Introduction
In Western countries, endometrial carcinoma (EC) is
the most common type of malignant tumor in the field of gynecology
(1,2). A state of persistent high estrogen, as
observed with ovulation disorders and obesity, is considered a risk
factor (3–5). The standard treatments for EC are
total hysterectomy and bilateral adnexectomy.
EC is a disease that frequently affects
perimenopausal females, however approximately 10% of affected
females are ≤40 years old, and the incidence in young females has
recently been on the increase (6).
As in Western countries, the number of ≤40-year-old females with EC
in Japan is on the rise (7). Thus,
the number of patients who select to undergo fertility-sparing
treatment is growing.
Studies at various institutions have reported the
results of fertility-sparing treatment for EC using progesterone
preparations (8–17). However, as the number of cases have
been low, the efficacy of such treatment has yet to be clarified.
Furthermore, few studies have closely described the clinical
background of young females with early-stage EC wishing to undergo
fertility-sparing treatment.
The present study investigated the effects of
treatment, prognosis, pregnancy status and other factors, in order
to demonstrate the therapeutic outcomes of current
fertility-sparing treatment. Furthermore, patient background
factors were investigated to elucidate the characteristics of the
patient group.
Patients and methods
Patients
A total of 59 patients with EC who underwent
fertility-sparing treatment, at either the Jichi Medical University
Hospital or the Kitasato University Hospital over the 21-year
period from 1987 to 2007, were retrospectively investigated.
Atypical endometrial hyperplasia was excluded in this study.
Selection criteria of the treatment were: i) highly differentiated
(G1) endometrioid adenocarcinoma; ii) no appearance of myometrial
invasion (stage I a: FIGO, 1988) on magnetic resonance imaging;
iii) unmarried or strong desire to have a child; iv) no current or
past history of thrombotic disease. As a general rule, blood
clotting and fibrinolysis were normal. However, when patients
strongly desired, fertility-sparing treatment was performed on
patients with stage I b (in which myometrial invasion may be
suspected) or II a (in which cervical mucosa invasion may be
suspected). In all patients, treatment was administered only after
written informed consent was obtained. Pathological specimens were
examined by experienced pathologists in each institute.
Treatments
In the fertility-sparing treatment, a dose of
400–600 mg/day of medroxyprogesterone acetate (MPA) was
administered orally, and the entire surface was curetted between 8
and 12 weeks to confirm the therapeutic effects and absence of
disease progression. Following administration of the drug for 16–24
