Introduction
Melanoma is a malignant tumor of melanocytes and is
considered to be the most invasive and dangerous cutaneous cancer
(1). The median 5-year survival rate
is <5% following metastasis and the incidence of melanoma has
increased over the past few decades (2). Traditional therapies including surgery,
chemotherapy and radiation have not significantly increased in
overall survival for these patients over the past 10 years
(3,4).
Patients with metastasis or recurrence present a formidable
challenge despite new therapeutic treatments, such as immunotherapy
and molecular-targeted chemotherapy (5–7).
Therefore, it is necessary and urgent to develop new strategies for
the patients with melanoma.
Traditional Chinese herbal medicines have been
discovered to have excellent anticancer activity in recent decades
(8–11). Andrographolide (Andro), the active
ingredient of the traditional Chinese medicine Andrographis
paniculata, has been used primarily for analgesic (12). It has been proved that Andro possess
various biological activities such as anti-inflammation,
anti-infection, immune system regulation, anti-cardiovascular
disease, and anticancer effects (13–15).
Previous studies have shown that Andro exhibited potential
antitumor activity in various malignancies, including gastric
cancer (16), chondrosarcoma
(17) and colorectal cancer (18). However, whether Andro suppresses the
growth of human melanoma cells and its potential molecular
mechanisms were still not well investigated.
In the present study, we evaluated that Andro can
effectively inhibit the proliferation of melanoma cells by causing
G2/M cell cycle arrest, and lead to cell death by inducing
apoptosis. Furthermore, the underlying molecular mechanisms were
discussed by JNK and p38 signaling pathways.
Materials and methods
Cells and reagents
Human malignant melanoma A375 and C8161 cell lines
were purchased from American Type Culture Collection (ATCC;
Manassas, VA, USA). All cells were grown in high-glucose Dulbecco's
modified Eagle's medium (DMEM; Hyclone, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin
and 100 µg/ml streptomycin (all from Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C and 5% CO2.
Andrographolide (MF: C8H8O4, MW:
168.15, purity >98%) was purchased from Shanghai Yuanye
Biotechnology, Co., Ltd. (Shanghai, China) and dissolved in
dimethyl sulfoxide (DMSO) at a stock concentration of 80 mM.
Antibodies against cleaved-PARP, cleaved-caspase-3, phospho-JNK,
JNK, p38, phospho-p38, and GAPDH were all obtained from Cell
Signaling Technology, Inc. (Beverly, MA, USA).
Cell viability assay
Inhibition of cell proliferation by Andro was
detected using the 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl
tetrazolium bromide (MTT) assay. Briefly, cells were trypsinized
and plated into 96-well plate at a density of 5×103
cells/well and incubated overnight at 37°C in a humidified
incubator, then treated with fresh medium containing various
concentration of Andro (5, 10, 20, 40, and 80 µM). And the cells
were incubated for 24, 48 h. Subsequently, final concentration of
0.5 mg/ml MTT was added directly to and incubated for 4 h at 37°C.
The plates were depleted and a total of 100 µl of DMSO was added to
each well, and the optical density was measured at 490 nm using
microplate reader iMark (Molecular Devices, LLC, Sunnyvale, CA,
USA). Data represented the mean of five replicates. Three
independent experiments were carried out in triplicate.
Cell cycle analysis by flow
cytometry
To examine whether Andro affects cell cycle
distribution, we used flow cytometer with PI/RNase staining buffer
(BD Biosciences, Mountain View, CA, USA). In brief,
5×105 cells were seeded in 6-well plates and incubated
with Andro for 24 h at 37°C. And then the cells were collected and
fixed with 70% ethanol at −20°C overnight. The cells were washed
and resuspended in cold PBS and followed by staining with 10 mg/ml
RNase and 1 mg/ml propidium iodide for 30 min at 37°C. Next, the
samples were detected on Accuri C6 (BD Biosciences) and the data
were analyzed by ModFit LT software (FACSCalibur).
