Introduction
Fibrosarcoma is a malignant neoplasm derived from
fibrous connective tissue and is characterized by immature or
undifferentiated anaplastic spindle cells (1). Fibrosarcoma may occur in soft tissues
such as muscles, connective tissue, blood vessels, fat and even in
bones. Generally, fibrosarcoma develop equally among males and
females. According to data compiled by the NCI in the SEER database
between 2000 and 2004, the age-adjusted incidence of all bone and
joint sarcoma was 0.9 per 100,000 individuals per year (2).
Although the incidence is low, long-term patient
survival rates have remained poor (3). The reason for this may be the
resistance of fibrosarcoma to radiation therapy and chemotherapy.
Fibrosarcoma mainly metastasizes to the lung and unless metastases
are completely resected, almost all patients with metastatic
disease succumb to the disease. In addition, although fibrosarcomas
have been studied for decades, their biological characteristics and
cellular origins have not been well elucidated.
Current opinion is that cells in a tumor are
hierarchically organized with respect to their capacity to initiate
and sustain tumor growth. Cancer stem cells (CSCs) are a rare
subpopulation of cancer cells that possess stem-like
characteristics. The CSC hypothesis proposes that CSCs are
responsible for forming the bulk of the tumor (4–6). CSCs
are similar to stem cells and are capable of renewal and
differentiation into all types of cells within a tumor.
Furthermore, it is also believed that CSCs may play a key role in
chemotherapeutic resistance, metastasis and recurrence (7).
Recent studies have identified CSCs in certain
epithelial tumors and sarcomas, including leukemia, breast cancer,
brain tumors, melanoma and Ewing’s sarcoma, but not fibrosarcoma
(8–12). Therefore, we aimed to detect the
possible presence of cells possessing stem cell-like properties in
human fibrosarcomas using the sphere-forming assay, which has been
previously used to isolate cells that acquire a colony-forming
capacity. The sarcospheres were then compared with adherent cells
in terms of their stem cell-like properties using cell self-renewal
assays, invasion assays, drug resistance assessments, western blot
analysis, real-time quantitative PCR and in vivo tumor
transplantation assay.
In the present study we show, for the first time,
that sarcospheres are observed in primary fibrosarcoma tumor cells.
Moreover, we demonstrated that these sphere-forming cells display
higher self-renewal capacity, invasiveness and drug resistance
compared with adherent cells. In addition, the sphere-forming cells
showed greater expression of the embryonic stem cell-related genes
and proteins. Taken together, our data suggest that stem-like cells
may be found in human fibrosarcoma. These data may be of paramount
importance in understanding the biology of stem cell-like cells as
well as for designing novel therapies for human fibrosarcoma.
Materials and methods
Ethics statement
The patient in this study provided written informed
consent for the publication of his case details. The protocol of
the study adhered to the tenets of the Declaration of Helsinki and
was approved by the institutional review board of Harbin Medical
University, Harbin, China. The animal experimentation was carried
out in accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals. The protocol was approved by
the Committee on the Use of Live Animals in Teaching and Research
of the Harbin Medical University, Harbin, China (SYSK
2011-009).
Primary tumor cells and HT1080 cell
line culture
A tumor sample from a 42-year-old male patient who
had been diagnosed with fibrosarcoma in the left thigh muscle was
obtained directly after surgical removal. The tumor sample was
mechanically dissociated, digested in collagenase II (Sigma,
Beijing, China) and incubated in a shaking water bath for 2 h at
37°C. Pre-separation filters (Miltenyi Biotec, Beijing, China) were
used to remove clumps and erythrolysis was performed in hypotonic
solution (0.2% NaCl followed by 1.2% NaCl to stop lysis). The
sample was purified with a dead cell removal kit (Miltenyi Biotec)
and prepared as a cell suspension.
The HT1080 fibrosarcoma cell line was purchased from
the American Type Culture Collection (Rockville, MA, USA). HT1080
cells and purified primary fibrosarcoma cells were maintained in
Dulbecco’s minimum essential medium (DMEM) with 10% fetal bovine
serum (FBS; Invitrogen, Beijing, China) at 37°C in a 5.0%
CO2 atmosphere.