weeks, the endometrium was again curetted, and therapeutic effects
were assessed. At this stage, if the lesion persisted, total
hysterectomy was selected as a general rule; if the lesion had
disappeared, the patient was monitored and allowed to become
pregnant. Even when the lesion persisted, MPA was continued if the
patient strongly preferred to preserve fertility. In such patients,
pathological tests were conducted every 4–8 weeks, and the duration
of therapy was determined individually.
Results
Patient characteristics
A total of 59 patients, including 44 patients with
stage I a (75%) and 15 with stage I b- II a (25%) were included in
the present study (Table I). The
median age was 31 years, and the mean body mass index was 23.3
kg/m2. The proportions of females with menstrual
irregularity, nulliparity and polycystic ovary syndrome (PCO),
which are considered risk factors for EC, were 63, 98 and 7%,
respectively. The cause of detection was metrorrhagia in 35
patients (59%), and incidental discovery of the lesion during
screening or treatment for infertility in 16 patients (27%).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Patient
characteristics, n=59 |
|---|
| Median age, years
(range) | 31 (21–42) |
| Mean BMI,
kg/m2 (range) | 23.3 (15–38) |
| Clinical stage
(cases) |
| I a | 44 (75%) |
| I b- II a | 15 (25%) |
| Risk factor
(cases) |
| Irregular
periods | 37 (63%) |
| Nulligravida | 58 (98%) |
| PCO syndrome | 4 (7%) |
| Detectability
(cases) |
| Metrorrhagia | 35 (59%) |
| Infertility
examination | 16 (27%) |
| Abnormal
mensesa | 6 (10%) |
MPA administration positively affects EC
patients despite recurrence
In the present study, the mean duration of MPA
administration until complete response (CR) was 24.9 weeks (range,
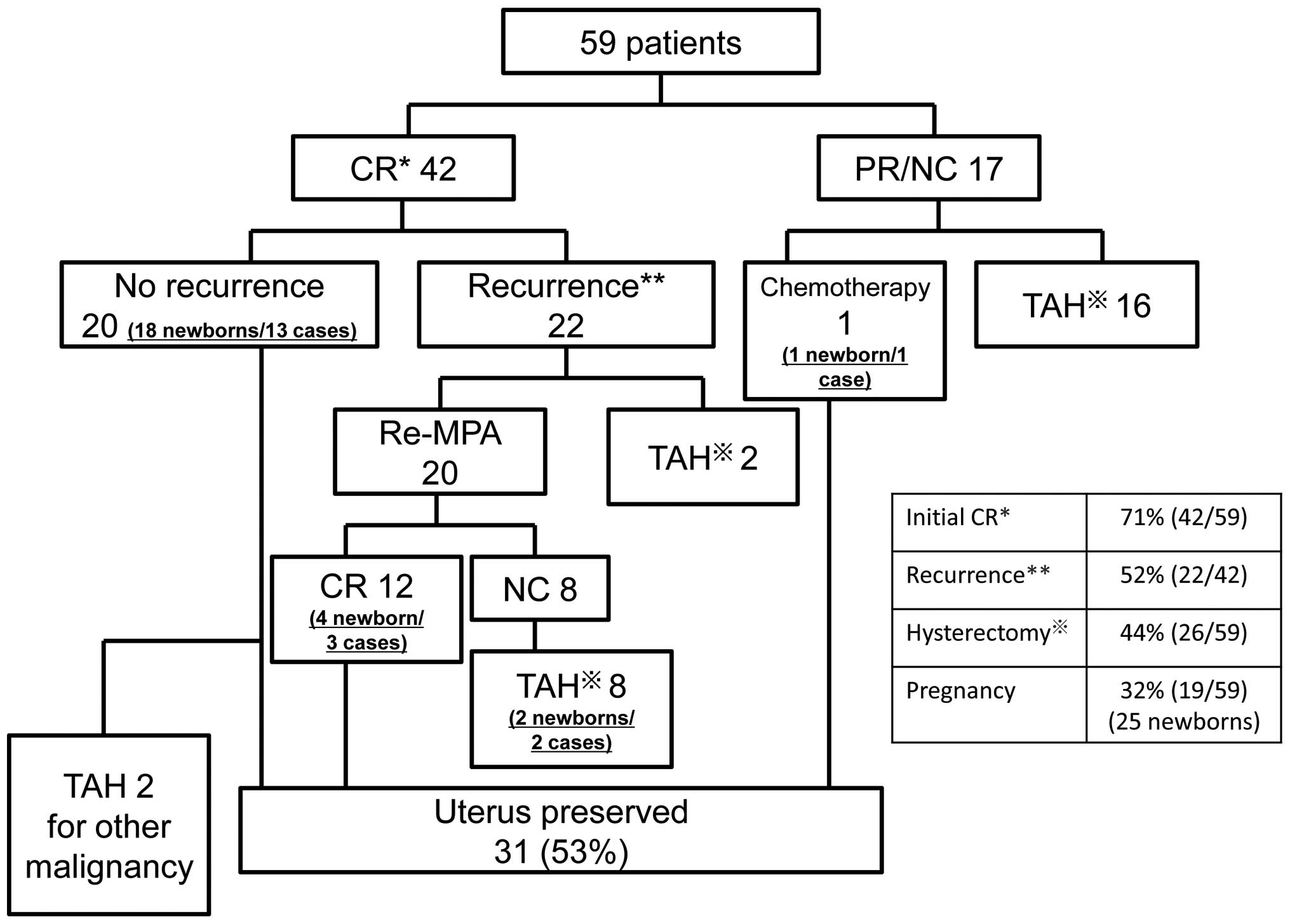
13–70). Fig. 1 shows the
therapeutic results. The median duration of follow-up was 66 months
(range, 11–251). The response to the initial treatment was CR in 42
patients (71%), and either partial response (PR) or no change (NC)
in 17 patients (29%). In 16 of the 17 patients without CR, total
hysterectomy was performed and fertility could not be preserved.
One patient refused surgery and underwent cytotoxic chemotherapy;
subsequently, the cancer went into remission. This patient gave
birth to a child and is currently alive and disease-free. Of the 16
patients who underwent surgery, 15 are currently alive and
disease-free, but cancer recurred in one patient, who succumbed to
recurrence 126 months after initial treatment.
A survey of the 42 initial CR patients revealed that
20 patients (48%) remain alive and are disease-free, with a median
recurrence-free survival of 62 months (range, 19–157). Of these 20
patients, 13 patients have given birth to a total of 18 children.
