Introduction
Ovarian cancer is highly malignant among
gynecological tumors and is associated with an insidious onset,
rapid progression and a complex early diagnosis, which leads to a
poor prognosis and high mortality rates. Chemotherapeutic agents
are an important means of adjuvant therapy for the treatment of
patients with ovarian cancer. For example, combination therapy
using cisplatin (DDP) and paclitaxel is considered to be the gold
standard of chemotherapy (1).
However, during clinical treatment, the majority of patients
develop a drug resistance (2),
which manifests as a tolerance to the chemotherapeutic agents or
recurrence following treatment, leading to chemotherapy failure and
a marked limitation of this treatment modality (3).
Previous clinical studies have shown that malignant
ovarian cancer cells express the proto-oncogene, c-Kit and that the
prognosis of patients exhibiting positive c-Kit gene expression is
usually poorer. Schmandt et al (4) demonstrated that c-Kit was highly
expressed in malignant ovarian tumors via immunohistochemistry, and
indicated that c-Kit expression was associated with the
histological tumor grade. The present study used a DDP-resistant
cell line with a high expression of c-Kit to establish an
orthotopic transplantation animal model, stimulating human ovarian
cancer with regard to onset, location, mechanism and histological
and biological characteristics, was used to investigate association
between c-Kit gene expression and drug resistance and degree of
malignancy in ovarian cancer.
Materials and methods
Experimental cells
The SKOV3 human ovarian cancer cell line and its
DDP-resistant variant, SKOV3/DDP were purchased from the Tumor Cell
Bank of the Chinese Academy of Medical Sciences (Beijing,
China).
Animals
BALB/c nude mice (weight, 18–20 g) were purchased
from the Model Animal Research Center of Nanjing University
(Nanjing, China). The mice were maintained in the animal facility
at the Institute of Biophysics, Chinese Academy of Sciences
(Beijing, China) under specific pathogen-free (SPF) conditions and
housed in plastic cages in groups of six in Animal Resource Service
facilities. The study was approved by the Institution of Animal
Care and Use Committee at the Institute of Biophysics, Chinese
Academy of Sciences (SYXK2013-02).
Experimental therapeutic agents and
reagents
Broad-spectrum antibiotic ampicillin sodium powder
(5 mg/bottle; North China Pharmaceutical Co., Ltd., Shijiazhuang,
China) and surgical anesthetic sodium pentobarbital solution (10
mg/ml) were administered to the nude mice following sterilization
via filtering through a 0.22-μm filter membrane.
Experimental instruments
A surgical dissection microscope (Nikon SMA800;
Nikon Corporation, Tokyo, Japan) was used to analyze the
microscopic morphology.
Cell culture and validation
SKOV3 and SKOV3/DDP cells were cultured in RPMI-1640
medium containing 10% fetal bovine serum. The cells were collected
in the logarithmic growth phase using centrifugation at 86 × g
(TDZ5-WS multicarrier auto-balancing centrifuge; Hunan XiangYi
Centrifuge Instrument Co., Ltd., Changsha, China), counted and
resuspended in fresh medium. MTT assay (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) and quantitative
polymerase chain reaction (qPCR) methods were used to validate the
DDP resistance and c-Kit gene expression in the SKOV3/DDP
cells.
Establishment of the animal model
Four nude mice were used for the subcutaneous tumor
model. Cultured SKOV3/DDP cells in the logarithmic growth phase
were harvested, prepared into a cell suspension at a cell density
of 2×107 cells/ml and injected subcutaneously into the
neck of the nude mice (0.2 ml/mouse). The mice were returned to
their cages for continuous feeding following confirmation that
there was no leakage at the injection site. The size of the
subcutaneous tumor was regularly measured using a vernier caliper
to monitor its growth. The tumors were excised and used for the
orthotopic implantation experiment when the diameter was >1
cm.
Six-week-old SPF-level nude mice (weight, 17–18 g)
were used for the orthotopic transplantation model. The animals
were allowed to adapt to the experimental environment for one week.