Sphere formation assay
At ∼80% confluence in DMEM/10% FBS medium, monolayer
cells were dissociated with trypsin-EDTA into single-cell
suspensions. The cells were then inoculated into N2-supplemented
DMEM/F12/1% methylcellulose medium without serum at a density of
1x105 cells/well in ultra-low-attachment six-well plates
(Corning, Inc., Corning, NY, USA). Fresh aliquots of human
recombinant epidermal growth factor (EGF; 10 ng/ml) and basic
fibroblast growth factor (bFGF; 10 ng/ml) were added every other
day. Following 10–14 days in culture, colonies that contained
>10 cells were quantitated by inverted phase contrast microscopy
(Olympus CK2; Tokyo, Japan).
Single-cell suspension assay
Fibrosarcospheres were mechanically dissociated and
adherent cells were digested into single-cell suspensions. The
cells were then reintroduced into 96-well ultra low-attachment
plates (Corning, Inc.) at a density of 1 cell/well in
anchorage-independent methylcellulose medium to investigate their
ability to self-renew through secondary sphere formation.
Assessment of drug resistance to
doxorubicin
Cell Counting Kit-8 assay
Fibrosarcospheres were mechanically dissociated, and
adherent cells were digested into single-cell suspensions. The
fibrosarcosphere cells (4x103/well) and adherent cells
(4x103/well) were then split into 96-well plates and
incubated overnight to allow the cells to adhere. The cells were
then were exposed to gradient doses of doxorubicin for 48 h. The
cells were then incubated with WST-8 solution at 37°C for 1 h and
the absorbance at 450 nm was measured on a microplate reader
(MPR-A4i, Tosoh Corporation, Tokyo, Japan). The cell viability
index was calculated according to the following formula:
experimental OD value/control OD value x 100%.
Crystal violet assay
Fibrosarcospheres and adherent cells
(5x104/well) were seeded into 6-well plates and cultured
overnight. The medium was then replaced with complete culture
medium containing doxorubicin (10 μmol/l) for an additional
48 h. The cells were then washed twice with pre-warmed PBS, and the
remaining cells were stained for 1 h with a crystal violet solution
(0.1% crystal violet, 20% methanol). Images were captured using a
camera.
Matrigel invasion assay
The Matrigel invasion assay was performed according
to the manufacturer’s instructions. Briefly, 1x105 PFT
or HT1080 sphere-forming cells were plated onto the Matrigel-coated
membrane in the top chamber (24-well insert; pore size, 8
μm; Corning, Inc.). The adherent cells were processed in the
same way as the control. All cells were added to the transwell
inserts suspended in 0.5 μl medium containing 1% FBS and the
inserts were placed in 750 μl complete medium. Following 48
h of incubation, cells that had migrated through the Matrigel were
stained with hematoxylin. Cells in five representative microscopic
fields were then counted and photographed.
Quantitative real-time PCR
analysis
Quantitative real-time PCR was performed as
previously described (13).
Briefly, total RNA was extracted using a Qiagen RNeasy kit (Qiagen,
Hilden, Germany) and then converted to cDNA with the Omniscript
First-Strand synthesis system (Qiagen) using random primers
(Qiagen). RT-PCRs were carried out using ABI Power SYBR Green mix
(ABI, Applied Biosystems, Inc., Foster City, CA, USA) on a BioRad
Chromo 4 instrument (BioRad, Richmond, CA, USA). Reactions were
carried out in triplicate with RT controls; the gene for the
ribosomal protein HL32 was used as a reference gene (14). The data were analyzed using the
modified ΔΔCt method.
Western blotting
Protein lysates of PFT and HT1080 cells were
prepared and separated onto SDS-polyacrylamide gels as previously
described (15). Blots were stained
with anti-β-actin antibody as an internal control for the amounts
of target proteins. Anti-STAT3, -Oct 3/4, -Nanog, -Sox 2, -Sox 10
and -MDR1 primary antibodies (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) were used.
Nude mice xenografts
Five-week-old athymic nude mice (BALB/c nu/nu; Vital
River Laboratory Animal Center, Beijing, China) were divided into
two groups. Trypsinized fibrosarcospheres and adherent cells
(5x102-1x105 unfractionated cells) were
subcutaneously injected into the left and right flank,
respectively. The mice were then inspected daily for 12 weeks.