Flow cytometric analysis of
apoptosis
To evaluate apoptosis induction, a FITC-Annexin
apoptosis detection kit (BD Biosciences, Sparks, MD, USA) was used.
Cells were seeded in 6-well plates and treated with or without
Andro for 24 h. Apoptotic cells were measured by using Annexin
V-FITC/PI double staining. Briefly, cells were collected and washed
with ice-cold PBS, and then resuspended in 500 µl of binding
buffer. These cells were stained with 5 µl of Annexin V/FITC
solution and 10 µl propidium iodide (PI) (1 g/ml). Cells were then
incubated for 15 min at 37°C in the dark. The samples were detected
by the Accuri C6.
Western blot analysis
Cells were cultured in 6-well plates and treated
with as indicated for 24 h. The cell were washed twice with cold
PBS solution and then resuspended in radioimmunoprecipitation assay
(RIPA) lysis buffer with phosphorylase and protease inhibitor. The
protein concentration was determined using using BCA Protein Assay
kit and equalized before loading. Equivalent amounts of total
protein (25–50 µg) were separated by SDS-PAGE and and transferred
onto a PVDF membrane (0.45 mm; Millipore, Bedford, MA, USA).
Following blocking with 5% fat-free milk for 60 min, membrane was
blotted with the following primary antibodies as follows:
Cleaved-PARP, cleaved-caspase-3, phospho-JNK, JNK, p38,
phospho-p38, and GAPDH at 4°C overnight. Horseradish
peroxidase-linked anti-mouse or anti-rabbit IgG was then used as
secondary antibody. And cells were incubated with horseradish
peroxidase-linked anti-mouse or anti-rabbit IgG (1:5,000 dilution)
for 1 h. Specific antibody binding was visualized using ECL
(Millipore, Plano, TX, USA) and digitalized by scanning.
Statistical analysis
All data represent at least three independent
experiments and were expressed as mean ± standard deviation (SD).
The statistical differences were calculated by one-way ANOVA
analysis of variance with Dunnett's test or unpaired Student's
t-test. All statistical analyses were performed using SPSS 19.0
software and significant difference was analyzed by Duncan's
multiple-range test. (SPSS Inc., Chicago, IL, USA). P-values
<0.05 were considered to indicate a statistically significant
difference.
Results
Cell growth inhibition of Andro on
human malignant melanoma cell lines
To investigate the antiproliferative activity of
Andro, A375 and C8161 cells were treated with different
concentration of Andro for 24 and 48 h (Fig. 1). The cell viability was measured by
MTT assay. The IC50 values of Andro for A375 were 23.08
µM (24 h), 12.07 µM (48 h), while the IC50 values for
C8161 were 20.31 µM (24 h), 10.92 µM (48 h). These results suggest
that Andro exhibits potent anti-proliferation effects in malignant
melanoma cells in a dose- and time-dependent manner.
Andro induces cell cycle G2/M arrest
in melanoma cell lines
To gain insights into the mechanism by which Andro
inhibits cell proliferation, we examined the effect of Andro on the
cell cycle progression. As shown in Fig.
2, in comparison to DMSO-treated cells, a significant increase
of cells in G2/M phase was observed after Andro treatment, while a
corresponding decrease in G0/G1 phases was observed after Andro
treatment. These data showed that Andro induces G2/M phase
arrest.
Andro induces apoptosis in melanoma
cell lines
To examine whether the cell growth inhibition
induced by Andro is also dependent on apoptosis, Andro-treated
cells were analysed by Annexin V-FITC/PI double staining. As shown
in Fig. 3A and B, in comparison to
DMSO-treated cells, Andro resulted in a significant increase of the
proportion of apoptosis the number of apoptotic cells in a
dosedependent manner. Western blot analyses showed that cleaved
poly(ADP-ribose) polymerase (PARP) and cleaved caspase-3 were
increased after treatment with Andro for 24 h (Fig. 3C). Overall, these results clearly
indicate that Andro provoks caspase-dependent apoptosis in melanoma
cell lines.
