Introduction
The serine/threonine protein kinase AKT (also known
as protein kinase B) mediates the cell survival signals coming
through the phosphoinositide 3-kinase (PI3K) pathway by
phosphorylation (1,2). Human AKT has three isoforms: AKT1,
AKT2 and AKT3. Once activated, AKT moves to the cytoplasm and
nucleus, where it phosphorylates, activates or inhibits numerous
downstream targets to regulate various cellular functions,
including cell proliferation. Increased expression and activation
of AKT is involved in a variety of human cancers. Although the
activation of AKT alone is believed to be insufficient for
tumorigenesis, it contributes to cancer progression by inhibiting
apoptosis, promoting the cell proliferation and regulating their
invasion abilities. Accordingly, AKT inhibitors are now in clinical
development for the treatment of cancers (3).
Certain herbs are believed to possess numerous
beneficial activities. The clinical use of herbal medications in
therapy is widespread as herbs have mild bioavailability and also
low toxicity (4). In one study, an
inverse correlation was observed between the consumption of certain
medicinal herbs, including sage and rosemary, and the incidence of
lung cancer (5). In addition,
traditional Chinese herbs have the characteristic of suppressing
the expression of several genes involved in cancer (6). The inhibition of AKT expression is
expected to interfere with the progression of cancer. We therefore
hypothesized that certain medicinal herbs or spices affect the
expression of AKT and therefore inhibit cell proliferation.
Materials and methods
Cell culture
The human cell lines K562, Daudi, Jurkat and U937
were maintained in RPMI-1640 supplemented with 10% fetal bovine
serum (FBS), penicillin and streptomycin at 37°C in a humidified
atmosphere containing 5% CO2.
Preparation of extracts and reagents
Herb and spice powders were purchased at a food
market in Japan. The powders were dissolved in 80% ethanol and
subsequently diluted in 40% ethanol at a stock concentration of 50
mg/ml. The mixtures were vortexed rigorously for 3 min followed by
3 min sonication. Following centrifugation at 1500 × g for 5 min,
the supernatants were collected and stored at −20°C until use. The
reagents used were dissolved in ethanol and subsequently diluted at
a stock concentration of 10 mM. The reagents were stored at −20°C
until use. For the cell treatments, a quantity of 0.5–10.0 μl was
added into 1 ml of cell culture medium.
Reverse transcriptase polymerase chain
reaction (RT-PCR)
Ubiquilin1, presenilin1 and GAPDH mRNAs were
analyzed by semi-quantitative RT-PCR. Total RNA was extracted using
an RNA isolation kit (Takara, Japan). Total RNA (2 μg)was reverse
transcribed using a Phusion RT-PCR kit (New England Biolabs,
Ipswich, MA, USA) as described in the manufacturer’s instructions.
Cycle-based PCR was used to semi-quantitate the ubiquilin1 and
presenilin1 gene level. GADPH was also used as an internal loading
control. Samples were determined within 3 months of collection. The
primers used for PCR were designed as follows: AKT1: forward,
TCTATGGCGCTGAGATTGTG; and reverse, CTTAATGTGCCCGTCCTTGT (expected
size, 116 bp); GAPDH: forward, TCCCATCACCATCTTCCA; and reverse,
CATCACGCCACAGTTTCC (expected size, 376 bp). For real-time PCR, the
reactions were performed in a real-time PCR system (Illumina, USA)
using KAPA SYBR FAST reaction mix (Genetics, Japan). Thermocycling
was performed according to the instructions at an annealing
temperature of 60°C in a final volume of 10 μl including Taq DNA
polymerase.
Western blot analysis
An equal amount of protein samples were used for
western blot analysis using anti-AKT1 (Cell Signaling Technology,
Inc.), anti-Rb2 (BD Transduction Laboratories) and anti-Erk2
(AnaSpec, Inc., Fremont, CA, USA) antibodies, and quantified by
densitometry. Western blots were repeated at least three times and
the representative data were shown.