Tumor size was measured with a caliper and tumor volume was
calculated using the formula (axb2)/2, with a being the
longest diameter and b the shortest diameter of the tumor.
Statistical analysis
The results are expressed as the mean ± standard
deviation and the Student’s t test was used to evaluate statistical
significance. P<0.05 was considered to indicate a statistically
significant result.
Results
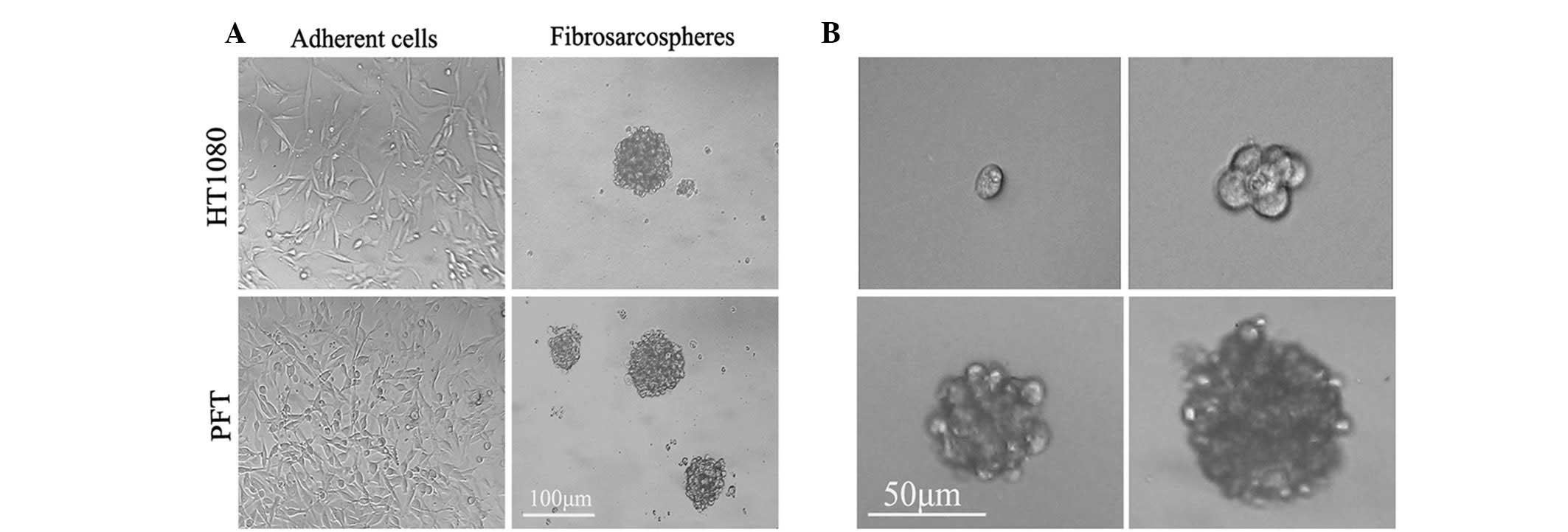
Fibrosarcosphere formation and
self-renewal from primary tumors and the HT1080 cell line
When cultured in serum-containing medium (DMEM with
10% FBS), primary fibrosarcoma culture (PFT) and HT1080 cells
showed an adherent growth pattern with a spindle-shaped morphology
(Fig. 1A).
The cells were then trypsinized and replated in
serum-free medium supplemented with bFGF and EGF in six-well
ultra-low-attachment plates, and their growth characteristics and
morphology were monitored. Within 48 h of replating, the single
cells began to form loose clumps that continued to increase in
density. At day 4, the loose clumps aggregated. At day 8, spheroids
began to take shape. By day 12, spheroids had completely formed and
became well-rounded structures composed of numerous, compacted
cells (Fig. 1A). The PFT and HT1080
tumor cells eventually formed spheroids at a frequency of ∼1/100
(928.25±30.25 colonies/1x106 cells) and 1/130 (769.75±69
colonies/1x106 cells).