The mice with subcutaneous tumors were sacrificed via cervical
dislocation and the tumors in the neck were collected. Following
the removal of the capsule and connective tissues, the tumor was
cut into small sections (~1 mm3), which were placed in
ice-cold phosphate-buffered saline for further investigation. The
recipient nude mouse was anesthetized with sodium pentobarbital and
fixed on the operating table. The abdominal cavity of the mouse was
opened to expose the ovaries, which were cut under the Nikon SMA800
dissecting microscope (Nikon Corporation). The prepared tumor
sections were directly implanted in the right ovary and sutured
using an 8–0 absorbable suture. The abdomen was closed
layer-by-layer using sterile silk. Post surgery, the mouse was
placed on a warm pad, administered with anti-infection treatment
and maintained under SPF conditions.
Following orthotopic transplantation, the food
uptake, physical activities and mental conditions of the model nude
mice were monitored daily. Weight measurement and abdominal
palpation were performed every third day. When the abdominal mass
was obviously palpable, the tumor-bearing mice were sacrificed and
dissected to observe the in situ tumor growth, as well as
tumor infiltration and metastasis to other tissues and organs. The
tumor mass at the orthotopic transplantation site, as well as other
associated tissues, were dissected, sectioned, stained with
hematoxylin and eosin (H&E) and analyzed under a microscope