However, 2 patients subsequently underwent total hysterectomy; one
for new ovarian cancer and the other for new tubal cancer.
Recurrence was observed in 22 patients (52%), with a
median onset of recurrence of 12 months (range, 7–84). In 20 of
these 22 patients, MPA was again administered, and remission was
achieved in 12 patients without recurrence. A total of 3 of these
patients have given birth to a total of 4 children. In 8 patients,
CR could not be achieved despite additional MPA administration, and
total hysterectomy was performed. However, 2 of these 8 patients
had each given birth to a child prior to recurrence. Of the 22
patients with recurrence, 2 patients stopped visiting the hospital
and have not been followed up.
Fig. 2 shows the
timing of recurrence following remission and the recurrence-free
survival curve. In 18 (82%) of the 22 patients who had recurrence,
cancer recurred within 2 years, and the longest time to recurrence
was 7 years and 1 month.
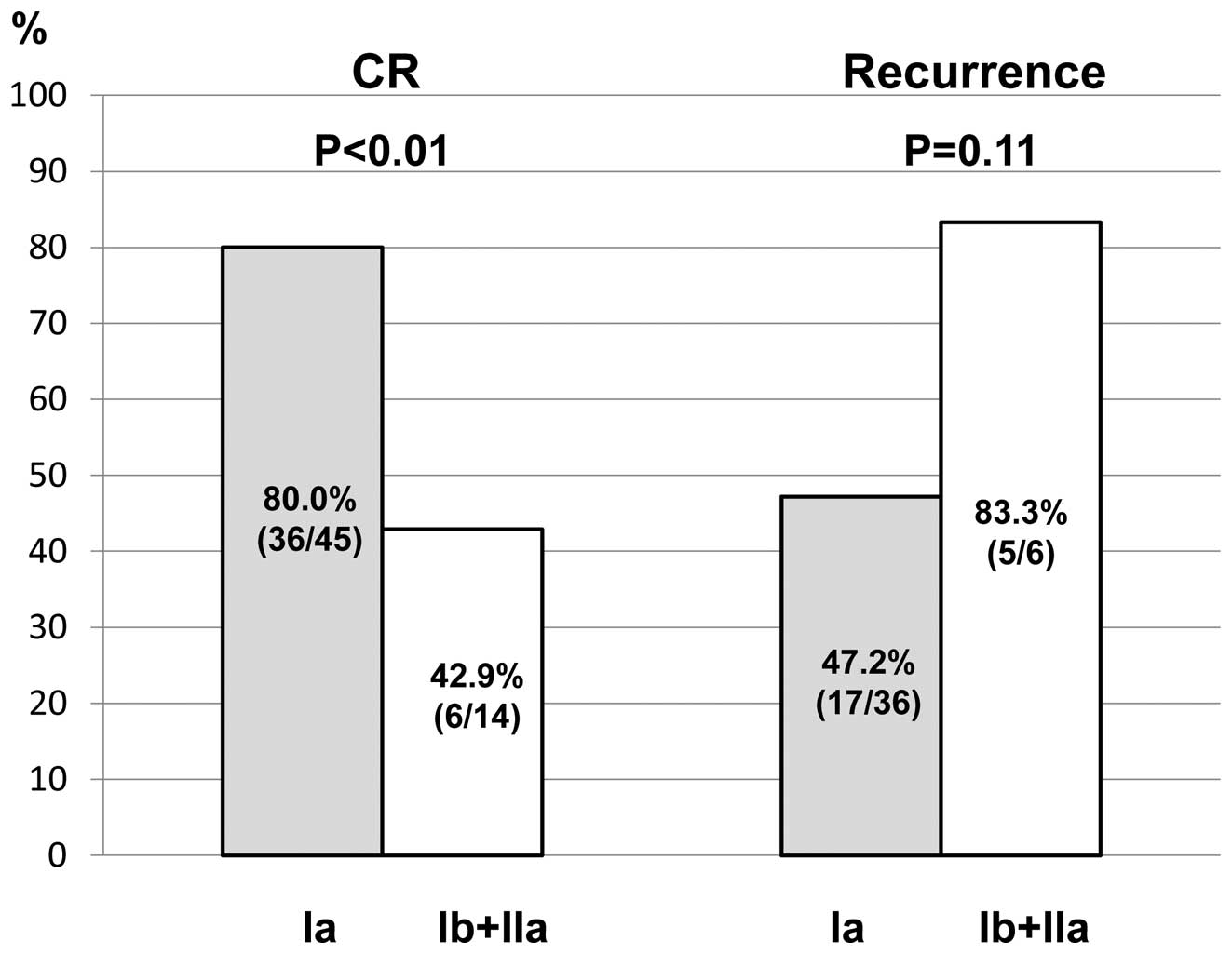
The initial CR rate and the rate of
recurrence following CR in relation to the clinical stage
For patients with stage Ia, the CR rate was
significantly higher than that for the other stages (80.0 vs.