To further investigate the self-renewal capacity of
spherical cells, the single fibrosarcosphere cells were replated
into 96-well ultra-low-attachment plates (1 cell/well) under
serum-starved conditions. Both sphere-forming cells demonstrated
self-renewal through the formation of secondary spheres at a
frequency of approximately 1/100 (Fig.
1B). These data suggest that human fibrosarcomas possess the
ability to generate suspended spherical clones and contain a
population of self-renewing primitive cells as well as populations
of differentiating cells.
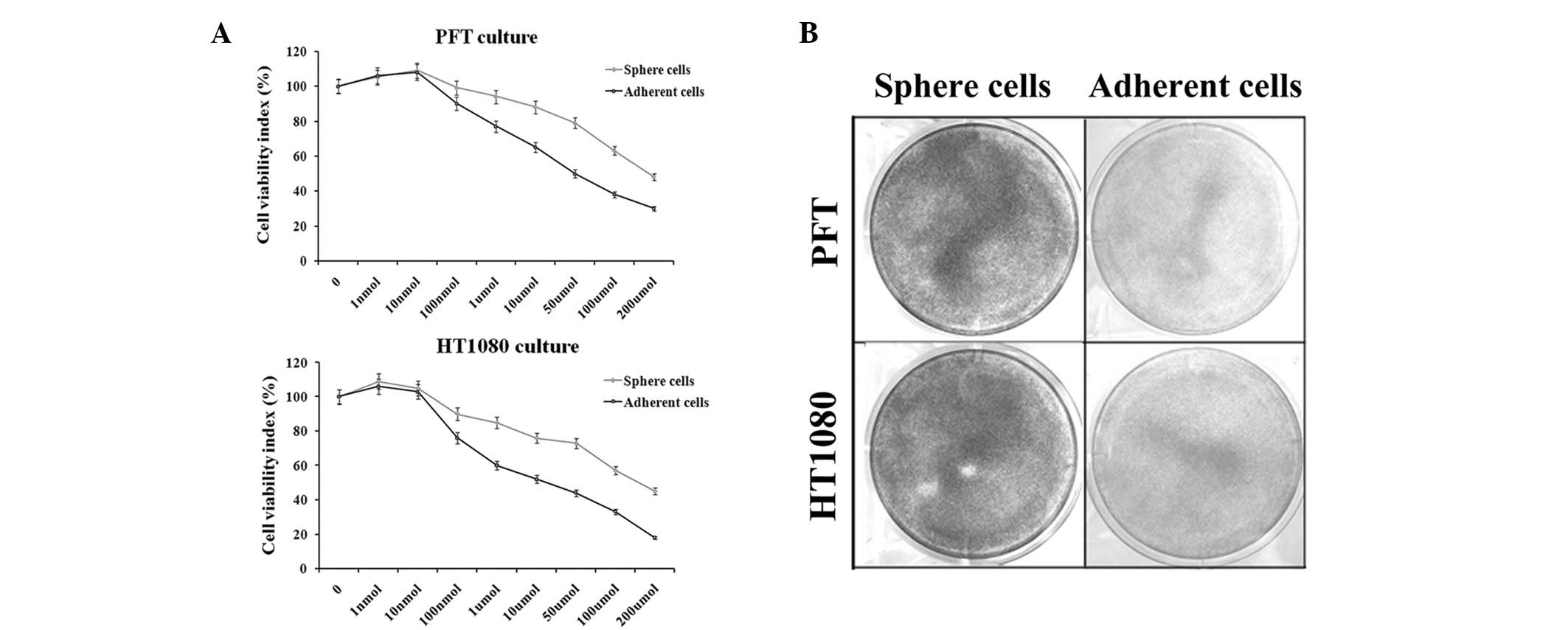
Fibrosarcosphere cells are more
resistant to the chemotherapeutic agent doxorubicin
In this study, the Cell Counting Kit-8 assay was
used to assess the cell viability rate. When exposed to gradient
doses of doxorubicin for 48 h, the growth of PFT and HT1080
cultures was inhibited in a dose-dependent manner. The difference
in growth inhibition rate between spherical and adherent cells was
statistically significant. For example, 10 μM doxorubicin
inhibited PFT and HT1080 cell growth by up to 38 and 48% in
adherent cultures and 11 and 25% in spherical cultures,
respectively (Fig. 2A). These
results were further confirmed by the crystal violet assay
(Fig. 2B).