(SCN400; Leica, Mannheim, Germany).
Statistical Analysis
Data are presented as the mean ± standard deviation
(n=5) for individual experiments, unless noted otherwise.
Differences between the variables of groups were compared using
Student’s t-test. Statistical analysis was performed using SPSS
11.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference for MTT. However,
for qPCR, P<0.01 was considered to idicate a statistically
significant difference.
Results
Cell culture and verification
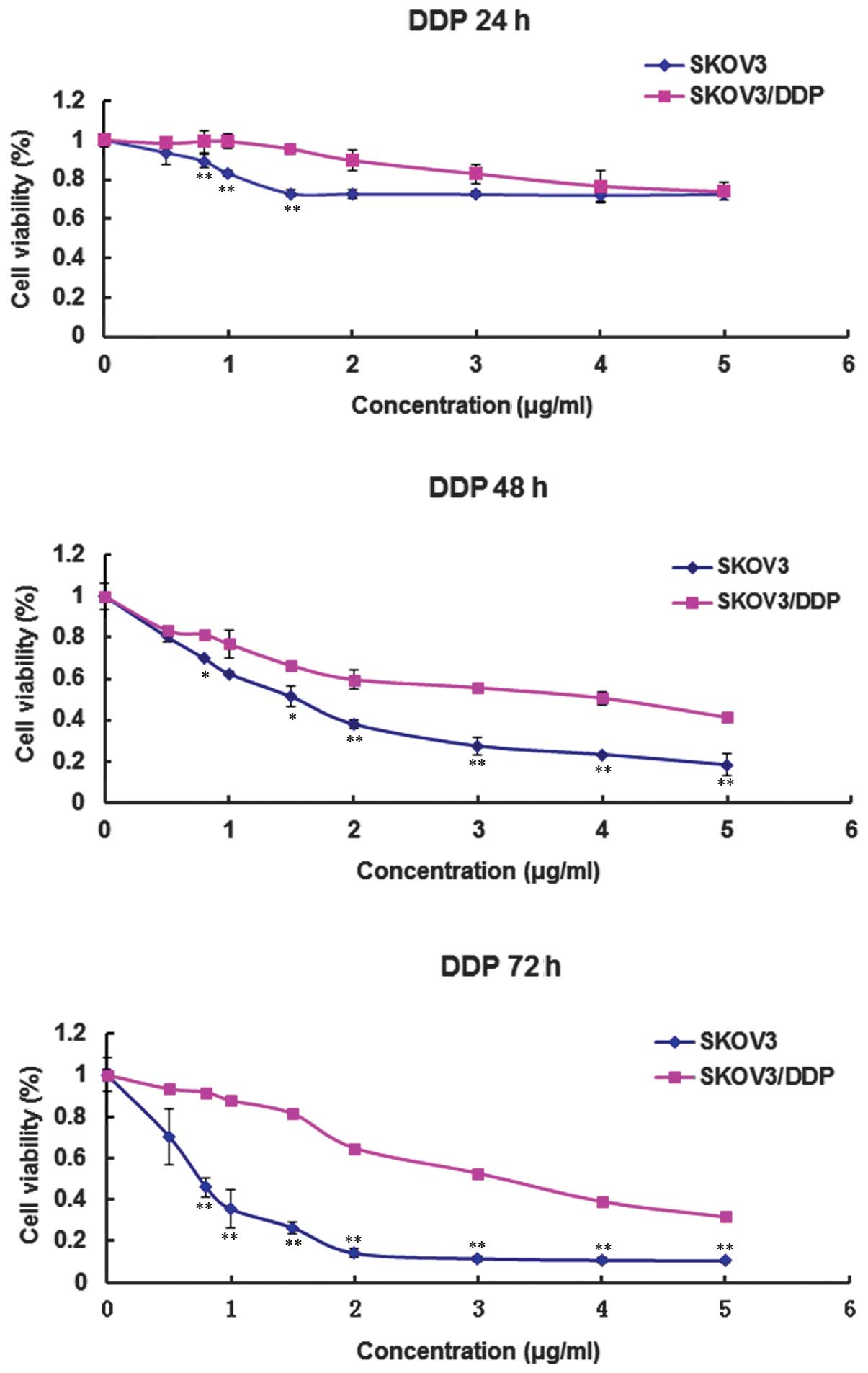
The dose- and time-dependent cytotoxic effects of
DDP on the cultured SKOV3 and SKOV3/DDP cells were determined using
an MTT colorimetric method. Cell growth and survival curves were
then plotted (Fig. 1). As shown in
Fig. 1, DDP resistance was
significantly higher in the SKOV3/DDP cells compared with the SKOV3
cells following treatment with DDP for 24, 48 and 72 h.
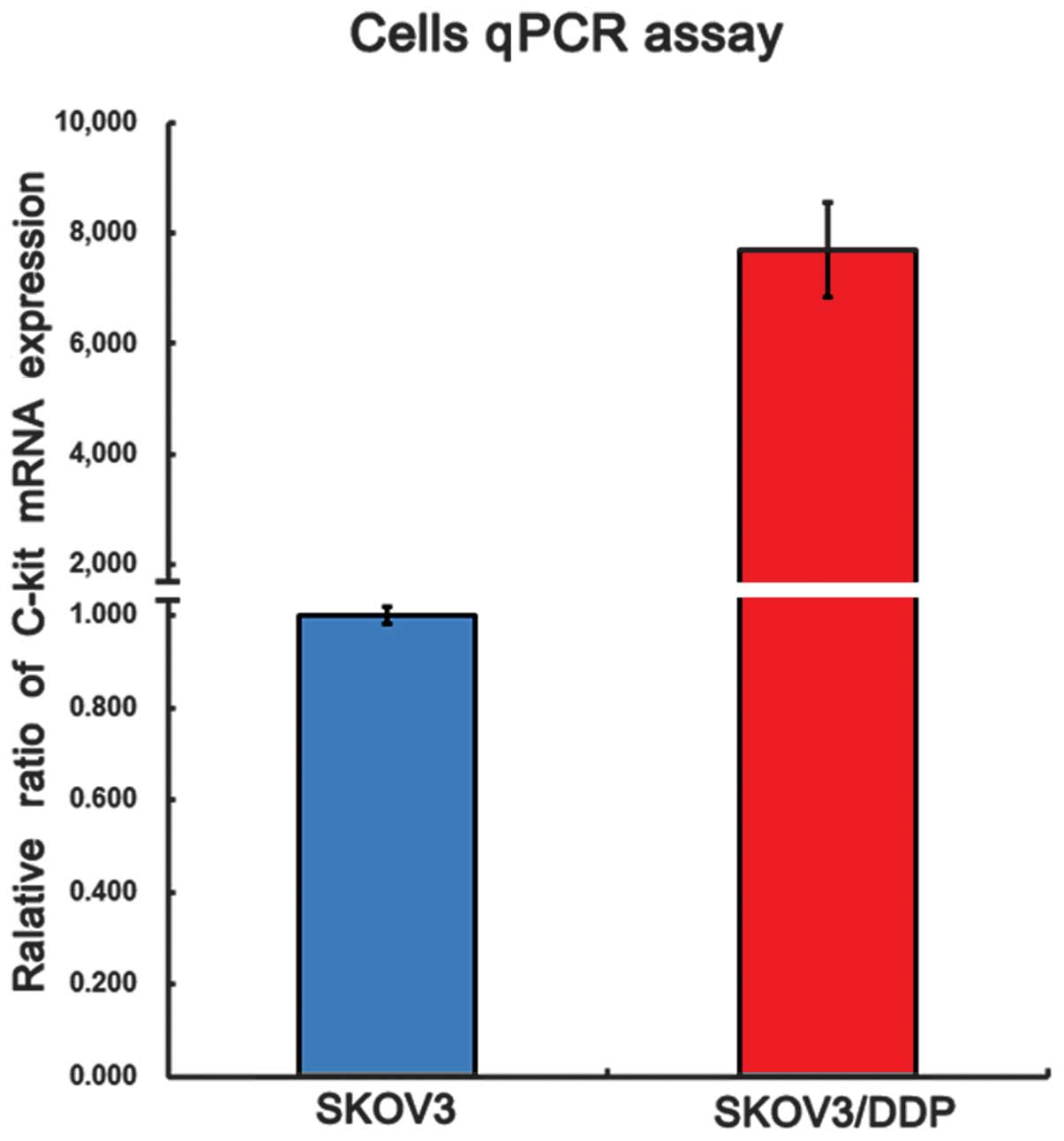
Furthermore, qPCR analysis (Fig. 2)
revealed that c-Kit mRNA was expressed in the SKOV3 and SKOV3/DDP
cells, and that the expression of c-Kit in the DDP-resistant
SKOV3/DDP cells was significantly higher compared with that in the
non-resistant SKOV3 cells.
Growth and metastasis of tumors
transplanted orthotopically
A total of 16 nude mice were used to establish an
orthotopic tumor transplantation model with SKOV3/DDP ovarian
cancer cells. Two mice succumbed within one week of surgery;