Cell proliferation assay
Cell proliferation activity was examined using Tetra
Color One (Seikagaku Corporation, Japan). Cells were seeded onto
96-well microplates (1,000 cells/well) and treated with extracts
for 0, 24, 48, 72 and 96 h. Following treatment, Tetra Color One
was added according to the manufacturer’s instructions. The optical
density value of each well was measured using a microplate reader
(BioRad iMark) with a test wavelength of 450 nm.
Cytotoxicity assay
Cytotoxicity was examined using Annexin V-EGFP
(Abcam) and 7-AAD Red (Enzo Life Sciences, Inc.). These detection
reagents were used according to the manufacturer’s instructions.
Stained cells were detected by fluorescence microscopy (Eclipse Ti,
Nikon).
Results and Discussion
Extracts of rosemary, green tea, sage, kuro-shitimi
(red pepper), ginger, zingiber mioga or perilla frutescens were
added into the cell culture media of the K562, Daudi, Jurkat or
U937 cells, and the level of genes, including AKT1, was examined.
RT-PCR analysis was employed to quantify the expression level of
the gene. Total RNA was isolated 48 h after herbal extract
treatment for the detection of AKT1, and the levels of mRNA were
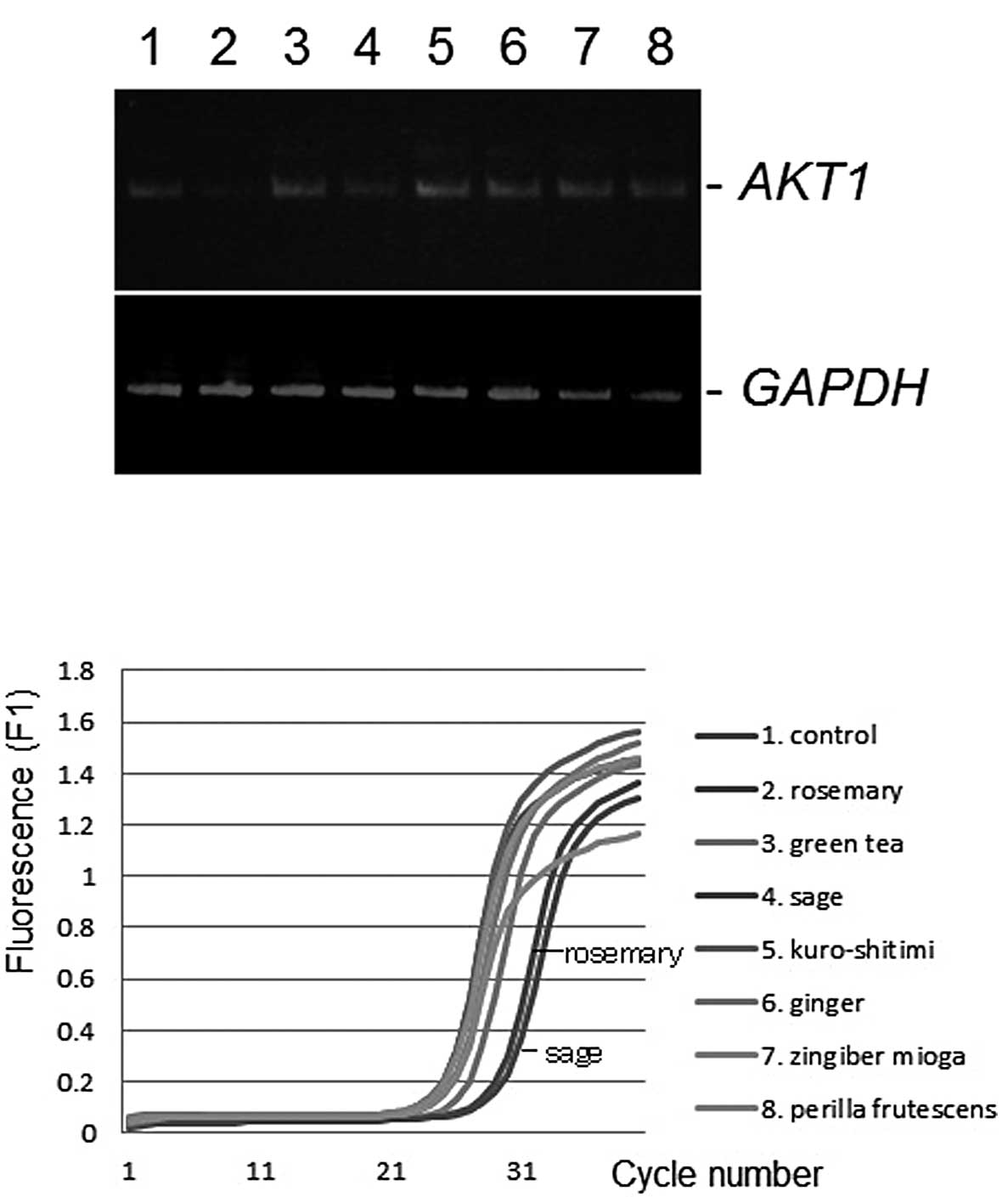
determined by conventional semi-quantitative RT-PCR (7). As shown in Fig. 1A, the AKT1 gene expression level was