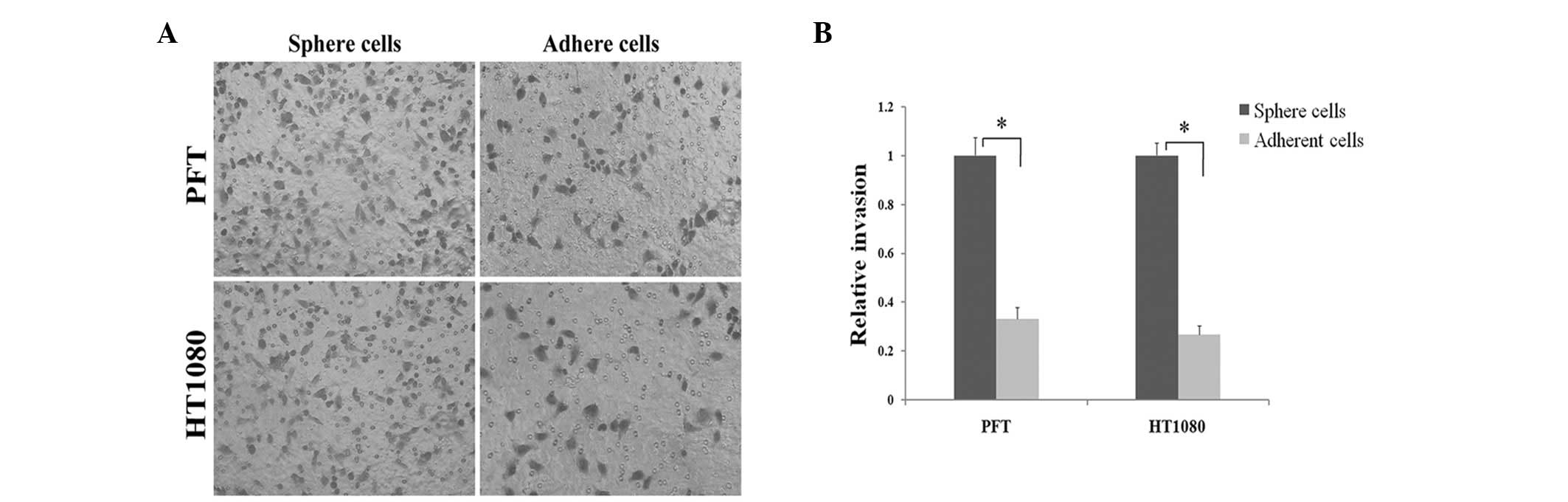
Fibrosarcosphere cells possess
stronger invasive characteristics than adherent cells in Matrigel
assays
Certain studies have shown that CSCs in tumors are
more invasive compared with their adherent counterparts, which is
defined as an inherent characteristic of CSCs (7,12). As
shown in Fig. 3, after 48 h of
incubation PTF and HT1080 spherical cells readily migrated through
the Matrigel chamber in relatively high numbers, whereas their
adherent counterparts exhibited a marked reduction in invasion.
Fibrosarcospheres express markers of
pluripotent embryonic stem cells
In this study, as shown in Fig. 4A, PFT and HT1080 fibrosarcospheres
grown in the serum-free, anchorage-dependent system showed a
significantly greater (P<0.05) expression of Oct3/4, Nanog, Sox2
and Sox10 than cells in adherent culture. Notably, both
fibrosarcosphere and adherent cultures showed similar levels of
STAT3 mRNA.
Consistent with the real-time quantitative PCR
results, sphere cells from PFT and HT1080 cultures showed a higher
protein expression of Oct3/4, Nanog, Sox2 and Sox10 than adherent
cells (Fig. 4B). Moreover, MDR1, a
protein related to chemotherapy resistance in tumors, was detected
in both cultures. MDR1 expression in sphere cells was higher than
that in adherent cells. This result was consistent with the result
from the drug resistance assessment assay.
