Introduction
Microvenular hemangioma (MH) is an acquired benign
vascular tumor, occurring on the trunk and limbs in young to
middle-aged adults without any gender predilection. MH usually
presents as a solitary, purple-to-red papule/plaque measuring 5–20
mm in diameter. MH was first described by Bantel et al in
1989 (1). The etiology of MH is
unknown. MH is rare, with <50 cases reported to date. Even in
these reported cases, accounts of multiple MH are extremely
uncommon. The present study reports a case of multiple MH. Informed
consent was obtained from the patient.
Case report
Patient presentation
A 35-year-old male presented to Cangzhou Central
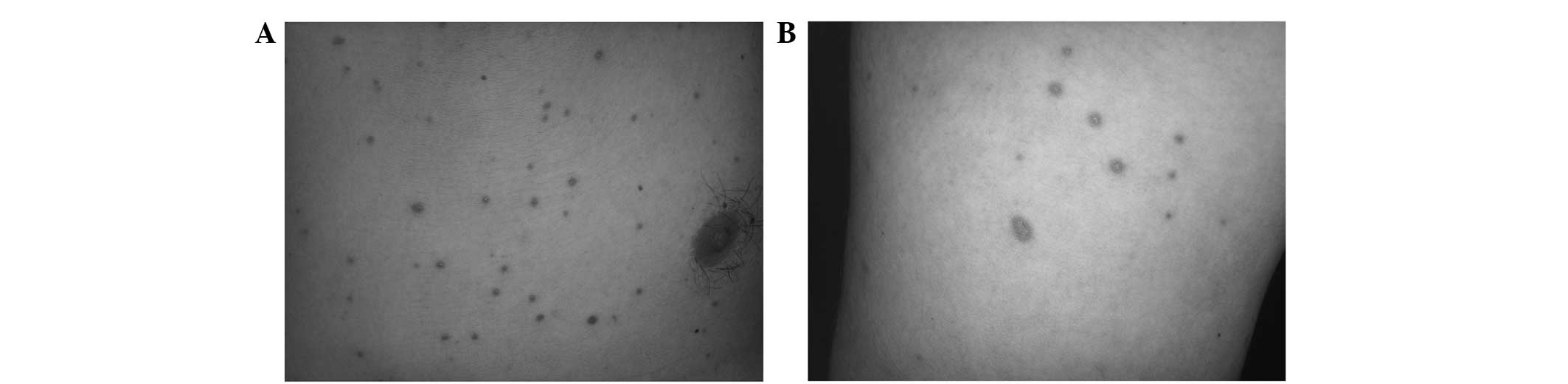
Hospital (Cangzhou, China) in September 2009 with multiple dark red
maculopapules on the trunk and limbs (Fig. 1). The patient reported a history of
their presence on the trunk and limbs for the past 5 years and with
the absence of any precipitating factor. Initially, the lesions
were the size of rice granules, painless and itchy, although not
troublesome enough to persuade the patient to attend a
consultation. Thereafter, the size of the lesions gradually
enlarged and also increased in number. Subsequently, the individual
sought medical advice at a local hospital and was prescribed oral
antihistamines and topical corticosteroids that proved of no
benefit. No other family member demonstrated such lesions.
Lesion examination
Upon examination, numerous dark red, circular,
non-scaly maculopapules measuring 5 mm in diameter were noted on
the chest, back, abdomen, buttocks and upper and lower extremities.
The lesions had a diffuse distribution with clear borders and were
painless. An attempt to count the number of lesions found >100.
The lesions were found in a greater concentration on the chest and
back. The head, face, palms and soles were unaffected.
Clinical and pathological analyses
Nothing abnormal was found on chest X-ray or
abdominal B-mode ultrasound scan. The patient’s blood profile,
urine and stool routine, liver function, renal function and
electrolyte levels were also normal.