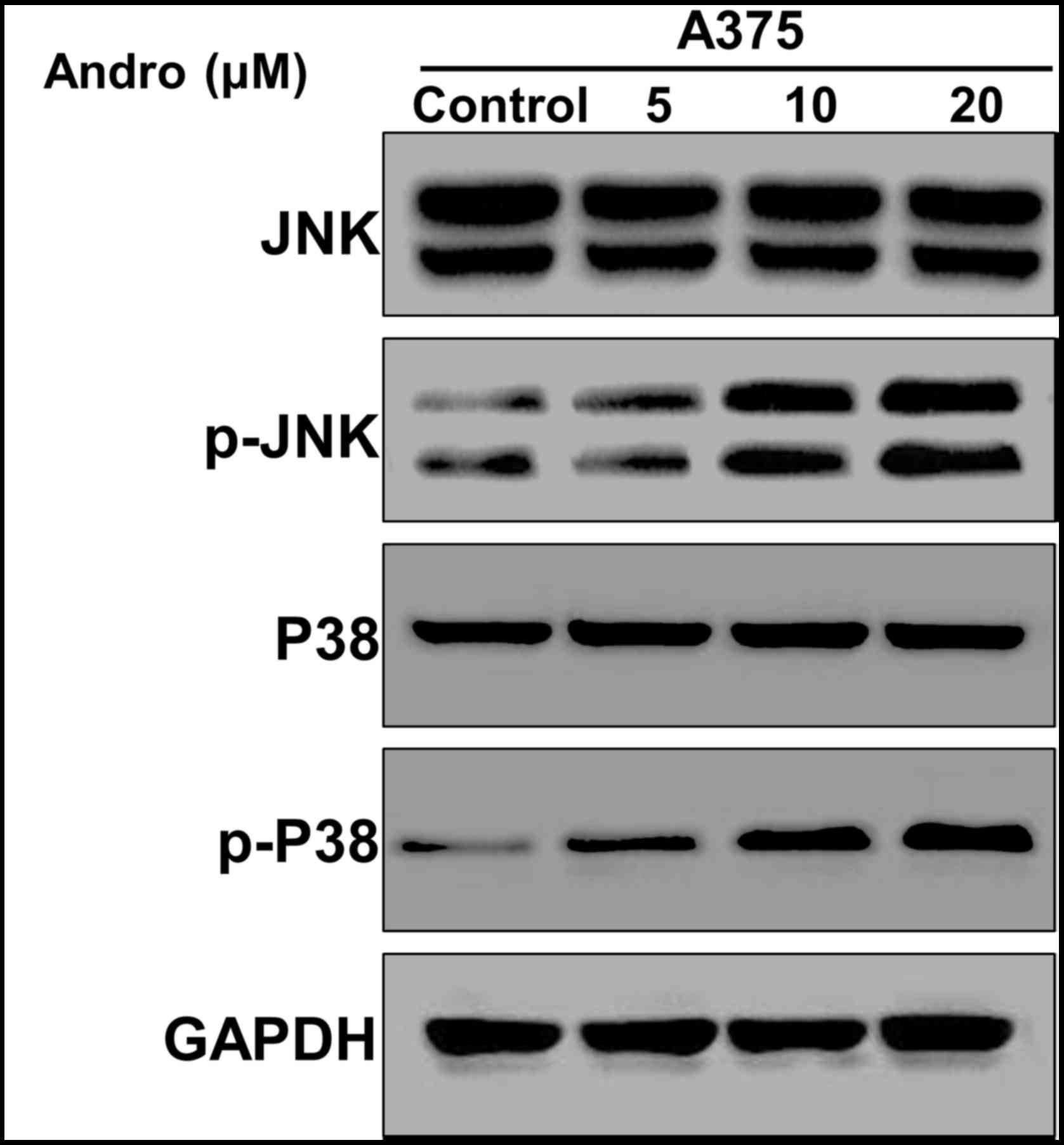
Andro activates JNK and p-P38
signaling pathway
Recent studies have suggested JNK signaling pathway
plays important roles in many cellular events, such as regulating
cell cycle, cell apoptosis and autophagy (19,20). To
further elucidate the effect of Andro on JNK signal pathway, we
measured the expression of JNK an p-P38 genes. As shown in Fig. 4, phosphorylation of JNK and p-P38 were
induced in a dose-dependently manner after Andro treatment. These
results clearly indicate that Andro activated the JNK and p-P38
signaling pathway.