Spherical cells show higher
tumorigenic potential in xenografts
To study the tumor-initiating capability of the
spherical cells, we generated xenografts using nude mice. As shown
in Table I, spherical cells formed
visible xenograft tumors in certain mice 2 weeks after injection,
whereas their adherent counterparts did not form any visible
tumors. Notably, despite the largest xenograft tumors initiated by
spherical cells having reached 2.5±0.4 cm in diameter at week 12,
few tumors were observed with the adherent cells. Moreover, as
little as 500 PFT spherical cells were capable of initiating a
tumor, whereas no tumor was found with >5x103 PFT
adherent cells (Fig. 4C).
 | Table I.Tumorigenicity of fibrosarcosphere
cells and adherent cells of PFT and HT1080 cultures in nude
mice. |
Table I.
Tumorigenicity of fibrosarcosphere
cells and adherent cells of PFT and HT1080 cultures in nude
mice.
| Cell type | Cell numbers
injected | Tumor
incidencea | Latency
(days)b |
|---|
| Fibrosarcosphere
cells of PFT culture |
1x102 | 0/3 | - |
|
5x102 | 1/3 | 45 |
|
1x103 | 1/3 | 38 |
|
5x103 | 2/3 | 32 |
|
5x104 | 3/3 | 21 |
|
1x105 | 2/2 | 13 |
| Adherent cells of
PFT culture |
5x102 | 0/3 | - |
|
5x103 | 0/3 | - |
|
5x104 | 1/3 | 25 |
|
1x105 | 2/2 | 18 |
| Fibrosarcosphere
cells of HT1080 culture |
1x102 | 0/3 | - |
|
5x102 | 0/3 | - |
|
5x103 | 1/3 | 35 |
|
5x104 | 2/3 | 23 |
|
1x105 | 2/2 | 14 |
| Adherent cells of
HT1080 culture |
5x102 | 0/3 | - |
|
5x103 | 0/3 | - |
|
5x104 | 1/3 | 28 |
|
1x105 | 1/2 | 19 |
Discussion
Previous studies have suggested that the
characteristics of normal stem cells, including self-renewal, the
ability to differentiate and the activation of anti-apoptotic
pathways, may be shared by tumor cells. In a tumor, CSCs comprise a
relatively small subpopulation of cells and possess primitive
phenotypes, the capability of initiating tumor formation,
resistance to chemotherapy and invasion into other tissues
(16). Therefore, CSCs may survive
therapy and begin to differentiate and reform a bulk tumor. Hence,
CSCs are proposed to be responsible for chemoresistance, recurrence
and progression in a number of tumors (5,16).
In the present study, we demonstrated that HT1080
and PFT cells have the ability to form fibrosarcospheres and to
self-renew in a culture system previously developed to isolate stem
cells from brain and breast tumors (10,17).
This anchorage-independent, serum-free culture system yielded
clonogenic stem-like cells that possessed many attributes common to
normal stem cells. Furthermore, we found that fibrosarcosphere
cells presented the characteristics of tumor stem cells previously
described for CSCs present in other tumor types, including
osteosarcoma, melanoma, breast and colon tumors.
By RT-PCR analysis, the fibrosarcosphere cells were
found to highly express the stemness-related genes compared with
adherent cells. This result was confirmed by western blot detection
of their gene products. Oct3/4 is a POU family homeoprotein
initially expressed in the inner cell mass of embryos and is
essential for the maintenance of pluripotency. After maturity,
Oct3/4 is only observed in certain early progenitor cells, but not
in somatic cells (18). Nanog is a
divergent homeoprotein that is capable of maintaining self-renewal
in ES cells. Overexpression of Nanog is associated with an
increased self-renewal capacity of ES cells (19,20).
Together, our data suggest that fibrosarcospheres contain cells
that possess pluripotent and self-renewal capacity.
In the Cell Counting Kit-8 and crystal violet
assays, we demonstrated that fibrosarcosphere cells showed higher
resistance potential to the chemotherapeutic agent doxorubicin.
Although the mechanisms of drug resistance remain to be elucidated,
several studies have revealed a possible involvement of the
ATP-binding cassette (ABC) drug transporters (21). In this study, spherical cells highly
expressed multidrug resistance transporter (MDR1). Goodell et
al demonstrated that ABC transporters may be involved in the
efflux capacity of CSCs (22). This
transporter protein has been found to contribute to Hoechst dye
efflux and produce a cancer stem cell phenotype in a wide variety
of tissues (21). The expression of
ABC transporters has been analyzed in various malignancies in
relation to the drug resistance of CSCs (23). As such, the higher expression of
MDR1 may be a mechanism of the resistance of fibrosarcosphere cells
to the chemotherapeutic agents that was observed in our study.