42.9%, respectively; p<0.01). The rate of recurrence was lower
for stage I a patients than recurrence for other stages (47.2 vs.
83.3%, respectively; p=0.11), however there was no statistically
significant difference (Fig.
3).
Following exclusion of the 2 patients who underwent
total hysterectomy for other types of cancer, the 26 patients who
underwent total hysterectomy for the primary disease were examined.
Clinical and pathological stages matched in 14 patients (54%),
however, pathological stages were more advanced in the remaining 10
patients (38%). In 2 patients (8%), the grade as ascertained by
biopsy prior to MPA therapy differed from the postoperative grade;
grade G1 prior to MPA therapy, but a final postoperative diagnosis
of G2 and G3 in each patient, respectively.
As mentioned earlier, double cancer was confirmed in
2 cases (ovarian and tubal cancer), with an overall frequency of
3.3%. In the 2 cases, residual endometrioid adenocarcinoma was not
observed in the excised uterus.
Other than mild body weight increases, no adverse
reactions were observed with the present treatment. No venous
thrombosis was recorded in the patients.
Discussion
The therapeutic results and prognosis of
fertility-sparing treatment using MPA for EC were reviewed
retrospectively. Table II
summarizes findings from past studies. While differences exist
among institutions in terms of response rates (range, 53–92%) and
recurrence rates (range, 11–53%), the present findings were
comparable. Our response rate exceeded 70%, and 19 of the patients
(32% of enrolled patients) gave birth to a total of 25 children.
This suggests that the present treatment is sufficient for the
preservation of fertility.
 | Table IISummary of studies on patients treated
with progestins. |
Table II
Summary of studies on patients treated
with progestins.
| Author/Year
(Refs.) | Number of cases | Complete response
(%) | Recurrence (%) |
|---|
| Kim et al,
1997 (9) | 7 | 4 (57) | 2 (50) |
| Randall et al,
1997 (10) | 12 | 9 (75) | 0 |
| Imai et al,
2001 (11) | 15 | 8 (53) | 3 (37.5) |
| Kaku et al,
2001 (12) | 12 | 9 (75) | 2 (22) |
| Wang et al,
2002 (13) | 9 | 8 (89) | 4 (50) |
| Niwa et al,
2004 (14) | 12 | 12 (100) | 8 (67) |
| Ushijima et
al, 2007 (15) | 39 | 26 (67) | 14 (54) |
| Yamazawa et
al, 2007 (16) | 9 | 7 (78) | 2 (29) |
| Hahns et al,
2009 (17) | 35 | 22 (63) | 9 (41) |
| Present study | 59 | 42 (71) | 22 (52) |
| Total | 209 | 147 (70) | 66 (45) |
However, patients and doctors require sufficient
understanding prior to the initiation of treatment that the
recurrence rate for this fertility-preserving treatment is high. As
surgery is the standard therapy for EC, the present treatment
should be performed only after obtaining written informed consent.
As for the preoperative criteria for patient selection, the present
study found differences in the rates of initial remission and the
rate of recurrence after remission between stages Ia and more
advanced stages, and therapeutic results for stages Ia were
markedly improved. These results suggest that fertility-sparing
treatment for EC should be limited to patients with stages up to I
a.
Our data suggest that for the first 2 years after
treatment, patients should be followed up relatively frequently,
approximately every 1–3 months, to check for recurrence. As cancer
recurred after a long period of remission in certain cases,
patients should be followed up for longer than after the first 2
years. Cases have been documented in which EC recurred after more
than 10 years (18) or after
childbirth (19). In the present
study, EC recurred 5 years and 5 months after treatment in one
patient (3 years and 10 months after childbirth). Thus, the
possibility of recurrence is likely even after several years of
remission, as well as after childbirth. These findings suggest that
total hysterectomy can be performed as a preventive measure in
females in remission who have given birth and do not want to have
more children.
