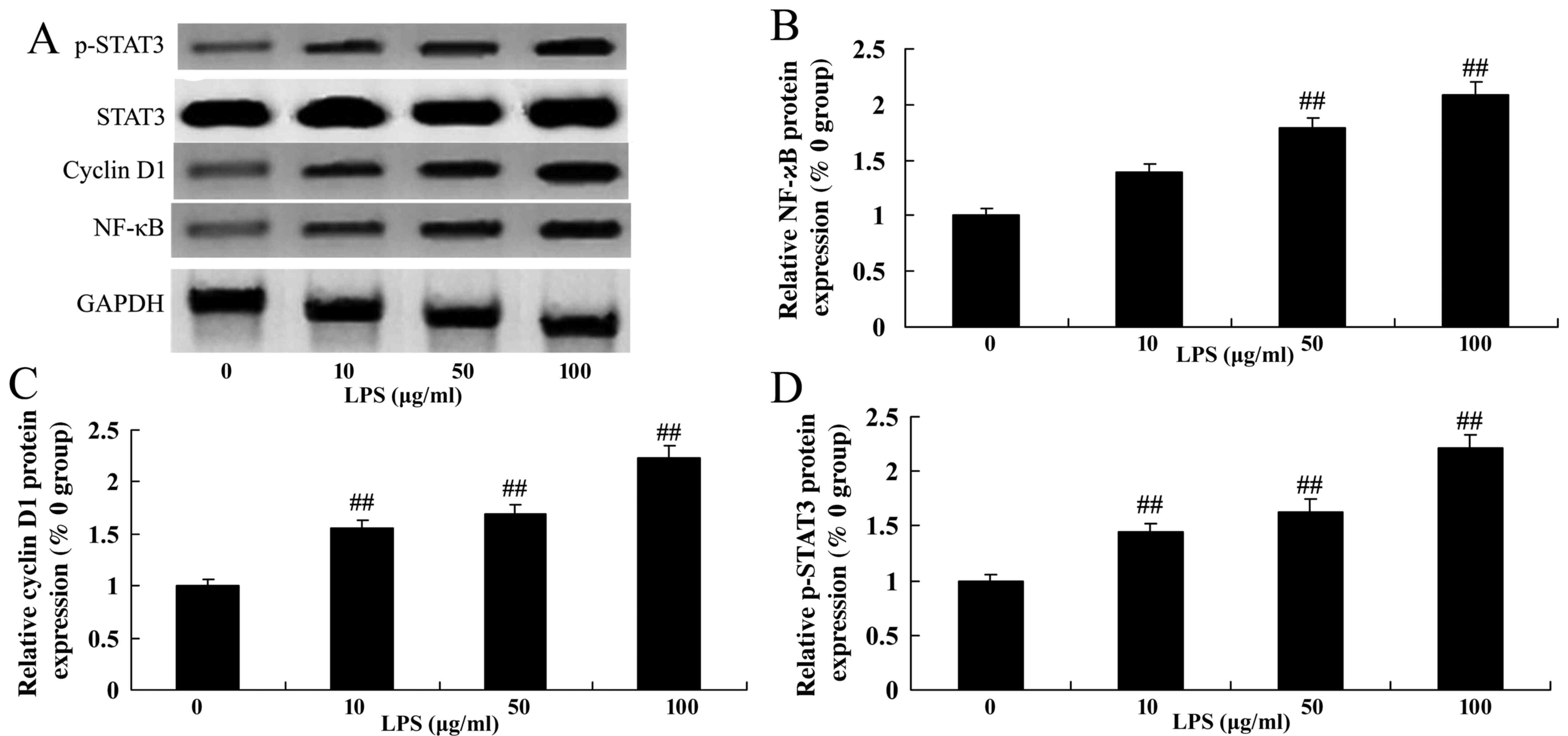
Level of NF-κB, cyclin D1 and p-STAT3
expression in LPS-stimulated SiHa cervical cancer cells
In addition, the expression level of NF-κB, cyclin
D1 and STAT3 was detected by western blotting in cervical cancer
SiHa cells that were treated with LPS. Treatment with LPS (50 and
100 µg/ml) was able to significantly increase the level of NF-κB,
cyclin D1 and p-STAT3 expression in SiHa cells compared with the
control group (0 µg/ml LPS; Fig.
7).
Discussion
The 5-year survival rates of cervical cancer are
~30%, which is a serious risk to the health and lives of females
(12). Consequently, it has become a
focus to investigate the occurrence, progression and metastasis of
cervical cancer (12). In the present
study, it was revealed that LPS increased cell proliferation but
did not affect the rate of apoptosis and caspase-3 activity in SiHa
cells.
