Introduction
Rhabdomyosarcoma (RMS) is a rare and aggressive
malignancy possibly originating from primitive mesenchymal cells
that arise anywhere in the body, including sites where striate
muscle is not found (1). The annual
incidence of RMS in children is reported to be 4.3 cases per
million (2). RMS is the most common
type of soft-tissue sarcoma in young children, representing 5% of
all childhood malignancies (3). By
contrast, RMS occurs less frequently in adults (4).
Almost half of RMS occur in the head and neck
(5–8) and three different primary sites of
head and neck RMS (HNRMS) have been recognized in the following
locations: parameningeal (PM), non-PM (NPM) and orbital (ORB)
(9). In addition, surviving
hereditary retinoblastoma patients have an increased risk of
craniofacial second primary tumor (SPT), such as RMS, particularly
following treatment with external beam radiotherapy. RMS is one of
the most common types of craniofacial SPT in irradiated hereditary
retinoblastoma patients, which develops in specific locations (such
as the ethmoid sinus and temporal fossa) (10). HNRMS is commonly confused with other
types of rapidly progressive malignant tumors of the head and neck,
including lymphoma, nasopharyngeal carcinoma (NPC), primitive
neuroectodermal tumors, Langerhans cell histiocytosis, olfactory
neuroblastoma (ONB), osteosarcoma and metastasis (1,10–13).
The aim of the present study was to investigate the
computed tomography (CT) and magnetic resonance imaging (MRI)
features of HNRMS and analyze the correlation between the imaging
observations and the pathological subtypes.
Patients and methods
Subjects
Patients who underwent treatment for RMS at the East
Hospital affiliated to Tongji University School of Medicine
(Shanghai, China) between 2007 and 2013 were identified from the
pathology and health record databases in agreement with the
recommendations of the East Hospital ethics committee. The
following inclusion criteria were used: i) Availability of adequate
CT or MRI information; and ii) histopathological confirmation of
RMS. A total of 10 HNRMS patients (three males and seven females;
median age, 16 years), who were histologically diagnosed by biopsy
(n=8) or surgery (n=2), were included in this retrospective study.
The patients had no medical history of hereditary retinoblastoma or
treatment with radiotherapy. In addition, their age, gender,
symptoms and pathological subtype were recorded.
CT and MRI technique
In patients with HNRMS, CT is predominantly
performed to assess for the absence or presence of bony
destruction, calcification and lung metastases. Eight patients
underwent CT using a 64-slice spiral CT system (Philips Brilliance;
Philips Medical Systems, Best, The Netherlands). The CT scanner
parameters were as follows: 250 mAs; 120 kVp; rotation time, 0.75
sec; pitch, 1.204; 25-cm field of view; matrix size, 512×512; slice
thickness, 1.5 mm; and detector configuration, 64×0.625 mm. In
addition, dual-phase dynamic enhanced scanning (30 and 65 sec) was
performed in four patients to obtain images of the arterial and
venous stages following the intravenous administration of the
contrast agent (Omnipaque 300; 300 mgI/ml; dose, 1.5 ml/kg body
weight; injection rate, 2.5 ml/sec) purchased from Nycomed Amersham
(Princeton, NJ, USA).
In total, nine patients underwent MRI using a
3.0-Tesla system (Philips Achieva; Philips Medical Systems) and a
combined head and neck coil. The parameters of the MRI scanner were
as follows: 23-cm field of view; matrix size, 256×192; and slice
thickness, 3 mm. T1-weighted spin-echo (SE) images were obtained in
the axial plane [repetition time (TR)/echo time (TE), 279/2.3 msec
of two excitations]. In addition, T2-weighted fast SE images
(TR/TE, 3,118/80 msec of one excitation) and T2-weighted short time
inversion recovery in the axial and coronal planes were obtained
prior to injection of the contrast material. Following the
intravenous administration of gadopentetate dimeglumine (Gd-DTPA:
Magnevist®; Bayer Schering Pharma AG, Berlin, Germany;
dose, 0.1 mmol/kg body weight; injection rate, 1.5 ml/sec),
fat-saturated T1-weighted SE images were obtained in the axial,
coronal and sagittal planes with the same parameters that were used
prior to the Gd-DTPA injection. In seven out of the 10 HNRMS cases,
CT and MRI were available.
Image interpretation
On CT examination, the attenuation of each tumor was
recorded as hypo-, iso- or hyperdense as compared with the adjacent
muscle. On MRI, the signal intensity of each tumor was recorded as
hypo-, iso- or hyperintense as compared with the adjacent
muscle.