Discussion
Melanoma is one of the most aggressive and mortal
cancers that occurs frequently with a significant contribution of
environmental factors to its etiology (21,22).
Traditional therapies include cryotherapy, surgery, and
chemotherapy, which is difficult to accord with the clinical
requirement (23,24). Some nonsurgical treatments are usually
limited to auxiliary treatment. Therefore, it is urgent to search
for natural drug to further improve the outcome of melanoma
patients. Andro, one of the major components of Andrographis
paniculata, has been used as an effective, safe and antitumor
drug for several centuries (25). In
the present study, we revealed that Andro could inhibit
proliferation of human melanoma cell lines through inducing G2/M
cycle arrest and cell apoptosis. Also, we found that Andro
simultaneously induces apoptosis by activating JNK pathway.
Imbalance of cell cycle regulation is one
characteristic of cancer. Many studies have highlighted that
induction of cell cycle arrest especially G2/M cycle phase may be
an effective anticancer therapy (9,26). Many
anticancer agents inhibit tumor cell proliferation by causing G2/M
cycle arrest, such as celastrol (27), ophiopogonin D (26). The G2 checkpoint regulats cell
caryomitosis when DNA is destroyed, which provides repair
opportunities for damaged cells (28,29). Our
studies showed that Andro decreased the cell proportion in G0/G1
and S phases while Andro induced G2/M phase arrest in human
malignant melanoma A375 and C8161 cell lines. However, the
underlying mechanism need to be further explored.
Previous studies have showed that there are two main
apoptotic pathways in cell apoptosis: The extrinsic or death
receptor pathway and the intrinsic or mitochondrial pathway
(30,31). The apoptotic pathway is triggered by
activation of caspase-3 anderesults in DNA fragmentation and the
cleavage of PARP (32,33). Meanwhile, Apoptosis of cells can
further influence cell proliferation. In the current study, flow
cytometry with Annexin V/PI staining suggested that Andro induced
apoptosis in human malignant melanoma A375 cells. Accordingly, the
protein expression of cleaved-PARP and cleaved-caspase-3 were all
remarkably increased after treatment with Andro. These data
indicated that Andro induced cell apoptosis.
In addition, we explored the effect of Andro on the
upstream pathways. Considerable evidence has delineated that c-Jun
N-terminal kinases (JNKs) is an important intracellular signaling
pathway of MAPKs family and are involved in a wide spectrum of cell
physiology, such as cell proliferation, cycle arrest, apoptosis and
autophagy (34,35). JNK has three subtypes, JNK1, JNK2, and
JNK. The function of JNK is intricate, which may be depending on
cell subtype, external stimulus condition (36). In our current study, we found that
treatment with Andro induced a significant increase in JNK and
p-p38 phosphorylation in human malignant melanoma A375 cells, which
may be related to cell cycle arrest and apoptosis.
In conclusion, our study elucidated that antitumor
effects of Andro on human malignant melanoma and the underlining
molecular mechanisms. We also demonstrated Andro could inhibit cell
proliferation by causing G2/M phase arrest, and cell apoptosis. In
addition, we also demonstrated that Andro activated JNK and p38
signaling pathway. Further investigations are needed to
comprehensively explore the molecular mechanism of Andro in our
future study, which may better understand the function of Andro on
melanoma. To conclude, our findings suggests that Andro is a novel
anticancer drug candidate which can be used as a potential
operative agent for malignant melanoma patients.
Acknowledgements
This study was supported by the Science Foundation
of Shandong Province (no. S20160921).