however, the orthotopic tumor transplantation model was
successfully established in the remaining 14 mice, with a success
rate of 87.5%. Table I shows the
growth and metastasis findings of the tumor that was transplanted
orthotopically in vivo.
 | Table IOrgans with metastatic tumors in the
mouse model. |
Table I
Organs with metastatic tumors in the
mouse model.
| Mouse no. | Organs with
metastatic tumors |
|---|
| 1 | Bilateral ovaries,
fallopian tubes and mesentery |
| 2 | Bilateral ovaries and
fallopian tubes |
| 3 | Bilateral ovaries,
fallopian tubes and omentum |
| 4 | Bilateral ovaries,
fallopian tubes and mesentery |
| 5 | Bilateral ovaries,
fallopian tubes, mesentery and hepatic surface, with small number
of bloody ascites |
| 6 | Bilateral ovaries,
fallopian tubes and liver |
| 7 | Bilateral ovaries,
fallopian tubes, mesentery and intestinal surface |
| 8 | Bilateral ovaries,
fallopian tubes, mesentery and omentum |
| 9 | Bilateral ovaries,
fallopian tubes, mesentery and hepatic surface, with small number
of bloody ascites |
| 10 | Bilateral ovaries,
fallopian tubes and hepatic surface |
| 11 | Bilateral ovaries and
fallopian tubes |
| 12 | Bilateral
ovaries |
| 13 | Bilateral
ovaries |
| 14 | Ipsilateral
ovary |
Abdominal swellings gradually appeared in the nude
mice ~8–10 weeks after the successful orthotopic tumor
transplantation. In addition, the mice exhibited loss of body
weight and appetite, accompanied by decreased activity levels.