Two radiologists (specialists in head and neck
imaging), who were blinded to the diagnosis of HNRMS, independently
evaluated the CT and MRI images and were in agreement. The tumor
characteristics, including site, size, margin, local extent,
calcification, hemorrhaging, bony destruction and site of
metastasis, were recorded. In addition, the attenuation and
intensity, as well as the contrast enhancement pattern of the HNRMS
were evaluated.
The CT and MRI features together with the clinical
data of the 10 HNRMS patients were analyzed using the pathological
subtypes. All patients provided written informed consent for
participation in the study and for the review of their medical
records.
Results
Clinical features
The 10 patients (three males and seven females)
ranged in age between five and 77 years (median age, 16 years) and
70% of the patients were aged <20 years. The clinical symptoms
were not specific, however, they were associated with the tumor
site, which included nasal obstruction (n=5), purulent nasal
discharge (n=3), proptosis (n=3), visual disturbance (n=2),
epistaxis (n=1), hyposmia (n=1) and subcutaneous mass (n=1).
CT and MRI observations
The 10 HNRMSs were classified into embryonal (n=8)
and alveolar (n=2) subtypes, confirmed by surgery (n=2: Cases 6 and
10) and biopsy (n=8). Immunohistochemical analysis of the masses
revealed characteristic positivity for desmin (n=10) and MyoD1
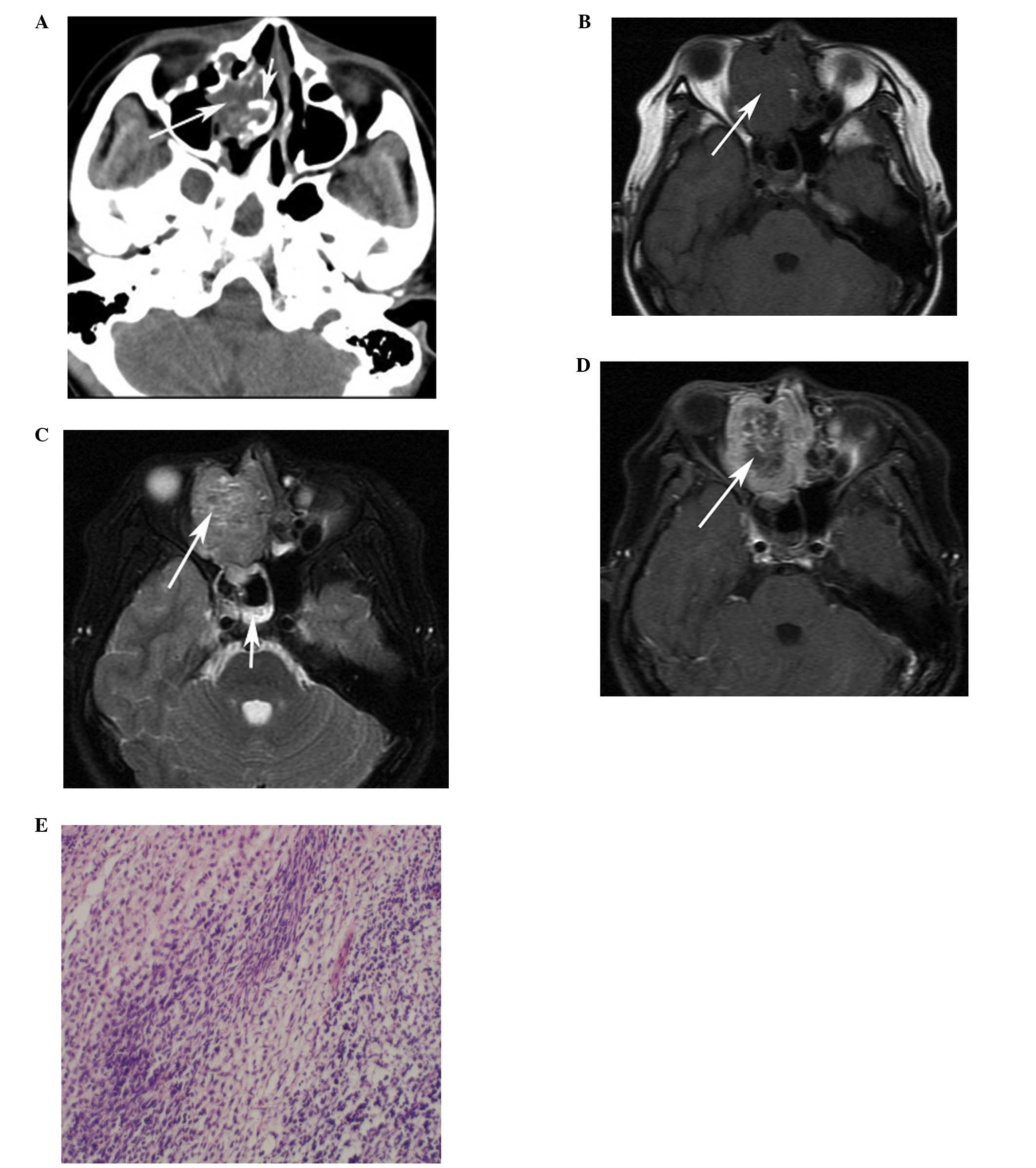
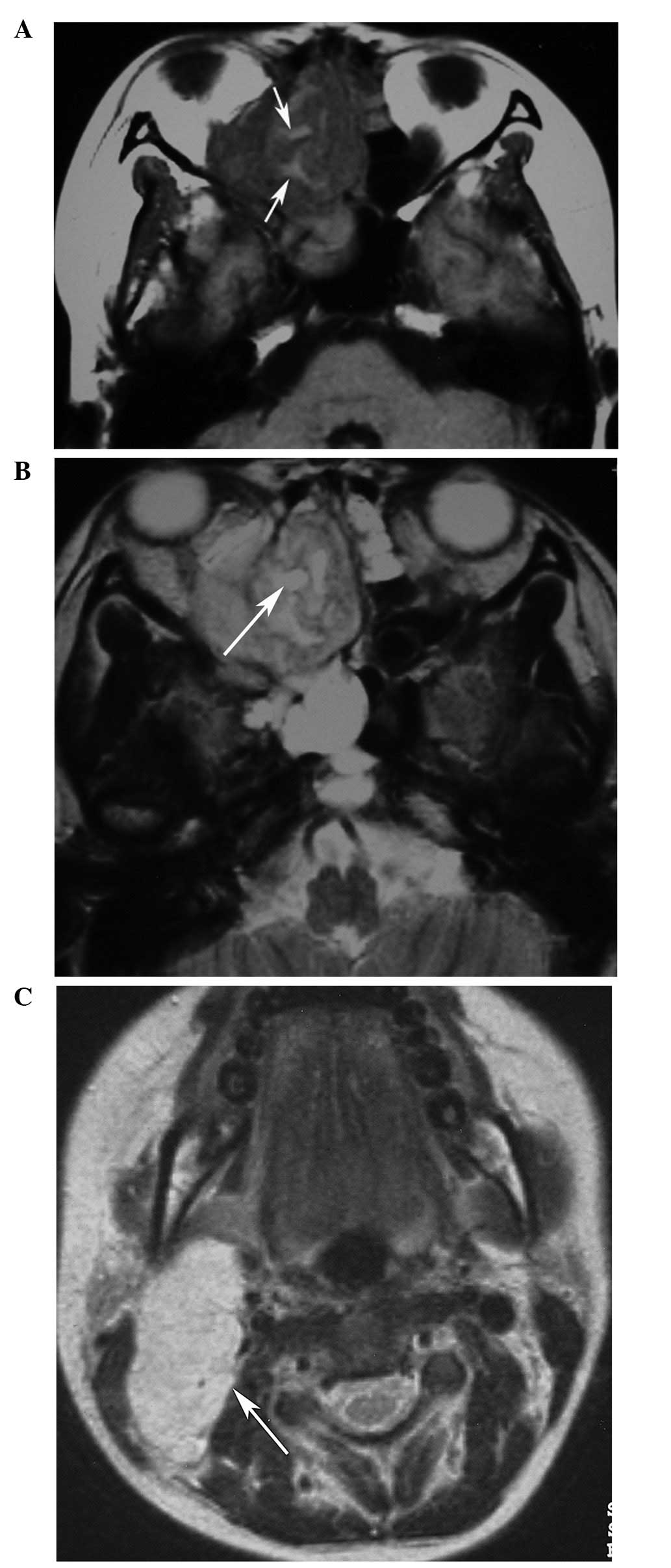
(n=10). The original locations of the HNRMSs were the ethmoid sinus
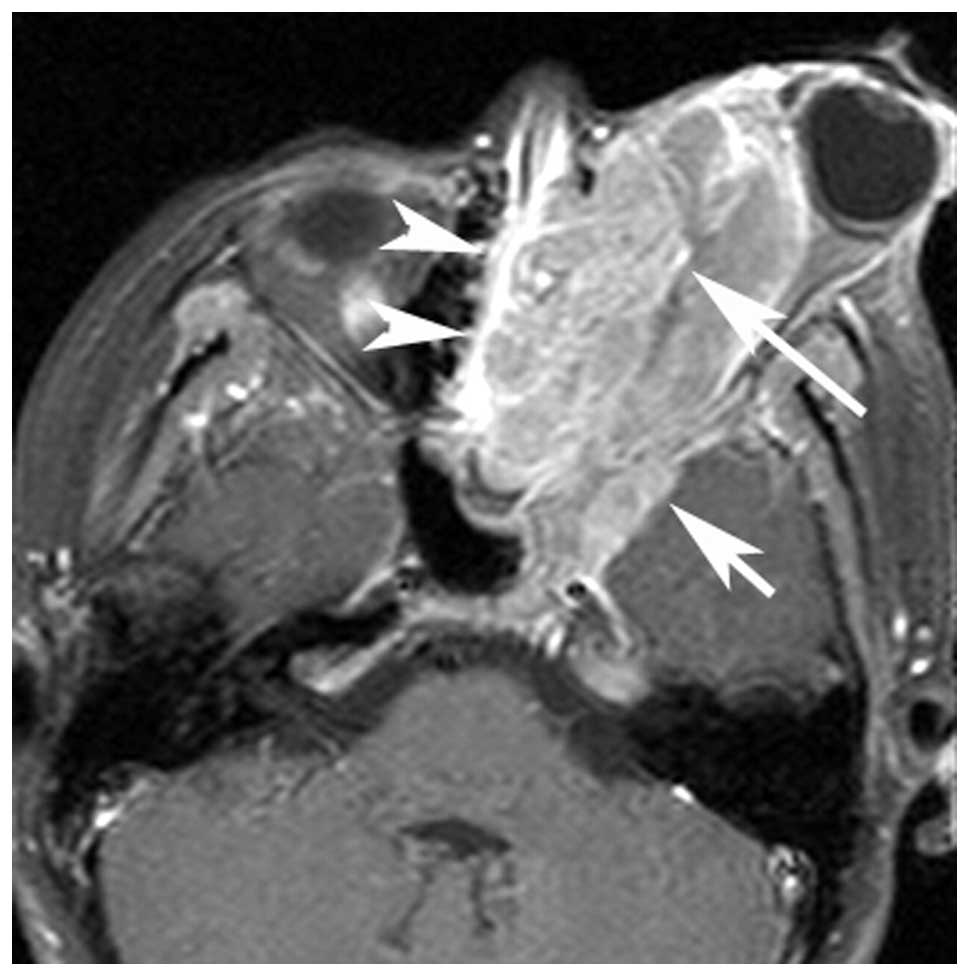
(n=4; Figs. 1 and 2), the maxillary sinus (n=1), orbit (n=3;
Fig. 3), nasopharynx (n=1) and the
frontotemporal subcutaneous area (n=1). The average tumor diameter
was 4.5 cm (range, 2.9–7.1 cm). On the CT (n=8) and MRI (n=9)
images, 90% (9/10) of the patients exhibited ill-defined
soft-tissue masses. The tumors appeared as isodense (n=6) or
slightly hypodense (n=2) on the precontrast CT images, and
isointense on the T1-weighted images (WI; n=9, one tumor exhibited
multiple hyperintensity signals, the others exhibited a homogeneous
signal). On T2WI, the tumors showed homogeneous moderate
hyperintensity (n=5), homogeneous marked hyperintensity (n=1) and
heterogeneous moderate hyperintensity (n=3). In addition, the
masses exhibited homogeneous enhancement [n=7, one patient on
post-contrast CT images, three patients on contrast-enhanced (CE)
T1WI and three patients on CT and CE-T1WI] or heterogeneous
enhancement (n=3, CE-T1WI only). Three embryonal RMSs (cases 1–3)
originating from the ethmoid sinus demonstrated heterogeneous
hyperintensity on T2WI and heterogeneous enhancement with multiple
small rings, resembling nodules (Figs.
1 and 2). Unilateral or
bilateral sinusitis was also observed in five patients.
Bony destruction (n=10) was ubiquitous and sclerosis
was identified in two tumors originating from the ethmoid sinus
(Fig. 1A). The tumors destroyed
adjacent bony structures and extended into the surrounding spaces,
including the paranasal sinus (n=6), nasal cavity (n=5), cranial
cavity (n=5), orbit (n=4) and infratemporal fossa (n=1).