Furthermore, we found that certain cells derived
from fibrosarcospheres were able to reform spherical colonies with
a similar or greater frequency in the cell renewal assay in
vitro, indicating that the fibrosarcospheres contain
self-renewing daughter cells and differentiating cells through
asymmetric cell divisions. We also found that as few as 500
fibrosarcosphere cells were able to initiate tumor formation in
nude mice. Collectively, these results suggest that both PFT and
HT1080 cell populations contain enriched stem-like cells that
retain self-renewal, chemotherapeutic resistance, invasive and
strong tumor-initiating abilities. Thus, these cells have a number
of characteristics indicative of ‘stem-like cancer cells’.
Although the cell of origin has still not been
clearly determined, specific molecular markers for cancer stem cell
populations in certain epithelium-derived tumors have been
identified, such as CD133 (prominin-1), which was used initially as
a marker for neuroepithelial stem cells and subsequently as a maker
for many CSCs, including those of the brain and colon (24–28).
To develop CSC-targeted therapy, it is important to specifically
isolate the CSCs. Although we have demonstrated the existence of a
cell population in human fibrosarcomas that has stem cell
characteristics, no specific molecular markers of human
fibrosarcoma CSCs were determined in this study. Therefore, future
studies will employ immunochemistry and fluorescence-activated cell
sorting (FACS) to investigate specific molecular markers for the
isolation of the CSCs in human fibrosarcomas.
In conclusion, our study results suggest that both
human fibrosarcoma primary tumors and cell lines contain a
subpopulation of stem cell-like cells that possess a self-renewal
capacity, strong tumor-initiating ability, higher resistance to
chemotherapy and greater invasiveness, as well as primitive
phenotypes of embryonic stem cells. These data may lead to a
considerable increase in our understanding of the biology of human
fibrosarcoma, raise the possibility that human fibrosarcoma is a
stem cell malignancy and provide key insight into improved drug
design and therapy in the future.
Acknowledgements
We would like to thank the Department
of Orthopedics (Affiliated Tumor Hospital of Harbin Medical
University) for providing us with the human fibrosarcoma tumor
specimens. This study was supported by grants from the National
Natural Scientific Foundation of China (30471471, 30872605).
References
|
1.
|
HD DorfmanB CzerniakBone
CancersCancer75203210199510.1002/1097-0142(19950101)75:1+%3C203::AID-CNCR2820751308%3E3.0.CO;2-V8000997
|
|
2.
|
WK TaconisTG van RijsselFibrosarcoma of
long bones. A study of the significance of areas of malignant
fibrous histiocytomaJ Bone Joint Surg Br6711111619852981883
|
|
3.
|
C WibmerA LeithnerN ZielonkeM SperlR
WindhagerIncreasing incidence rates of soft tissue sarcomas? A
population-based epidemiologic study and literature reviewAnn
Oncol2111061111201010.1093/annonc/mdp41519858086
|
|
4.
|
MF ClarkeJE DickPB DirksCancer stem cells
-perspectives on current status and future directions: AACR
Workshop on cancer stem cellsCancer
Res6693399344200610.1158/0008-5472.CAN-06-3126
|
|
5.
|
I IschenkoH SeeligerM SchafferK-W JauchCJ
BrunsCancer stem cells: how can we target them?Curr Med
Chem1531713184200810.2174/09298670878684854119075661
|
|
6.
|
U KochM KrauseM BaumannCancer stem cells
at the crossroads of current cancer therapy failures - radiation
oncology perspectiveSemin Cancer
Biol20116124201010.1016/j.semcancer.2010.02.00320219680
|
|
7.
|
VA SiclariL QinTargeting the osteosarcoma
cancer stem cellJ Orthop Surg
Res578201010.1186/1749-799X-5-7820979639
|
|
8.
|
D BonnetJE DickHuman acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cellNat
Med3730737199710.1038/nm0797-7309212098
|
|
9.