Patient diagnosis
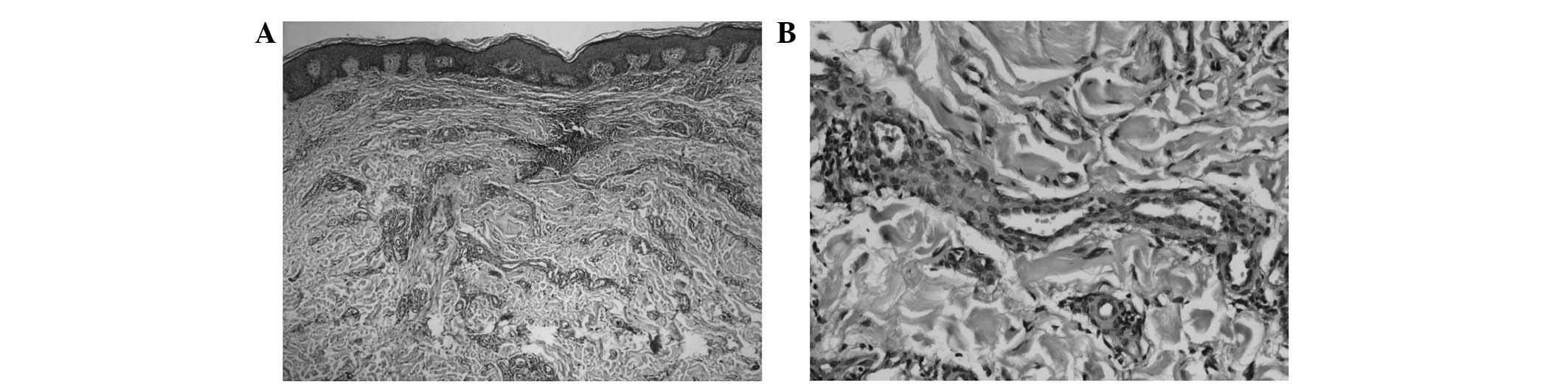
Two lesions of the chest were removed completely for
diagnosis. The tumors were of the same histopathology and
demonstrated proliferation of irregularly branched, thin-walled
venules infiltrating the sclerotic dermal collagen and the presence
of few blood vessels with inconspicuous lumina (Fig. 2A). A mixture of flat and plump
endothelial cells was observed (Fig.
2B), however, there was an absence of cellular atypia and
mitotic figures. Perivascular infiltration of few lymphocytes was
noted.
Immunohistochemically, the endothelial cells of the
proliferating vessels expressed CD31 (Fig. 3A), CD34 (Fig. 3B) and factor VIII (Fig. 3C). The pericytes expressed SMA
(Fig. 3D) and HHF-35 (Fig. 3E). The diagnosis was microvenular
hemangioma. The patient was treated with recombinant human
interferon α-2b gel, twice a day. There was no clear effect after a
month.
Discussion
MH is an acquired benign vascular tumor. MH was
first described by Bantel et al in 1989. At that time, the
study reported microcapillary angiomas in three female patients
(1). In 1991, similar cases were
described by Hunt et al using current terminology (2). MH is rare, with ≤50 cases reported to
date.
The etiology of MH is unknown, but in specific
cases, MH has been observed following changes in hormonal
contraception or during pregnancy (1). Sex hormone imbalance may also account
for certain cases (3). MH shows a
predilection for young to middle-aged adults, but is also
identified in children (4). It is
most frequently located on the trunk and upper extremities, and
less frequently on the toes (5). MH
grows slowly and usually presents as a solitary, purple to red
papule/plaque measuring 5–20 mm in diameter. Alternatively, it can
also present as a pale red nodule, which clinically resembles
cutaneous inflammation (6).
Generally, the lesion is asymptomatic, but slight tenderness has
also been noted in specific cases (7). Multiple MHs, as in the present case,
are extremely rare. To date, Xu et al has already reported
four cases of patients from China who exhibited a rapidly
progressive abrupt onset of numerous MHs numbering in the tens to
hundreds (8).
Upon dermoscopic examination, multiple
well-demarcated red globules are observed with the presence of a
fine pigmented network at the periphery (9). Histologically, MH is composed of
thin-walled, irregularly branching venules with inconspicuous
vascular lumina. The collagen bundles in the dermis are thickened.
The endothelial cells are surrounded by pericytes (10) and may present as a mixture of flat
or plump cells, but with a lack of cellular atypia, pleomorphism or
mitotic figures (11).
Immunohistochemically, the endothelial cells of an MH are positive
for CD31, CD34 and factor VIII, and the pericytes are positive for
SMA (9,12), but both stain negative for
podoplanin (13).
The tumor may exhibit infiltrative growth throughout
the dermis. Therefore, pathologists and clinicians must be aware of
the existence of this type of infiltrative growth accompanying the
hemangioma and hence, avoid overdiagnosis and overtreatment
(11).
There have been multiple accounts of MH associated
with other systemic diseases in the medical literature. An
association with a case of POEMS syndrome was previously reported
by Hudnall et al(14) where
HHV-8 was directly demonstrated within the endothelial cells of MH.
It is worth noting that the hemangiomas in this case responded to
chemotherapy with cyclophosphamide and prednisone.
A previously reported case of MH in a young child
with acute myelogenous leukemia (4)
demonstrated an association with systemic immunosuppression. In
addition, Rikihisa et al reported a case of MH in a patient
with Wiskott-Aldrich syndrome (15).