Multi-cavity growth (cavities, n≥2) was identified in 70% (7/10) of
patients (Fig. 3). Dural
enhancement (thickness, >5 mm) (1), which was interpreted as intracranial
extension, was noted in five patients (Fig. 3). Calcification and hemorrhaging
were not identified in any of the patients. Unilaterally enlarged
cervical lymph nodes (>1 cm in short diameter) without necrosis
were observed in two patients (cases 3 and 9) and identified as
metastatic by ultrasound-guided fine-needle cytology (Fig. 2C), however, no distant metastasis
was identified. The CT and MRI observations of the 10 HNRMS
patients together with their clinical data are summarized in
Tables I and II.
 | Table IClinical data and imaging results of
10 patients with head and neck rhabdomyosarcoma. |
Table I
Clinical data and imaging results of
10 patients with head and neck rhabdomyosarcoma.
| Case | Age,
years/gender | Pathology
subtype | Tumor origin | Tumor border | Size, cm | CT (n=8) | MRI (n=9) | Lymph | Tumor extent |
|---|
|
|
|---|
| Density | CE | T1WI | T2WI | CE-T1WI |
|---|
| 1 | 14/F | Embryonal | ES (R) | Ill-defined | 7.1 | Hypo | NA | Isointense | Hypera | Nodular | − | MS, NC, CC |
| 2 | 15/F | Embryonal | ES (R) | Ill-defined | 6.9 | Hypo | NA | Isointense | Hypera | Nodular | − | MS, NC, O, CC |
| 3 | 17/F | Embryonal | ES (R) | Ill-defined | 4.0 | Isodense | NA | Isointense | Hypera | Nodular | + | MS, SS, NC, O |
| 4 | 45/F | Embryonal | ES (L) | Ill-defined | 5.6 | Isodense | NA | Isointense | Hyperb | Homo | − | MS, NC, O, CC |
| 5 | 36/M | Alveolar | MS (R) | Ill-defined | 3.5 | Isodense | Homo | NA | NA | NA | − | ES, NC, O, ITF |
| 6 | 19/F | Embryonal | O (L) | Ill-defined | 3.1 | Isodense | Homo | Isointense | Hyperb | Homo | − | - |
| 7 | 6/F | Embryonal | O (L) | Ill-defined | 2.9 | NA | NA | Isointense | Hyperb | Homo | − | - |
| 8 | 13/F | Embryonal | O (L) | Ill-defined | 5.7 | Isodense | Homo | Isointense | Hyperb | Homo | − | ES, MS, CC |
| 9 | 5/M | Embryonal | NP (R) | Ill-defined | 3.6 | NA | NA | Isointense | Hyperc | Homo | + | CC |
| 10 | 77/M | Alveolar | S (R) | Well-defined | 3.5 | Isodense | Homo | Isointense | Hyperb | Homo | − | - |
 | Table IISummary of the clinical and imaging
results of 10 patients with head and neck rhabdomyosarcoma. |
Table II
Summary of the clinical and imaging
results of 10 patients with head and neck rhabdomyosarcoma.
| Characteristic | n (%) |
|---|
| Age, years |
| <20 | 7/10 (70.0) |
| ≥20 | 3/10 (30.0) |
| Gender |
| Male | 3/10 (30.0) |
| Female | 7/10 (70.0) |
| Tumor origin |
| Paranasal
sinus | 5/10 (50.0) |
| Orbit | 3/10 (30.0) |
| Other | 2/10 (20.0) |
| Pathological
subtype |
| Embryonal | 8/10 (80.0) |
| Alveolar | 2/10 (20.0) |
| Tumor border |
| Ill-defined | 9/10 (90.0) |
| Well-defined | 1/10 (10.0) |
| Computed tomography
attenuation |
| Slightly
hypodense | 2/8 (25.0) |
| Isodense | 6/8 (75.0) |
| Homogeneous | 8/8 (100.0) |
| Homogeneous
enhancement | 4/4 (100.0) |
| T1WI |
| Isointense | 9/9 (100.0) |
| Homogeneous
enhancement | 6/9 (66.7) |
| Nodule-shaped
enhancement pattern | 3/9 (33.3) |
| T2WI |
| Moderately
hyperintense | 8/9 (88.9) |
| Markedly
hyperintense | 1/9 (11.1) |
| Homogeneous | 6/9 (66.7) |
| Heterogeneous | 3/9 (33.3) |
| Tumor extent |
| 1 cavity | 3/10 (30.0) |
| ≥2 cavities | 7/10 (70.0) |
| Bony
destruction | 10/10 (100.0) |
| Cervical lymph node
metastases | 2/10 (20.0) |
Discussion
The incidence rate of HNRMS is uncertain between
males and females (5–7,14,15);
however, the present study exhibited a female predominance (70%). A
previous study reported that 43% of RMSs occur prior to reaching
five years of age and 78% occur prior to reaching 12 years of age
(6), which is consistent with the
current study where the median age of patients was 16 years, with
70% of patients <20 years old. RMS exhibits a predilection for
the head and neck regions, however, HNRMS in the PM, NPM and ORB
locations are involved in ~50, 25 and 25% of cases, respectively
(9). In the current study, the PM
(60%; cases 1–5 and 9) and ORB (30%; cases 6–8) locations were the
most common sites and the clinical symptoms were not specific,
however, they were associated with the tumor site.