|
HD HemmatiI NakanoJA LazareffCancerous
stem cells can arise from pediatric brain tumorsProc Natl Acad Sci
USA1001517815183200310.1073/pnas.203653510014645703
|
|
10.
|
D PontiA CostaN ZaffaroniIsolation and in
vitro propagation of tumorigenic breast cancer cells with
stem/progenitor cell propertiesCancer
Res6555065511200510.1158/0008-5472.CAN-05-062615994920
|
|
11.
|
T SchattonGF MurphyNY FrankIdentification
of cells initiating human
melanomasNature451345349200810.1038/nature0648918202660
|
|
12.
|
ML SuvaN RiggiJC StehleIdentification of
cancer stem cells in Ewing’s sarcomaCancer Res69177617812009
|
|
13.
|
C ChenY WeiM HummelEvidence for
epithelialmesenchymal transition in cancer stem cells of head and
neck squamous cell carcinomaPLoS
One6e16466201110.1371/journal.pone.001646621304586
|
|
14.
|
CK EaD BaltimoreRegulation of NF-kappaB
activity through lysine monomethylation of p65Proc Natl Acad Sci
USA1061897218977200910.1073/pnas.091043910619864627
|
|
15.
|
CP GibbsVG KukekovJD ReithStem-like cells
in bone sarcomas: implications for
tumorigenesisNeoplasia7967976200510.1593/neo.0539416331882
|
|
16.
|
C TangBT AngS PervaizCancer stem cell:
target for anti-cancer therapyFASEB
J2137773785200710.1096/fj.07-8560rev17625071
|
|
17.
|
SK SinghID ClarkeM TerasakiIdentification
of a cancer stem cell in human brain tumorsCancer
Res6358215828200314522905
|
|
18.
|
RR PochampallyJR SmithJ YlostaloDJ
ProckopSerum deprivation of human marrow stromal cells (hMSCs)
selects for a subpopulation of early progenitor cells with enhanced
expression of OCT-4 and other embryonic
genesBlood10316471652200410.1182/blood-2003-06-1967
|
|
19.
|
I ChambersThe molecular basis of
pluripotency in mouse embryonic stem cellsCloning Stem
Cells6386391200410.1089/clo.2004.6.38615671667
|
|
20.
|
S GidekelG PizovY BergmanE PikarskyOct-3/4
is a dose-dependent oncogenic fate determinantCancer
Cell4361370200310.1016/S1535-6108(03)00270-814667503
|
|
21.
|
H FujiiK HonokiT TsujiuchiA KidoK
YoshitaniY TakakuraSphere-forming stem-like cell populations with
drug resistance in human sarcoma cell linesInt J
Oncol3413811386200919360350
|
|
22.
|
MA GoodellK BroseG ParadisAS ConnerRC
MulliganIsolation and functional properties of murine hematopoietic
stem cells that are replicating in vivoJ Exp
Med18317971806199610.1084/jem.183.4.17978666936
|
|
23.
|
LY BourguignonK PeyrollierW XiaE
GiladHyaluronan-CD44 interaction activates stem cell marker Nanog,
Stat-3-mediated MDR1 gene expression, and ankyrin-regulated
multidrug efflux in breast and ovarian tumor cellsJ Biol
Chem2831763517651200810.1074/jbc.M80010920018441325
|
|
24.
|
S BaoQ WuRE McLendonGlioma stem cells
promote radioresistance by preferential activation of the DNA
damage responseNature444756760200610.1038/nature0523617051156
|
|
25.
|
CA FargeasD CorbeilWB HuttnerAC133
antigen, CD133, prominin-1, prominin-2, etc.: prominin family gene
products in need of a rational nomenclatureStem
Cells21506508200310.1634/stemcells.21-4-50612832703
|
|
26.
|
L Ricci-VitianiDG LombardiE
PilozziIdentification and expansion of human
colon-cancer-initiating
cellsNature445111115200710.1038/nature0538417122771
|
|
27.
|
SK SinghC HawkinsID ClarkeIdentification
of human brain tumour initiating
cellsNature432396401200410.1038/nature0312815549107
|
|
28.
|
V TirinoV DesiderioR d’AquinoDetection and
characterization of CD133+ cancer stem cells in human solid
tumoursPLoS One3e34692008
|