References
|
1
|
Liu J, Gu J, Feng Z, Yang Y, Zhu N, Lu W
and Qi F: Both HDAC5 and HDAC6 are required for the proliferation
and metastasis of melanoma cells. J Transl Med. 14:72016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kircher DA, Silvis MR, Cho JH and Holmen
SL: Melanoma brain metastasis: Mechanisms, models and medicine. Int
J Mol Sci. 17:E14682016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lincoln R, Kohler L, Monro S, Yin H,
Stephenson M, Zong R, Chouai A, Dorsey C, Hennigar R, Thummel RP
and McFarland SA: Exploitation of long-lived 3IL excited states for
metal-organic photodynamic therapy: Verification in a metastatic
melanoma model. J Am Chem Soc. 135:17161–17175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
George DD, Armenio VA and Katz SC:
Combinatorial immunotherapy for melanoma. Cancer Gene Ther.
24:141–147. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Asmar R, Yang J and Carvajal RD: Clinical
utility of nivolumab in the treatment of advanced melanoma. Ther
Clin Risk Manag. 12:313–325. 2016.PubMed/NCBI
|
|
7
|
Wu KJ, Huang JM, Zhong HJ, Dong ZZ,
Vellaisamy K, Lu JJ, Chen XP, Chiu P, Kwong DWJ, Han QB, et al: A
natural product-like JAK2/STAT3 inhibitor induces apoptosis of
malignant melanoma cells. PLoS One. 12:e01771232017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang T, Li J, Yin F, Lin B, Wang Z, Xu J,
Wang H, Zuo D, Wang G, Hua Y and Cai Z: Toosendanin demonstrates
promising antitumor efficacy in osteosarcoma by targeting STAT3.
Oncogene. 36:6627–6639. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang G, Zhang T, Sun W, Wang H, Yin F,
Wang Z, Zuo D, Sun M, Zhou Z, Lin B, et al: Arsenic sulfide induces
apoptosis and autophagy through the activation of ROS/JNK and
suppression of Akt/mTOR signaling pathways in osteosarcoma. Free
Radic Biol Med. 106:24–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan Z, Shao HX, Liu T, Lu XY and Fan XH:
Research progress of 5-hydroxymethylfurfural, a safety-related
substance in traditional Chinese medicine injections. Zhongguo
Zhong Yao Za Zhi. 42:1842–1846. 2017.(In Chinese). PubMed/NCBI
|
|
11
|
Song J, Zhong RL, Xia Z, Wu H, Zhong QX,
Zhang ZH, Wei YJ, Shi ZQ, Feng L and Jia XB: Research and
application of hepatotoxicity evaluation technique of traditional
Chinese medicine. Zhongguo Zhong Yao Za Zhi. 42:41–48. 2017.(In
Chinese). PubMed/NCBI
|
|
12
|
Chakravarti RN and Chakravarti D:
Andrographolide, the active constituent of Andrographis paniculata
Nees; a preliminary communication. Ind Med Gaz. 86:96–97.
1951.PubMed/NCBI
|
|
13
|
Jotwani R, Eswaran SV, Moonga S and Cutler
CW: MMP-9/TIMP-1 imbalance induced in human dendritic cells by
Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 58:314–321.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mishra SK, Tripathi S, Shukla A, Oh SH and
Kim HM: Andrographolide and analogues in cancer prevention. Front
Biosci (Elite Ed). 7:255–266. 2015.PubMed/NCBI
|
|
15
|
Seo JY, Pyo E, An JP, Kim J, Sung SH and
Oh WK: Andrographolide activates Keap1/Nrf2/ARE/HO-1 pathway in
HT22 cells and suppresses microglial activation by Aβ42 through
Nrf2-related inflammatory response. Mediators Inflamm.