Due to the rarity of HNRMS, the majority of the
available CT and MRI information is derived from small case series.
Lee et al (7) reported that
10 HNRMSs appeared as isodense (100%; 10/10) on pre-contrast CT and
homogeneously enhanced (60%; 6/10) on post-contrast CT. In
addition, Hagiwara et al (6)
presented eight HNRMSs with isointensity (37.5%) and slight
hyperintensity (62.5%) on T1WI, and homogeneous (12.5%) and
heterogeneous hyperintensity (87.5%) on T2WI, and heterogeneous
enhancement (100%) on CE-T1WI. In the current study, the tumors
appeared as isodense (75%; 6/8) or slightly hypodense (25% 2/8) on
pre-contrast CT and homogeneous enhancement (100%, 4/4) was
demonstrated on post-contrast CT. On MRI, the tumors demonstrated
isointensity (100%; 9/9) on T1WI, homogeneously moderate to marked
hyperintensity (66.7%; 6/9) or heterogeneously moderate
hyperintensity (33.3%; 3/9) on T2WI, and homogeneous enhancement
(66.7%; 6/9) or heterogeneous enhancement (33.3%; 3/9) on CE-T1WI.
The imaging results of the HNRMS in the present study differ from
previous studies. This discrepancy may be a result of the lack of
HNRMS cases, however, it may be due to the different pathological
subtypes.
The current histological classification for RMS
includes the embryonal, alveolar and pleomorphic subtypes; the
botryoid type is classified as embryonal (5). Allen et al (4) reported that RMSs in adults (n=26)
demonstrate prominent heterogeneity and extreme hyperintensity on
T2WI in the alveolar and pleomorphic subtypes. However, according
to Franco et al (5), RMSs do
not exhibit these features in children. The results of the present
study revealed one embryonal RMS (11.1%; 1/9) with marked
hyperintensity and three embryonal RMSs (33.3%; 3/9) with
heterogeneously moderate hyperintensity on T2WI. These results
indicate that HNRMS exhibit different signaling features on T2WI.
Hagiwara et al (6) reported
that the ‘botryoid sign’ on CE-MRI correlates with RMS. In the
current study, nodule-shaped enhancement patterns were observed in
three HNRMSs with heterogeneous hyperintensity on T2WI. All three
RMSs with nodule-shaped enhancement patterns originated from the
ethmoid sinus and were of the embryonal subtype. However, the
remaining RMSs without nodule-shaped enhancement patterns, arising
in the ethmoid sinus, maxillary sinus, orbit, nasopharynx and
subcutaneous area, belonged to the embryonal (n=5) and alveolar
(n=2) subtypes.
The embryonal subtype predominantly occurs in the
head and neck in patients aged <10 years and accounts for 30–80%
of RMSs, which are commonly composed of spindle or botryoid cells
(4,7,16,17).
Botryoid RMS accounts for ~5% of cases and is identified
macroscopically by the presence of nodule-shaped polypoid masses,
which are found in the mucosa-lined organs of the nasopharynx,
paranasal sinus, genitourinary and gastrointestinal tracts
(18). In the present study,
embryonal RMSs with heterogeneous hyperintensity on T2WI and
nodule-shaped enhancement patterns on CE-T1WI were only located in
the ethmoid sinus. In addition, the signals of these three tumors
were homogeneously or heterogeneously isointense with isodensity on
CT, which could not be interpreted as hemorrhaging or necrosis.
This indicated that the tumor contained mucus and that the tumor
cells may have grown along the ethmoidal cells, which may have
resulted in the existence of this mucus in the RMS, particularly in
the botryoid RMS. We speculate that a mass in the ethmoid sinus,
that exhibits heterogeneous hyperintensity on T2WI and
nodule-shaped enhancement patterns on CE-T1WI, presents the
botryoid subtype of embryonal RMS. The three embryonal RMSs with
nodule-shaped enhancement patterns identified in the present study
may be mixed subtypes composed of botryoid and spindle cells.