TLR4 is a sub-type of TLRs. By activating immune
cells, TLR is able to generate an endogenous or exogenous immune
response (6). It has previously been
demonstrated that TLR4 is closely associated with the occurrence
and progression of tumors (6). A
previous study revealed that TLR4 is expressed in various types of
malignant cancer, including gastric carcinoma, hepatocellular
carcinoma, prostatic cancer and lymphoma. In particular, genetic
polymorphism of Asp299Gly of TLR4 is highly associated with the
occurrence and progression of tumors (6). In addition, TLR4 serves a fundamental
role in the apoptosis of tumor cells (9). By inhibiting apoptosis, TLR4 enhances
the immune evasion of tumor cells. A previous study reported that
TLR4 is positively expressed in epithelial ovarian cancer tissues
or cell lines, which may provide a novel therapeutic target for
treatment of cervical cancer (9). In
the present study, LPS upregulated the expression of TLR4 protein
in SiHa cells. These results indicated that TLR4 may mediate the
growth of cervical cancer SiHa cells.
The TRAF6/TAK1 signaling pathway serves an important
role in mechanisms of innate and acquired immunity (13). The TRAF6/TAK1 signaling pathway also
participates in the immune response pathway mediated by TCR. A
previous study suggested that the expression levels of TRAF6
correspond with those of TAK1 (13).
By activating the immune modulatory pathways of NF-κB and
mitogen-activated protein kinase (MAPK) and inducing the release of
IL-6, TNF-α and interferon-α/β, the activation of B cells and the
maturity and proliferation of T cells may be promoted by the
TRAF6/TAK1 signaling pathway (13).
Additionally, the TRAF6/TAK1 signaling pathway may participate in
the regulation of the activation and maturation of dendritic cells
(14). Therefore, antitumor immune
functions can be induced (14). In
the present study, it was revealed that treatment with LPS was able
to significantly enhance IL-6, TNF-α and MCP-1 mRNA expression
levels in SiHa cells.
Following binding with MyD88, members of the
interleukin 1 receptor associated kinase (IRAK) family are
recruited by TLRs, including IRAK1, IRAK2, IRAK4 and IRAK-M. Once
IRAK4 is phosphorylated, it would separate from MyD88 and interact
with TRAF6 (14). The binding of TAK1
leads to the activation of two downstream signaling pathways,
including the IκB kinase (IKK) complex and MAPK family. Composed of
IKK-d, IxB, IKK-p and IKK-y/NF-κB essential modulator, IKK complex
is able to catalyze the phosphorylation of the IκB protein
(14,15). This type of phosphorylation can
trigger the degradation of IкBs and the activation of NF-κB, as
well as inducing the upregulation of anti-apoptosis. In the present
study, it was revealed that treatment with LPS was able to
significantly activate MyD88 protein expression and promote TRAF6
and TAK1 mRNA expression in SiHa cervical cancer cells. These
results indicated that the effect of TLR4 on cervical cancer may be
mediated by the MyD88-TRAF6-TAK1 signaling pathway. Zeng et
al suggested that MC13 was able to protect against
neuro-inflammatory injury through suppression of the
TRAF6-TAK1-NF-κB signaling pathways (16).
TRAF is a multi-functional adaptor molecule in the
cytoplasm. TRAF6 is an ubiquitin-ligating enzyme, which is able to
activate TAK1 (17). TAK1 is a
serine/threonine kinase, which is involved in various signal
transduction pathways. Activated TAK1 is able to phosphorylate and
activate the NF-κB-MAPK signaling transduction pathway and finally
activate transduction factors, including NF-κB and adaptor protein
complex-1 (18). The expression of
TRAF6 and TAK1 were induced in malignant types of cancer and
chronic or acute inflammatory diseases, including breast cancer,
colorectal cancer, bone metastasis, lung injuries, rheumatoid
arthritis and multiple sclerosis (19). In the present study, it was revealed
that treatment with 50 and 100 µg/ml LPS was able to significantly
induce NF-κB protein expression in SiHa cervical cancer cells.
Cyclin D1 and p27 protein serve a particular and
interactive role in tumor progression and have certain implications
for the evaluation of the progression, metastasis and prognosis of
cancer (20). The G1/S
checkpoint is the most important regulatory point in the cell cycle
process. As a rate-limiting regulator in G1 cell cycle
phase, a high level of cyclin D1 expression shortens the length of
the G1 phase (21). The
regulation of cyclin D1 to G1 phase is mediated by the
regulation to phosphorylated retinoblastoma protein (22). The results of the present study
indicated that the treatment with LPS significantly increased
cyclin D1 protein expression in SiHa cells.