Histologically, the most common differential
diagnosis of MH is the patch stage of Kaposi’s sarcoma (KS)
(2). However, in KS, irregular
vascular spaces undergo anastomosis rather than collapse, as seen
in MH. The presence of accompanying atypical endothelial cells,
eosinophilic hyaline globules, plasma cells and fascicles of
spindle cells favors the diagnosis of KS over MH (9).
Additional differential diagnoses include
multinucleate cell angiohistiocytoma (MCA) and reactive
angioendotheliomatosis (RAE). In MCA, the characteristic cells are
large, with irregular, unusually-shaped, scalloped or angular
margins (16). The RAE is
characterized histologically by the proliferation of endothelial
cells within the vascular lumina, resulting in the obliteration of
the involved vessels secondary to intravascular thrombi, thereby,
making it easier to determine the diagnosis. In addition, MH must
be distinguished from other entities, including targetoid
hemosiderotic hemangioma, tufted angioma, sclerosing angioma and
granuloma pyogenicum (9,10,12).
The distinctive feature of the present case that
makes it an important academic observation is that, besides the
multiple skin lesions, there are also epidermal changes on the
histopathology, which include spreading and interdigitating
epidermal processes and an increase in the basal lamina
pigmentation. Furthermore, the course of this case was longer than
usually observed in MH.
For cases of this disease with minor solitary
lesions, excision has been associated with cure and usually no
recurrence is observed. In cases with major or multiple skin
eruptions, regular follow-ups should be performed, although the
disease is self-limiting in nature. In the present case, some
primary lesions disappeared without further treatment; however, a
few new eruptions appeared on the patient’s back and chest.
Acknowledgements
This study was supported by a grant from the Tianjin
Foundation of Natural Science (no. 09JCZDJC18300).
References
|
1
|
Bantel E, Grosshans E and Ortonne JP:
Understanding microcapillary angioma, observations in pregnant
patients and in females treated with hormonal contraceptives. Z
Hautkr. 64:1071–1074. 1989.(In German).
|
|
2
|
Hunt SJ, Santa Cruz DJ and Barr RJ:
Microvenular hemangioma. J Cutan Pathol. 18:235–240. 1991.
View Article : Google Scholar
|
|
3
|
Satge D, Grande-Goburdhun J and Grosshans
E: Microcapillary hemangioma. Ann Dermatol Venereol. 120:297–298.
1993.(In French).
|
|
4
|
Chang SE, Roh KH, Lee MW, et al:
Microvenular hemangioma in a boy with acute myelogenous leukemia.
Pediatr Dermatol. 20:266–267. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang IJ, Cho HR, Hong KK and Kim NI: A
case of microvenular hemangioma clinically mimicking Kaposi’s
sarcoma. Korean J Dermatol. 44:652–654. 2006.
|
|
6
|
Miyashita H, Yokoyama A and Tanaka K: A
case of microvenular hemangioma with presentation resembling
inflammatory skin tumor. J Plast Reconstr Aesthet Surg.
62:e166–e167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim YC, Park HJ and Cinn YW: Microvenular
hemangioma. Dermatology. 206:161–164. 2003. View Article : Google Scholar
|
|
8
|
Xu XL, Xu CR, Chen H, et al: Eruptive
microvenular hemangiomas in 4 Chinese patients: clinicopathologic
correlation and review of the literature. Am J Dermatopathol.
32:837–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scalvenzi M, De Natale F, Francia MG and
Balato A: Dermoscopy of microvenular hemangioma: report of a case.
Dermatology. 215:69–71. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aloi F, Tomasini C and Pippione M:
Microvenular hemangioma. Am J Dermatopathol. 15:534–538. 1993.
View Article : Google Scholar
|
|
11
|
Fukunaga M and Ushigome S: Microvenular
hemangioma. Pathol Int. 48:237–239. 1998. View Article : Google Scholar
|
|
12
|
Stefanaki C, Stefanaki K, Floros K,
Rontogiani D and Georgala S: Microvenular hemangioma: a rare
vascular lesion. J Dermatol. 32:402–404. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fernandez-Flores A: Lack of expression of
podoplanin by microvenular hemangioma. Pathol Res Pract.
204:817–821. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hudnall SD, Chen T, Brown K, Angel T,
Schwartz MR and Tyring SK: Human herpesvirus-8-positive
microvenular hemangioma in POEMS syndrome. Arch Pathol Lab Med.
127:1034–1036. 2003.PubMed/NCBI
|
|
15
|
Rikihisa W, Yamamoto O, Kohda F, et al:
Microvenular haemangioma in a patient with Wiskott-Aldrich
syndrome. Br J Dermatol. 141:752–754. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jaconelli L, Kanitakis J, Ktiouet S, Faure
M and Claudy A: Multinucleate cell angiohistiocytoma: Report of
three new cases and literature review. Dermatol Online J.
15:42009.PubMed/NCBI
|