However, it was not possible to identify the pathological features,
as all three patients were diagnosed by biopsy, which may not have
included the portion of nodule-shaped enhancement patterns.
Calcification and hemorrhaging are rare in HNRMS
(2,4–7,15,19)
and accordingly, these features were not present radiologically or
pathologically in the current study. RMS is an aggressive
malignancy, which spreads via three routes; direct extension,
lymphatic metastasis and hematogenous metastasis. In total, ≤14% of
patients with RMS exhibit metastatic disease at presentation
(20). In addition, bony
destruction has been a common imaging feature of RMS in previous
studies (4–7,19,21).
In the current study, bony erosion was frequently observed and
sclerosis was identified in two tumors, which is a rare sign in
HNRMS (5,7,19). On
CT and MRI, the present study clearly demonstrated multi-cavity
growth (70%) of HNRMS and three types of direct intracranial
extension, including nasocranial, ORB-cranial and
nasopharyngeal-cranial communication. The frequency of lymphatic
metastasis was 10–20% for HNRMS and is more common in other sites
(5,7,15,19,22).
In addition, cervical lymph node metastases were detected in two
patients (20%, 2/10) with the embryonal subtype, however, no
distant metastasis was observed.
With the exception of HNRMS, other rapidly growing
malignant tumors of the head and neck may be encountered in
children and adults. Lymphoma usually exhibit intermediate signal
intensity and homogeneous CE (11,23).
In addition, NPC appear as homogeneous masses with infiltration of
the adjacent soft tissue and erosion of the skull base (12). However, <20% of NPC cases occur
in children (8). Furthermore,
bilaterally enlarged neck nodes are common in lymphoma and NPC, and
rarely occur in HNRMS (1).
Osteosarcoma commonly exhibits areas of calcification, ossification
or sclerosis (24). ONB is an
aggressive type of neuroendocrine tumor located high in the nasal
cavity. Peripheral areas of cystic degeneration and calcific foci
are radiological features that are associated with ONB (13). Although these tumors exhibit such
features, the CT and MRI observations are similar to the imaging
manifestations of HNRMS. Thus, the differential diagnosis of
malignant tumors is difficult when based solely on CT and MRI
observations (25,26), therefore, the majority of masses
require a biopsy to confirm the diagnosis of HNRMS.
The present study had certain limitations. Firstly,
a small number of patients was included owing to the rarity of
HNRMS and secondly, 80% of patients were diagnosed by biopsy.
Therefore, further multicenter cooperation on the radiological
diagnosis of HNRMS is required.
In conclusion, HNRMS is a rare and aggressive
malignancy. MRI accurately demonstrates the location and extent of
HNRMS; however, HNRMSs may exhibit certain imaging features that
are similar to other tumors in the head and neck regions. The
present study indicated that a tumor with heterogeneous
hyperintensity on T2WI and nodule-shaped enhancement patterns in
the ethmoid sinus may be considered as specific MRI features, which
clearly indicate the botryoid subtype of embryonal RMS.
Acknowledgements
The current study was supported by the Foundation of
Shanghai Science and Technology Committee (grant no. 41902502) and
the Shanghai Health Bureau (grant no. 2012198).
References
|
1
|
Freling NJ, Merks JH, Saeed P, et al:
Imaging findings in craniofacial childhood rhabdomyosarcoma.
Pediatr Radiol. 40:1723–1738. 2010.
|
|
2
|
Karcioglu ZA, Hadjistilianou D, Rozans M
and DeFrancesco S: Orbital rhabdomyosarcoma. Cancer Control.
11:328–333. 2004.
|
|
3
|
McCarville MB, Spunt SL and Pappo AS:
Rhabdomyosarcoma in pediatric patients: the good, the bad, and the
unusual. AJR Am J Roentgenol. 176:1563–1569. 2001.
|
|
4
|
Allen SD, Moskovic EC, Fisher C and Thomas
JM: Adult rhabdomyosarcoma: cross-sectional imaging findings
including histopathologic correlation. AJR Am J Roentgenol.
189:371–377. 2007.
|
|
5
|
Franco A, Lewis KN and Lee JR: Pediatric
rhabdomyosarcoma at presentation: can cross-sectional imaging
findings predict pathologic tumor subtype? Eur J Radiol.