STAT3 with high degrees of phosphorylation do not
exist in normal ovarian epithelial cells lines or benign ovarian
tumor cell lines, and abnormal activation of the STAT3 signal
transduction pathway may induce a malignant phenotype (23). Previous studies investigating the
downstream target gene of the STAT3 transduction pathway have
provided increasing evidence, which suggested that the STAT3
transduction pathway serves an important role in a number of
processes, including overproliferation, inhibition of apoptosis,
vascular reconstruction, invasion and metastasis and drug
resistance (24). In addition, in the
present study, it was revealed that treatment with LPS was able to
significantly induce the expression of p-STAT3 protein in SiHa
cells. Chen et al (22)
demonstrated that treatment with Schizandrin A was able to suppress
microglia-mediated neuron-inflammation via LPS-induced TRAF6-NF-κB
and Jak2-Stat3 signaling.
In conclusion, the results of the present study
demonstrated that treatment with LPS was able to effectively
increase cell proliferation by regulating TLR4 expression in SiHa
cells but did not have an effect on rate of apoptosis and caspase-3
activity. It was also indicated that TLR4 was able to regulate the
growth of SiHa human cervical cancer cells via the MyD88-TRAF6-TAK1
and NF-κB-cyclin D1-STAT3 signaling pathways. The results suggest
that TLR4 antagonists may be potential therapeutic agents for the
treatment of human cervical cancer by modulating the
MyD88-TRAF6-TAK1 and NF-κB-cyclin D1-STAT3 signaling pathways,
however further studies are required.
References
|
1
|
Symonds RP, Gourley C, Davidson S, Carty
K, McCartney E, Rai D, Banerjee S, Jackson D, Lord R, McCormack M,
et al: Cediranib combined with carboplatin and paclitaxel in
patients with metastatic or recurrent cervical cancer (CIRCCa): A
randomised, double-blind, placebo-controlled phase 2 trial. Lancet
Oncol. 16:1515–1524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braicu EI, Fotopoulou C, Chekerov R,
Richter R, Blohmer J, Kümmel S, Stamatian F, Yalcinkaya I, Mentze
M, Lichtenegger W and Sehouli J: Role of serum concentration of
VEGFR1 and TIMP2 on clinical outcome in primary cervical cancer:
Results of a companion protocol of the randomized, NOGGO-AGO phase
III adjuvant trial of simultaneous cisplatin-based
radiochemotherapy vs. carboplatin and paclitaxel containing
sequential radiotherapy. Cytokine. 61:755–758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burtness B, Bourhis JP, Vermorken JB,
Harrington KJ and Cohen EE: Afatinib versus placebo as adjuvant
therapy after chemoradiation in a double-blind, phase III study
(LUX-Head & Neck 2) in patients with primary unresected,
clinically intermediate-to-high-risk head and neck cancer: Study
protocol for a randomized controlled trial. Trials. 15:4692014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muwonge R, Wesley RS, Nene BM, Shastri SS,
Jayant K, Malvi SG, Thara S and Sankaranarayanan R: Evaluation of
cytology and visual triage of human papillomavirus-positive women
in cervical cancer prevention in India. Int J Cancer.
134:2902–2909. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DeCarlo CA, Rosa B, Jackson R, Niccoli S,
Escott NG and Zehbe I: Toll-like receptor transcriptome in the
HPV-positive cervical cancer microenvironment. Clin Dev Immunol.
2012:7858252012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Werner J, Decarlo CA, Escott N, Zehbe I
and Ulanova M: Expression of integrins and Toll-like receptors in
cervical cancer: Effect of infectious agents. Innate Immun.
18:55–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L, Cheng FW, Wang F, Jia B, Luo X and
Zhang SQ: The activation of TLR7 regulates the expression of VEGF,
TIMP1, MMP2, IL-6, and IL-15 in Hela cells. Mol Cell Biochem.