An investigation of patients who underwent surgery
for recurrence revealed that preoperative clinical stages differed
from actual pathological stages in certain cases. Disease stages
may have advanced during therapy or preoperative investigations
might have missed myometrial invasion. Several studies have
revealed the limitations of preoperative staging of EC (8,20).
These limitations should be clearly explained to patients prior to
therapy, and if a patient does not respond to therapy, the
possibility that the stage is more advanced than Ia should be
considered. In certain cases, the grade advanced from G1 to G2 or
G3 postoperatively. Ota et al (21) documented grade progression from G1
to G2 in patients, who were unresponsive to MPA and underwent total
hysterectomy. Thigpen et al (22) reported that higher grade is
associated with lower MPA response rate. As MPA is a long-term
treatment, periodic pathological tests are required during
follow-up. If grades advance, termination of pharmacotherapy
requires consideration versus continuing MPA. As abovementioned,
the only patient who succumbed to disease following the present
treatment began therapy at stage Ib of G1 endometrioid
adenocarcinoma. Surgery was performed due to lack of response to
MPA, but by the time of surgery the tumor had advanced to G3
endometrioid adenocarcinoma and had metastasized to the pelvic
lymph nodes (pT1cN1M0). Kothari et al (23) also reported a patient in whom cancer
recurred after fertility-sparing treatment, categorized as stage IV
at the time of surgery. Patients need to consider that the present
treatment is strictly optional, and that cancer stage can advance
during or after MPA.
EC patients who are ≤40 years old are susceptible to
complications of ovarian and peritoneal cancer (24). In the present study, patients
developing ovarian or tubal cancer were documented. Throughout
regular follow-up, sufficient examination of the adnexa of the
uterus is required. Furthermore, when totally excising the uterus
by radical surgery, the aforementioned types of cancer should be
considered when deciding whether to resect the bilateral adnexa,
and informed consent must be obtained.
In general, symptoms, including metrorrhagia and
postmenopausal bleeding, are observed in a number of EC patients.
However, in the present study, approximately 60% of young EC
patients who received fertility-sparing treatment had metrorrhagia.
Additionally, in approximately 30% of patients, EC was accidentally
identified during visits to a hospital for infertility, even though
the patient had been asymptomatic during fertility tests. We have
already reported that fertility tests detected EC in young females
at a higher rate than those for EC in Japanese patients (25). In the group of patients who received
fertility-sparing treatment in the present study, EC was detected
by fertility tests in a number of patients, suggesting that
fertility tests provide an opportunity for the early detection of
EC. Healthcare professionals who conduct fertility tests should
remain aware that they are dealing with patients at high risk of
EC.
By retrospectively investigating fertility-sparing
treatment using MPA for EC, the results and characteristics of
patient backgrounds were established. The response rate was high,
and the present treatment was considered acceptable for the purpose
of enabling patients to give birth. However, the rate of recurrence
is also high, thus results remain less effective when compared to
surgery, the standard therapy for EC. In addition, standard
treatment methods, including daily dose and administration period,
have yet to be established. At present, treatments are administered
various doese at different institutions. Thus, more studies are
required for a standardized treatment to be established.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics. CA Cancer J Clin. 60:277–300. 2010.
|
|
2
|
Amant F, Moerman P, Neven P, Timmerman D,
Van Limbergen E and Vergote I: Endometrial cancer. Lancet.
366:491–505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parazzini F, La Vecchia C, Bocciolone L
and Franceschi S: The epidemiology of endometrial cancer. Gynecol
Oncol. 41:1–16. 1991. View Article : Google Scholar
|
|
4
|
Brinton LA, Berman ML, Mortel R, et al:
Reproductive, menstrual, and medical risk factors for endometrial
cancer: results from a case-control study. Am J Obstet Gynecol.
167:1317–1325. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lurain JR: Uterine Cancer. Berek &
Novak’s Gynecology. Berek JS: 14th edition. Lippincott Williams
& Wilkins; Philadelphia: pp. 1343–1401. 2007
|
|
6
|
Crissman JD, Azoury RS, Barnes AE and
Schellhas HF: Endometrial carcinoma in women 40 years of age or
younger. Obstet Gynecol. 57:699–704. 1981.PubMed/NCBI
|
|
7
|
Matsuda T, Marugame T, Kamo K, Katanoda K,
Ajiki W and Sobue T; Japan Cancer Surveillance Research Group.
Cancer incidence and incidence rates in Japan in 2005: based on
data from 12 population-based cancer registries in the Monitoring
of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol.