80:e446–e450. 2011.
|
|
6
|
Hagiwara A, Inoue Y, Nakayama T, et al:
The ‘botryoid sign’: a characteristic feature of rhabdomyosarcomas
in the head and neck. Neuroradiology. 43:331–335. 2001.
|
|
7
|
Lee JH, Lee MS, Lee BH, et al:
Rhabdomyosarcoma of the head and neck in adults: MR and CT
findings. AJNR Am J Neuroradiol. 17:1923–1928. 1996.
|
|
8
|
LIoyd C and McHugh K: The role of
radiology in head and neck tumours in children. Cancer Imaging.
10:49–61. 2010.
|
|
9
|
Stevens MC, Rey A, Bouvet N, et al:
Treatment of nonmetastatic rhabdomyosarcoma in childhood and
adolescence: third study of the International Society of Paediatric
Oncology - SIOP Malignant Mesenchymal Tumor 89. J Clin Oncol.
23:2618–2628. 2005.
|
|
10
|
Rodjan F, Graaf PD, Brisse HJ, et al:
Second cranio-facial malignancies in hereditary retinoblastoma
survivors previously treated with radiation therapy: Clinic and
radiologic characteristics and survival outcomes. Eur J Cancer.
49:1939–1947. 2013.
|
|
11
|
Gufler H, Laubenberger J, Gerling J,
Nesbitt E, Kommerell G and Langer M: MRI of lymphomas of the orbits
and the paranasal sinuses. J Comput Assist Tomogr. 21:887–891.
1997.
|
|
12
|
Yabuuchi H, Fukuya T, Murayama S, et al:
CT and MR features of nasopharyngeal carcinoma in children and
young adults. Clin Radiol. 57:205–210. 2002.
|
|
13
|
Yu T, Xu YK, Li L, et al:
Esthesioneuroblastoma methods of intracranial extension: CT and MR
imaging findings. Neuroradiology. 51:841–850. 2009.
|
|
14
|
Wurm J, Constantinidis J, Grabenbauer GG
and Iro H: Rhabdomyosarcomas of the nose and paranasal sinuses:
treatment results in 15 cases. Otolaryngology Head Neck Surg.
133:42–50. 2005.
|
|
15
|
Healy JN and Borg MF: Paediatric
nasopharyngeal rhabdomyosarcoma: A case series and literature
review. J Med Imaging Radiat Oncol. 54:388–394. 2010.
|
|
16
|
Moon HS, Kwon SW and Lee JH: A case of
alveolar rhabdomyosarcoma of the ethmoid sinus invading the orbit
in an adult. Korean J Ophthalmol. 20:70–75. 2006.
|
|
17
|
Little DJ, Ballo MT, Zagars GK, et al:
Adult rhabdomyosarcoma: outcome following multimodality treatment.
Cancer. 95:377–388. 2002.
|
|
18
|
McHugh K and Boothroyd AE: The role of
radiology in childhood rhabdomyosarcoma. Clin Radiol. 54:2–10.
1999.
|
|
19
|
Kim EE, Valenzuela RF, Kumar AJ, Raney RB
and Eftekari F: Imaging and clinical spectrum of rhabdomyosarcoma
in children. Clin Imaging. 24:257–262. 2000.
|
|
20
|
Crist W, Gehan EA, Ragab AH, et al: The
third intergroup rhabdomyosarcoma study. J Clin Oncol. 13:610–630.
1995.
|
|
21
|
Chu Y, Liu HG and Yu ZK: Patterns and
incidence of sinonasal malignancy with orbital invasion. Chin Med J
(Engl). 125:1638–1642. 2012.
|
|
22
|
Tateishi U, Hosono A, Makimoto A, et al:
Comparative study of FDG PET/CT and conventional imaging in the
staging of rhabdomyosarcoma. Ann Nucl Med. 23:155–161. 2009.
|
|
23
|
Nakamura K, Uehara S, Omagari J, et al:
Primary non-Hodgkin lymphoma of the sinonasal cavities: correlation
of CT evalution with clinical outcome. Radiology. 204:431–435.
1997.
|
|
24
|
Madani G, Beale TJ and Lund VJ: Imaging of
sinonasal tumors. Semin Ultrasound CT MR. 30:25–38. 2009.
|
|
25
|
Holsinger FC, Hafemeister AC, Hicks MJ,
Sulek M, Huh WW and Friedman EM: Differential diagnosis of
pediatric tumors of the nasal cavity and paranasal sinuses: a
45-year multi-institutional review. Ear Nose Throat J. 89:534–540.
2010.
|
|
26
|
Saboo SS, Krajewski KM, Zukotynski K,
Howard S, Jagannathan JP, Hornick JL and Ramaiya N: Imaging
features of primary and secondary adult rhabdomyosarcoma. AJR Am J
Roentgenol. 199:W694–W703. 2012.
|