389:43–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhan Z, Xie X, Cao H, Zhou X, Zhang XD,
Fan H and Liu Z: Autophagy facilitates TLR4- and TLR3-triggered
migration and invasion of lung cancer cells through the promotion
of TRAF6 ubiquitination. Autophagy. 10:257–268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao J, Guo Q, Wang X, Xie F, Zhang H and
Sui L: Study on the expression and signification of TLR4/NO pathway
in cervical tumorigenesis with high risk HPV infection. Zhonghua Fu
Chan Ke Za Zhi. 50:41–47. 2015.(In Chinese). PubMed/NCBI
|
|
10
|
He A, Ji R, Shao J, He C, Jin M and Xu Y:
TLR4-MyD88-TRAF6-TAK1 complex-mediated NF-κB activation contribute
to the anti-inflammatory effect of V8 in LPS-induced human cervical
cancer SiHa cells. Inflammation. 39:172–181. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kirchheiner K, Nout RA, Czajka-Pepl A,
Ponocny-Seliger E, Sturdza AE, Dimopoulos JC, Dörr W and Pötter R:
Health related quality of life and patient reported symptoms before
and during definitive radio (chemo)therapy using image-guided
adaptive brachytherapy for locally advanced cervical cancer and
early recovery-a mono-institutional prospective study. Gynecol
Oncol. 136:415–423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tomomura M, Suzuki R, Shirataki Y,
Sakagami H, Tamura N and Tomomura A: Rhinacanthin C inhibits
osteoclast differentiation and bone resorption: Roles of
TRAF6/TAK1/MAPKs/NF-κB/NFATc1 signaling. PLoS One. 10:e01301742015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He W and Cronstein BN: Adenosine A1
receptor regulates osteoclast formation by altering TRAF6/TAK1
signaling. Purinergic Signal. 8:327–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bai C, Yang X, Zou K, He H, Wang J, Qin H,
Yu X, Liu C, Zheng J, Cheng F and Chen J: Anti-proliferative effect
of RCE-4 from Reineckia carnea on human cervical cancer HeLa cells
by inhibiting the PI3K/Akt/mTOR signaling pathway and NF-κB
activation. Naunyn Schmiedebergs Arch Pharmacol. 389:573–584. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng KW, Yu Q, Liao LX, Song FJ, Lv HN,
Jiang Y and Tu PF: Anti-neuroinflammatory effect of MC13, a novel
coumarin compound from condiment murraya, through inhibiting
lipopolysaccharide-induced TRAF6-TAK1-NF-κB, P38/ERK MAPKS and
Jak2-Stat1/Stat3 pathways. J Cell Biochem. 116:1286–1299. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nees M, Geoghegan JM, Hyman T, Frank S,
Miller L and Woodworth CD: Papillomavirus type 16 oncogenes
downregulate expression of interferon-responsive genes and
upregulate proliferation-associated and NF-kappaB-responsive genes
in cervical keratinocytes. J Virol. 75:4283–4296. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang L, Gu L, Li Z and Zhou M: Translation
of TRAF1 is regulated by IRES-dependent mechanism and stimulated by
vincristine. Nucleic Acids Res. 38:4503–4513. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Capalbo G, Mueller-Kuller T, Koschmieder
S, Klein HU, Ottmann OG, Hoelzer D and Scheuring UJ:
Characterization of ZC3H15 as a potential TRAF-2-interacting
protein implicated in the NFκB pathway and overexpressed in AML.
Int J Oncol. 43:246–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Y, Fu H, Zhang H, Huang H, Chen M,
Zhang L, Yang H and Qin D: Cyclin D1 (CCND1) G870A polymorphisms
and cervical cancer susceptibility: A meta-analysis based on ten
case-control studies. Tumour Biol. 35:6913–6918. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ni HJ, Chang YN, Kao PH, Chai SP, Hsieh
YH, Wang DH and Fong JC: Depletion of SUMO ligase hMMS21 impairs G1
to S transition in MCF-7 breast cancer cells. Biochim Biophys Acta.
1820:1893–1900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen J, Bai M, Ning C, Xie B, Zhang J,
Liao H, Xiong J, Tao X, Yan D, Xi X, et al: Gankyrin facilitates
follicle-stimulating hormone-driven ovarian cancer cell
proliferation through the PI3K/AKT/HIF-1α/cyclin D1 pathway.
Oncogene. 35:2506–2517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan Z, Cui H, Yu H, Ji Q, Kang L, Han B,
Wang J, Dong Q, Li Y, Yan Z, et al: MiR-125a promotes paclitaxel
sensitivity in cervical cancer through altering STAT3 expression.
Oncogenesis. 5:e1972016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang P, Yang B, Yao YY, Zhong LX, Chen
XY, Kong QY, Wu ML, Li C, Li H and Liu J: PIAS3, SHP2 and SOCS3
expression patterns in cervical cancers: Relevance with activation
and resveratrol-caused inactivation of STAT3 signaling. Gynecol
Oncol. 139:529–535. 2015. View Article : Google Scholar : PubMed/NCBI
|