downregulated by the administration of sage or rosemary extracts at
a final concentration of 50 μg/ml, compared with the control
ethanol vehicle. Similar results were obtained from the
quantitative real-time RT-PCR analysis (Fig. 1B). No product was amplified in the
no-template sample or when reverse transcriptions were omitted.
However, the expression of anti-oncogenes BRCA1, BRCA2, Rb1
and Rb2 (data not shown) and the housekeeping gene
GAPDH was unaltered (Fig.
1B). Differences among the gene expression profiles of K562,
Daudi, Jurkat and U937 cells were insignificant (data not
shown).
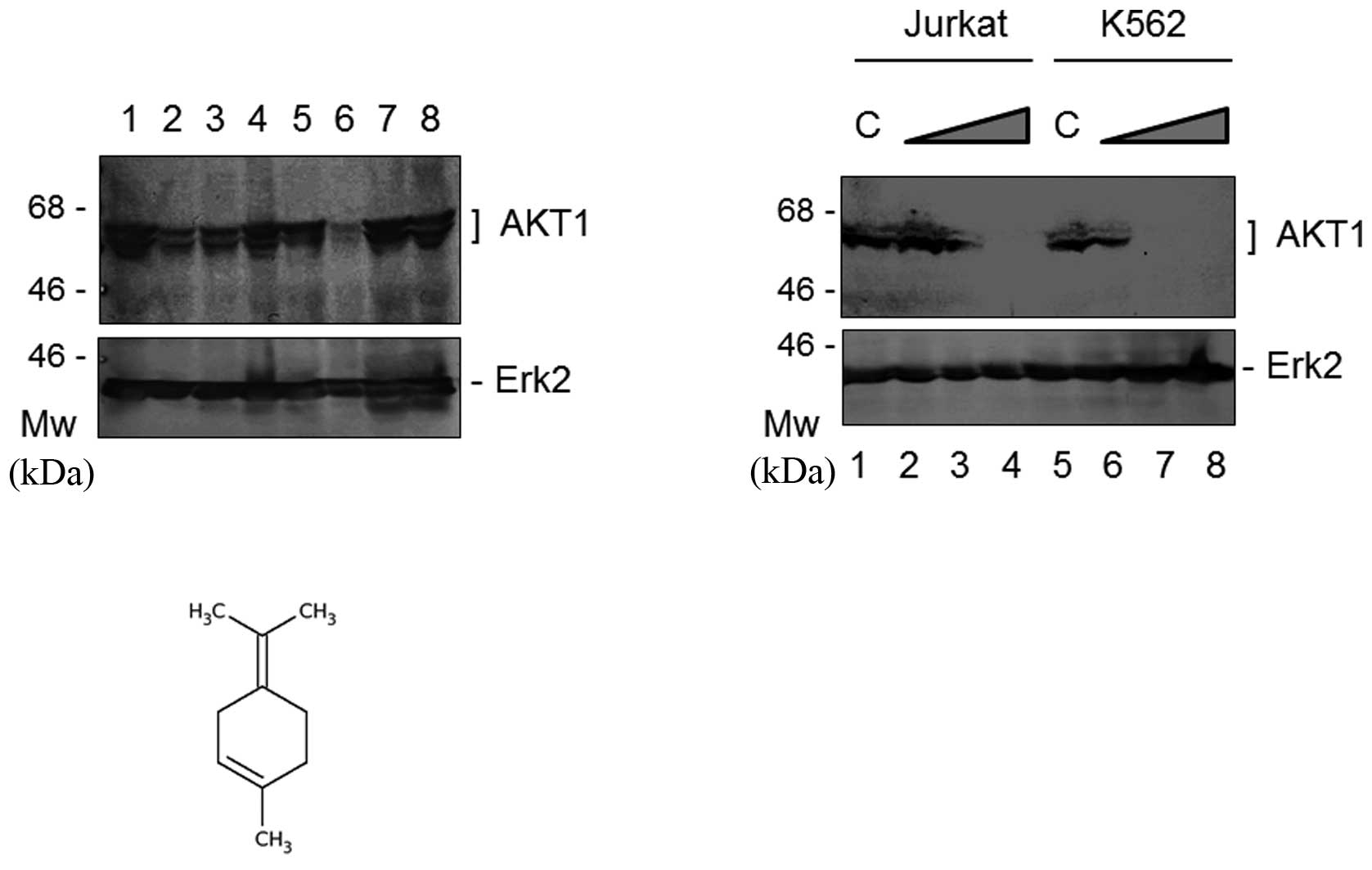
To examine the expression status at the protein
level, Western blot analysis was used to analyze the AKT1 protein
in the cells stimulated by sage or rosemary. As shown in Fig. 2, the sage or rosemary extracts
markedly reduced the protein expression of AKT1, but not Erk2, in
K562 cells, when cell cultures had been maintained in the herb
stimulation for 48 h. To rule out the possibility that sage or
rosemary had any effects other than protein expression, we also
checked several molecules relevant to cell proliferation. The
expression of BRCA1, BRCA2, Rb1, Rb2
and Erk2 was then subjected to evaluation, since AKT1
affected the function of anti-oncogene products in cancer cells
(8). We found that sage or rosemary
extracts markedly enhanced the protein expression of Rb2 (Fig. 2, middle panel). Similar results were
also obtained with BRCA2 (data not shown).
Notably, sage and rosemary did not function
synergistically to reduce AKT1 expression in K562 and other cells
(data not shown). We addressed the question of whether a component
of sage or rosemary was capable of reducing AKT1 expression. The
common components of sage and rosemary are linalool, carnosol and
terpinolene; these are expected to have anti-proliferative ability
(9,10). As shown in Fig. 3A, terpinolene markedly reduced the
protein expression of AKT1; however, linalool or carnosol did not
downregulate it. Following the treatment of the cells with various
concentrations of terpinolene, western blot analysis revealed that
the protein expression of AKT1, but not Erk2, was decreased with
the increasing concentrations of terpinolene in the K562 and Jurkat
cells (Fig. 3B). The final
concentration of 0.05% terpinolene inhibited AKT1 expression by
more than 95% in K562 and Jurkat cells (Fig. 3B). The chemical molecular structure
of terpinolene is shown in Fig. 3C.
It is produced by the alcoholic sulfuric acid treatment of pinene
or by fractionation of turpentine.