41:139–147. 2011. View Article : Google Scholar
|
|
8
|
Gadducci A, Spirito N, Baroni E, Tana R
and Genazzani AR: The fertility-sparing treatment in patients with
endometrial atypical hyperplasia and early endometrial cancer: a
debated therapeutic option. Gynecol Endocrinol. 25:683–691. 2009.
View Article : Google Scholar
|
|
9
|
Kim YB, Holschneider CH, Ghosh K, Nieberg
RK and Montz FJ: Progestin alone as primary treatment of
endometrial carcinoma in premenopausal women. Report of seven cases
and review of the literature. Cancer. 79:320–327. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Randall TC and Kurman RJ: Progestin
treatment of atypical hyperplasia and well-differentiated carcinoma
of the endometrium in women under age 40. Obstet Gynecol.
90:434–440. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Imai M, Jobo T, Sato R, Kawaguchi M and
Kuramoto H: Medroxyprogesterone acetate therapy for patients with
adenocarcinoma of the endometrium who wish to preserve the
uterus-usefulness and limitations. Eur J Gynaecol Oncol.
22:217–220. 2001.PubMed/NCBI
|
|
12
|
Kaku T, Yoshikawa H, Tsuda H, et al:
Conservative therapy for adenocarcinoma and atypical endometrial
hyperplasia of the endometrium in young women: central pathologic
review and treatment outcome. Cancer Lett. 167:39–48. 2001.
View Article : Google Scholar
|
|
13
|
Wang CB, Wang CJ, Huang HJ, et al:
Fertility-preserving treatment in young patients with endometrial
adenocarcinoma. Cancer. 94:2192–2198. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Niwa K, Tagami K, Lian Z, Onogi K, Mori H
and Tamaya T: Outcome of fertility-preserving treatment in young
women with endometrial carcinomas. BJOG. 112:317–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ushijima K, Yahata H, Yoshikawa H, et al:
Multicenter phase II study of fertility-sparing treatment with
medroxyprogesterone acetate for endometrial carcinoma and atypical
hyperplasia in young women. J Clin Oncol. 25:2798–2803. 2007.
View Article : Google Scholar
|
|
16
|
Yamazawa K, Hirai M, Fujito A, et al:
Fertility-preserving treatment with progestin, and pathological
criteria to predict responses, in young women with endometrial
cancer. Hum Reprod. 22:1953–1958. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hahn HS, Yoon SG, Hong JS, et al:
Conservative treatment with progestin and pregnancy outcomes in
endometrial cancer. Int J Gynecol Cancer. 19:1068–1073. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gotlieb WH, Beiner ME, Shalmon B, et al:
Outcome of fertility-sparing treatment with progestins in young
patients with endometrial cancer. Obstet Gynecol. 102:718–725.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mitsushita J, Toki T, Kato K, Fujii S and
Konishi I: Endometrial carcinoma remaining after term pregnancy
following conservative treatment with medroxyprogesterone acetate.
Gynecol Oncol. 79:129–132. 2000. View Article : Google Scholar
|
|
20
|
Kinkel K: Pitfalls in staging uterine
neoplasm with imaging: a review. Abdom Imaging. 31:164–173. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ota T, Yoshida M, Kimura M and Kinoshita
K: Clinicopathologic study of uterine endometrial carcinoma in
young women aged 40 years and younger. Int J Gynecol Cancer.
15:657–662. 2005.PubMed/NCBI
|
|
22
|
Thigpen JT, Brady MF, Alvarez RD, et al:
Oral medroxyprogesterone acetate in the treatment of advanced or
recurrent endometrial carcinoma: a dose-response study by the
Gynecologic Oncology Group. J Clin Oncol. 17:1736–1744.
1999.PubMed/NCBI
|
|
23
|
Kothari R, Seamon L, Cohn D, Fowler J and
O’Malley DM: Stage IV endometrial cancer after failed conservative
management: a case report. Gynecol Oncol. 111:579–582. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zivanovic O, Carter J, Kauff ND and
Barakat RR: A review of the challenges faced in the conservative
treatment of young women with endometrial carcinoma and risk of
ovarian cancer. Gynecol Oncol. 115:504–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujiwara H, Ogawa S, Motoyama M, et al:
Frequency and characteristics of endometrial carcinoma and atypical
hyperplasia detected on routine infertility investigations in young
women: a report of six cases. Hum Reprod. 24:1045–1050. 2009.
View Article : Google Scholar
|