Abdominal palpation revealed a palpable mass with poor mobility.
The tumor-bearing mice were sacrificed via cervical dislocation
when the abdominal mass was >2 cm in diameter. A laparotomy
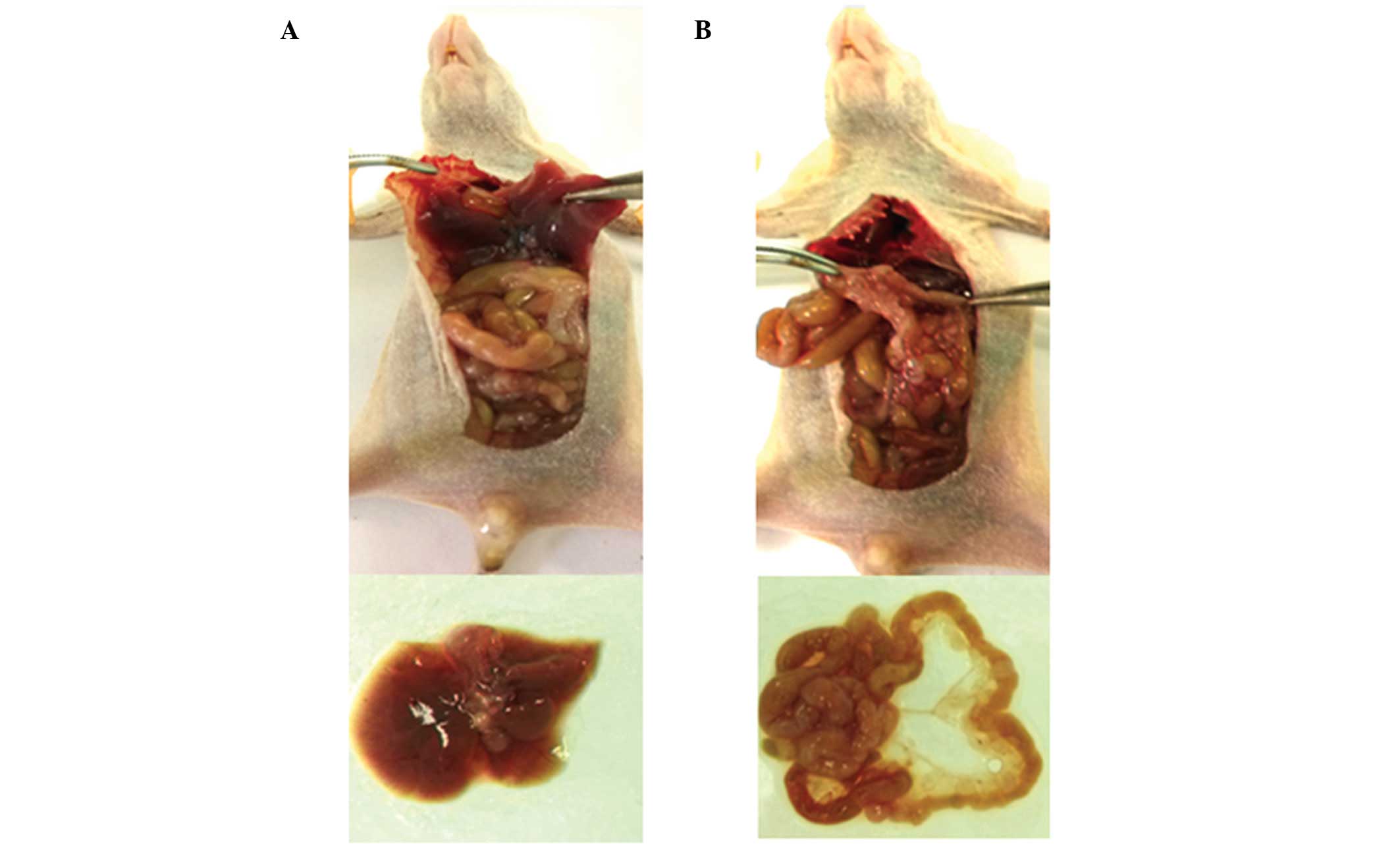
identified the formation of a relatively large mass in the
transplantation side (Fig. 3), with
a hard texture and varying degrees of adhesion to the surrounding
tissues. A small quantity of dark-red intraperitoneal fluid was
also observed. In certain mice, metastatic lesions were found in
the abdominal organs, including the liver and mesentery (Fig. 4).