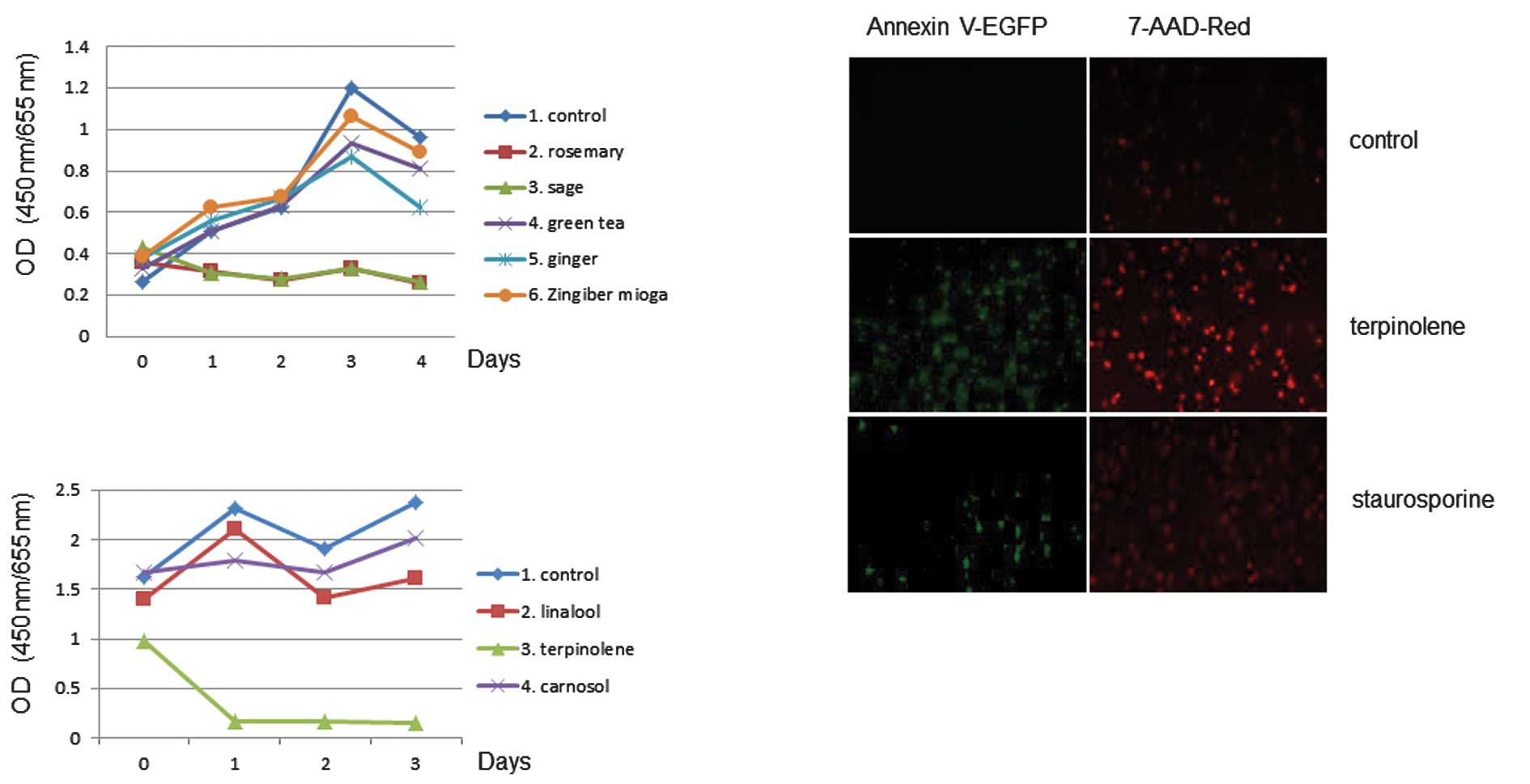
The effect of sage or rosemary on cell proliferation
activity was examined. The extracts were used in 3-day growth
curves to determine the effects of cell proliferation. The assay
revealed that cell proliferation activity was affected by sage or
rosemary treatment in K562 cells, as shown in Fig. 4. Similar results were obtained with
the use of Jurkat cells (data not shown). We again tested the
effects of terpinolene on K562 cell proliferation activity and
found that terpinolene significantly inhibited it (Fig. 4B). These results revealed the
significant effect of terpinolene on K562 cell growth. We
investigated whether terpinolene induced cytotoxicity in K562
cells. Results showed that terpinolene significantly induced cell
apoptosis as much as staurosporine (Fig. 4C), unlike other molecules such as
carnosol (data not shown).