2017:59061892017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai L, Wang G and Pan W: Andrographolide
inhibits proliferation and metastasis of SGC7901 gastric cancer
cells. Biomed Res Int. 2017:62421032017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang HT, Yang J, Liang GH, Gao XJ, Sang
Y, Gui T, Liang ZJ, Tam MS and Zha ZG: Andrographolide induces cell
cycle arrest and apoptosis of chondrosarcoma by targeting
TCF-1/SOX9 axis. J Cell Biochem. 118:4575–4586. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W, Guo W, Li L, Fu Z, Liu W, Gao J,
Shu Y, Xu Q, Sun Y and Gu Y: Andrographolide reversed 5-FU
resistance in human colorectal cancer by elevating BAX expression.
Biochem Pharmacol. 121:8–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong F, Han J, Jing G, Chen X, Yan S, Yue
L, Cao Z, Liu X, Ma G and Liu J: Dihydroartemisinin transiently
activates the JNK/SAPK signaling pathway in endothelial cells.
Oncol Lett. 12:4699–4704. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar A, Singh UK, Kini SG, Garg V,
Agrawal S, Tomar PK, Pathak P, Chaudhary A, Gupta P and Malik A:
JNK pathway signaling: A novel and smarter therapeutic targets for
various biological diseases. Future Med Chem. 7:2065–2086. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berwick M, Buller DB, Cust A, Gallagher R,
Lee TK, Meyskens F, Pandey S, Thomas NE, Veierød MB and Ward S:
Melanoma epidemiology and prevention. Cancer Treat Res. 167:17–49.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berwick M, Erdei E and Hay J: Melanoma
epidemiology and public health. Dermatol Clin. 27:205–214. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Higgins HW II, Lee KC, Galan A and Leffell
DJ: Melanoma in situ: Part I. Epidemiology, screening, and clinical
features. J Am Acad Dermatol. 73:181–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lasithiotakis KG, Petrakis IE and Garbe C:
Cutaneous melanoma in the elderly: Epidemiology, prognosis and
treatment. Melanoma Res. 20:163–170. 2010.PubMed/NCBI
|
|
25
|
Cheung HY, Cheung SH, Li J, Cheung CS, Lai
WP, Fong WF and Leung FM: Andrographolide isolated from
Andrographis paniculata induces cell cycle arrest and
mitochondrial-mediated apoptosis in human leukemic HL-60 cells.
Planta Med. 71:1106–1111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zang QQ, Zhang L, Gao N and Huang C:
Ophiopogonin D inhibits cell proliferation, causes cell cycle
arrest at G2/M, and induces apoptosis in human breast carcinoma
MCF-7 cells. J Integr Med. 14:51–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li HY, Zhang J, Sun LL, Li BH, Gao HL, Xie
T, Zhang N and Ye ZM: Celastrol induces apoptosis and autophagy via
the ROS/JNK signaling pathway in human osteosarcoma cells: An in
vitro and in vivo study. Cell Death Dis. 6:e16042015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stark GR and Taylor WR: Analyzing the G2/M
checkpoint. Methods Mol Biol. 280:51–82. 2004.PubMed/NCBI
|
|
29
|
Dothager RS, Putt KS, Allen BJ, Leslie BJ,
Nesterenko V and Hergenrother PJ: Synthesis and identification of
small molecules that potently induce apoptosis in melanoma cells
through G1 cell cycle arrest. J Am Chem Soc. 127:8686–8696. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang X, Chen Y, Jenkins LW, Kochanek PM
and Clark RS: Bench-to-bedside review: Apoptosis/programmed cell
death triggered by traumatic brain injury. Crit Care. 9:66–75.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mondal J, Samadder A and Khuda-Bukhsh AR:
Psorinum 6 × triggers apoptosis signals in human lung cancer cells.
J Integr Med. 14:143–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jia J, Yang F, Yang M, Wang C and Song Y:
P38/JNK signaling pathway mediates the fluoride-induced
down-regulation of Fam83h. Biochem Biophys Res Commun. 471:386–390.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goc A, Al-Husein B, Kochuparambil ST, Liu
J, Heston WW and Somanath PR: PI3 kinase integrates Akt and MAP
kinase signaling pathways in the regulation of prostate cancer. Int
J Oncol. 38:267–277. 2011.PubMed/NCBI
|
|
36
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|