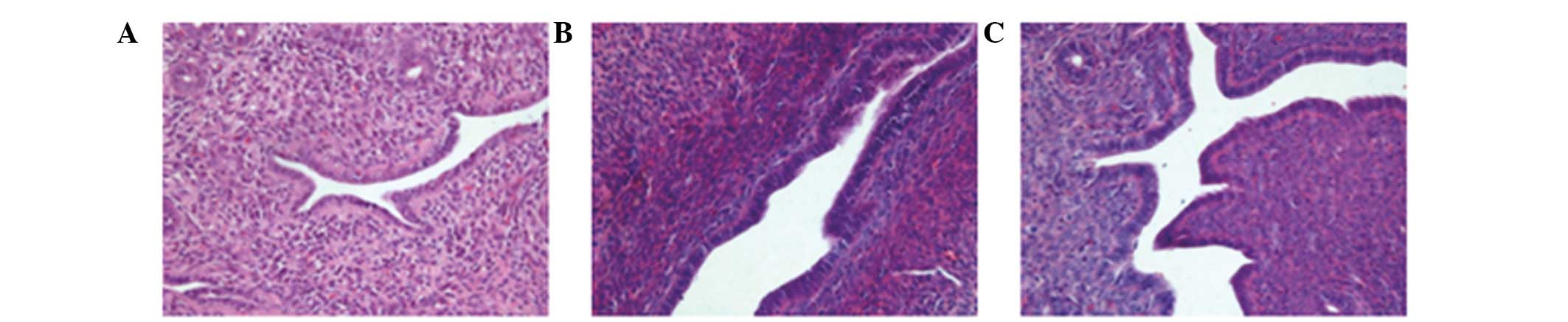
Pathology
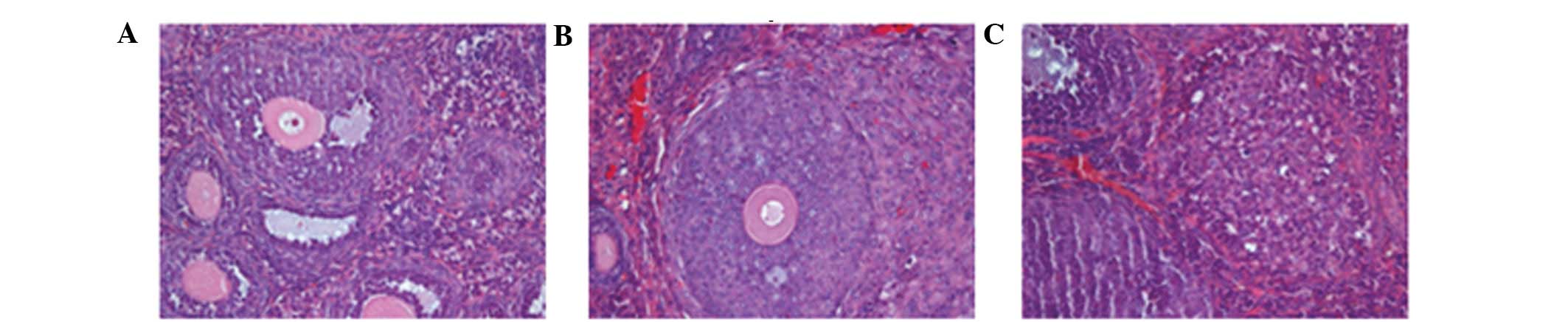
The orthotopic tumors and organs with suspected
metastatic tumors were resectioned, stained with H&E and
analyzed under a microscope. A large number of ovarian cancer cells
were observed in the bilateral ovaries and demonstrated
infiltrative growth. The cell morphology was observed to be the
same as that of the parental subcutaneous tumor, with large
dark-stained nuclei and a high karyoplasmic ratio. The cells
exhibited evident atypia, and a dense and disordered arrangement
with visible interstitial cells (Fig.