The possibility of interactions between natural
products and conventional prescription medicines is one of the most
crucial issues in pharmacotherapeutic safety. Terpinolene is also
used as a synthetic food flavoring additive or fragrance enhancer.
Thus, it has high potential for human exposure. Although we have
not yet identified the precise molecular mechanisms by which AKT1
expression is regulated, our findings provide new insight regarding
cancer chemoprevention via the PI3K pathway and may help to
establish strategies for a viable therapy.
Acknowledgements
This study was supported by Grants-in-Aid from the
Ministry of Education, Culture, Sports, Science and Technology in
Japan, and the Nara Women’s University Intramural Grant for Project
Research. In addition, this study was supported in part by a grant
from the Fuji Foundation for Protein Research.
References
|
1
|
Castaneda CA, Cortes-Funes H, Gomez HL and
Ciruelos EM: The phosphatidyl inositol 3-kinase/AKT signaling
pathway in breast cancer. Cancer Metastasis Rev. 29:751–759. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martelli AM, Evangelisti C, Chiarini F, et
al: The emerging role of the phosphatidylinositol
3-kinase/Akt/mammalian target of rapamycin signaling network in
normal myelopoiesis and leukemogenesis. Biochim Biophys Acta.
1803:991–1002. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pal SK, Reckamp K, Yu H and Figurelin RA:
Akt inhibitors in clinical development for the treatment of cancer.
Expert Opin Investig Drugs. 19:1355–1366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abad MJ, Bedoya LM and Bermejo P: An
update on drug interactions with the herbal medicine Ginkgo biloba.
Curr Drug Metab. 11:171–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson JJ: Carnosol: a promising
anti-cancer and anti-inflammatory agent. Cancer Lett. 305:1–7.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu CP, Tsai WJ, Lin YL, Liao JF, Chen CF
and Kuo YC: The extracts from Nelumbo Nucifera suppress cell cycle
progression, cytokine genes expression, and cell proliferation in
human peripheral blood mononuclear cells. Life Sci. 75:699–716.
2004. View Article : Google Scholar
|
|
7
|
Nishimura Y, Kitagishi Y, Yoshida H,
Okumura N and Matsuda S: Ethanol extracts of black pepper or
turmeric down-regulated SIRT1 protein expression in Daudi culture
cells. Mol Med Report. 4:727–730. 2011.PubMed/NCBI
|
|
8
|
Plo I, Laulier C, Gauthier L, Lebrun F,
Calvo F and Lopez BS: AKT1 inhibits homologous recombination by
inducing cytoplasmic retention of BRCA1 and RAD51. Cancer Res.
68:9404–9412. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cherng JM, Shieh DE, Chiang W, Chang MY
and Chiang LC: Chemopreventive effects of minor dietary
constituents in common foods on human cancer cells. Biosci
Biotechnol Biochem. 71:1500–1504. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshida N, Takada T, Yamamura Y, Adachi I,
Suzuki H and Kawakami J: Inhibitory effects of terpenoids on
multidrug resistance-associated protein 2- and breast cancer
resistance protein-mediated transport. Drug Metab Dispos.
36:1206–1211. 2008. View Article : Google Scholar
|