5). Certain mice exhibited visible bilateral tubal metastasis
of the tumor cells, with a morphology the same as that of the tumor
cells that were transplanted orthotopically (Fig. 6). In the liver, the hepatic cord
cells showed disarrangement, with evident inflammatory cell
infiltration and small nodule formation. There were no metastatic
tumor cells visible in the uterine tissue.
Discussion
Resistance to platinum-based chemotherapy in ovarian
cancer is a critical issue in the clinical setting. In a previous
clinical study, c-Kit gene expression was identified to be closely
associated with drug resistance and malignancy in ovarian cancer
(5), which was consistent with
previous findings (4). In the
preliminary experiments, using 4T1-LUC cells, an orthotopic
transplantation model of ovarian cancer was established. The
implanted ovarian tumor model exhibited biological characteristics,
and a metastatic rate and pattern that were closest to the clinical
state. To understand the association between c-Kit gene expression,
and drug resistance and malignancy in ovarian cancer, an animal
model that simulates human ovarian developmen cancer in terms of
onset, location, mechanism and histological and biological
characteristics was required.
In the present study, SKOV3 and SKOV3/DDP human
ovarian cancer cell lines were used and their sensitivity to DDP
and c-Kit expression was assessed via MTT assay and qPCR. The c-Kit
gene was identified to be expressed in the two cell lines and was
significantly highly expressed in the DDP-resistant SKOV3/DDP
cells, which correlated with its drug resistance. Using the
SKOV3/DDP cells, an orthotopic tumor transplantation model was
successfully established in the nude mice that closely simulated
the occurrence, development and metastasis of human ovarian cancer.
This model may facilitate further investigations into the
association between c-Kit gene expression and malignancy in ovarian
tumors. Furthermore, the model may provide a valuable tool for
investigating the underyling mechanism of drug resistance in
ovarian cancer, as well as the late-phase evaluation of the in
vivo efficacy of novel treatment plans.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
8117245/H1621).
References
|
1
|
Mundhenke C, Weigel MT, Sturner KH, et al:
Novel treatment of ovarian cancer cell lines with Imatinib mesylate
combined with Paclitaxel and Carboplatin leads to receptor-mediated
antiproliferative effects. J Cancer Res Clin Oncol. 134:1397–1405.
2008.
|
|
2
|
Seeber LMS and van Diest PJ: Epigenetics
in ovarian cancer. Methods Mol Biol. 863:253–269. 2012.
|
|
3
|
Li X, Ling V and Li PC: Same single cell
analysis for the study of drug efflux modulation of multidrug
resistant cells using a microfluidic chip. Anal Chem. 80:4095–4102.
2008.
|
|
4
|
Schmandt RE, Broaddus R, Lu KH, et al:
Expression of C-ABL, c-KIT, and platelet-derived growth factor
receptor-beta in ovarian serous carcinoma and normal ovarian
surface epithelium. Cancer. 98:758–764. 2003.
|
|
5
|
Yi C, Li L, Chen K, et al: Expression of
c-Kit and PDGFRα in epithelial ovarian tumors and tumor stroma.
Oncol Lett. 3:369–372. 2012.
|