Introduction
Pancreatic carcinoma is one of the most frequently
occurring gastrointestinal malignancies and the incidence rate is
showing an upward trend worldwide (1). The prognosis for patients with
advanced pancreatic carcinoma remains poor with a 5-year survival
rate of <5% (2). Among the most
significant determinants of the poor prognosis associated with this
malignancy are the highly aggressive loco-regional invasion and
early metastasis that characterize this malignancy, such that the
majority of patients present with advanced, surgically unresectable
disease (3). Gemcitabine and
erlotinib are the only agents that are approved for the treatment
of pancreatic carcinoma. However, both drugs induce a poor response
in patients and their use can result in patients developing
multiple drug resistance (4,5).
Although in recent years, great progress has been observed with
regard to investigations on the molecular pathogenesis of
pancreatic carcinoma, the clinical treatment of pancreatic
carcinoma remains a challenge. Therefore, novel therapeutic
approaches to this malignancy are needed.
Carcinoembryonic antigen-related cell adhesion
molecule 6 (CEACAM6), also known as CD66c or NCA-90, as well as
another 6 members of the CEACAM subgroup, belong to the human
carcinoembryonic antigen (CEA) family (6). Numerous lines of studies have
indicated that altered expression of CEACAM6 may have a role in
carcinogenesis and development. Increased CEACAM6 gene expression
has been found in lung, breast, colorectal, hepatocellular and
pancreatic carcinomas (7–12). Yet, the relationship between the
differentially expression of CEACAM6 in tumor compared to normal
tissue and its biological function needs further investigation. In
the present study, we used immunohistochemistry to explore CEACAM6
protein in pancreatic carcinomas. The relationship between clinical
and pathological parameters was also analyzed. Furthermore, we
examined the role of CEACAM6 gene expression in human pancreatic
carcinoma. The aim of the present study was to increase the
knowledge on the treatment of pancreatic carcinoma.
Materials and methods
Samples
A total of 42 cases of pancreatic carcinoma and 12
cases of non-cancerous specimens were selected between February
2005 and January 2010 from the Xiangya Hospital of Central South
University. The resected tissue samples were immediately cut into
small pieces and snap-frozen in liquid nitrogen until use. All
procedures were conducted in accordance with the Helsinki
declaration, and with approval from the Ethics Committee of Xiangya
hospital. Written informed consent was obtained from all the
participants.
Immunohistochemistry
Routine paraffin sectioning, dewaxing and hydration
using 3% hydrogen peroxide were performed to remove endogenous
peroxidase. Microwave antigen was retrieved and blocked with fetal
calf serum (FCS) for 2 h. Approximately 50 µl (1:25) of goat
polyclonal anti-human CEACAM6 was added, and the mixture was
incubated at 4°C overnight. Approximately 50 µl of
biotinylated goat anti-rabbit IgG secondary antibody working
solution was added, and the mixture was incubated 37°C for 30 min,
followed by diaminobenzidine coloration. The sample was dyed with
hematoxylin, separated using ethanol and hydrochloric acid,
saturated with lithium carbonate until the color returned to blue,
and then dehydrated with gradient alcohol as well as xylene.
Mounting with neutral resin followed. The negative control used was
phosphate-buffered saline (PBS) in place of the primary antibody.
The stained cells were divided into five grades based on cell
counting, namely, 0 (no staining), 1 (<25% stained cells), 2
(25–50% stained cells), 3 (50–75% stained cells), and 4 (>75%
stained cells). Based on both of the above scoring methods, the
immunohistochemistry results were classified into four, namely,
negative (0), weakly positive (1–2 score), positive (3–4 score),
and strongly positive (5–7 score) (13).
Cell lines
Pancreatic carcinoma cell lines, BxPC-3, SW1990 and
MIA PaCa-2, were maintained by our laboratory. They were cultured
in Dulbecco's modified Eagle's medium (DMEM; Gibco, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS; Gibco). Cells
were maintained at 37°C atmosphere of humidified air with 5%
CO2.
Semi-quantitative polymerase chain
reaction
RNA isolated from cells was reverse-transcribed and
amplified using the One-Step reverse transcription polymerase chain
reaction (RT-PCR) system (Fermentas). The sets of primers for
CEACAM6 receptor subunit are: sense, 5′-TACAAAGGCGAAAGAGTGGATGG-3′
and antisense, 5′-GTTAGAGGCTGCGTGGCAGGAGA-3′ (590 bp); sense,
5′-AATCCCATCACCATCTTCCA-3′ and antisense,
5′-CCTGCTTCACCACCTTCTTG-3′ for glyceraldehyde-3-phosphate
dehydrogenase (GAPDH, 580 bp). After heating at 95°C for 1 min,
samples were exposed to 28 cycles (GAPDH, 25 cycles) of 95°C for 30
sec, 58°C for 30 sec and 72°C for 1 min 30 sec with a final
extension at 72°C for 10 min. Reaction products were separated on
1% agarose gels containing ethidium bromide and the level of
amplification was analyzed using a Phosphor imager.
Western blot analysis
The cells were washed with cold PBS and lysed in
Laemmli buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 50
mM dithiothreitol and 0.01% bromophenol blue) for 5 min at 95°C.
Cell lysates were analyzed by SDS-PAGE and transferred
electrophoretically to polyvinylidene difluoride membrane. The
blots were probed with specific antibodies by a secondary detection
step. The immunoreactive proteins were revealed by an ECL kit.
Western blot analysis was carried out using the following
antibodies: rabbit anti-CEACAM6 antibody (Santa Cruz Biotechnology,
Santa Cruz, CA, USA), rabbit anti-cyclin D1 antibody (Cell
Signaling Biotechnology, Danvers, CA, USA), rabbit anti-cyclin E1
antibody (Abcam Biotechnology), rabbit anti-CDK4 antibody and
rabbit anti-CDK2 antibody (both from Santa Cruz Biotechnology).
Knockdown and overexpression of CEACAM6
vector construction
To knock down CEACAM6 expression, we used GV102
vector encoding a small hairpin RNA directed against the target
gene in BxPC-3 and SW1990. The target sequences for CEACAM6 were:
5′-GTATTGGTTACAGCTGGTA-3′. As a negative control, we used shRNA
vector without hairpin oligonucleotides (NC).
To transfection of the plasmid expression vector
encoding human CEACAM6, the DNA sequencing containing the
full-length CEACAM6 (1034 bp) open reading frame flanked by
XHoI (sense) and BamHI (antisense) restriction sites
was PCR amplified from BxPC-3 cells. Primer sequences used were
sense 5′-CCGCTCGAGACCCTGGGGGGAGTCGGGGAGGGAC-3′ and antisense
5′-CGCGGATCCCTATATCAGAGCCACCCTGGCCAGC-3′. The resulting fragment
was inserted into pcDNA3.1(+) to generate pcDNA3.1-CEACAM6. The
desired sequence was confirmed by direct DNA sequencing.
Cell transfection
For transfection, the human pancreatic carcinoma
cell lines were plated onto 24-well plates, and transfected with
these vectors using FuGENE6 (Roche, Indianapolis, IN, USA)
according to the instructions of the manufacturer, followed by 200
µg/ml of neomycin selection. The cells were harvested 15
days later to analyze the knockdown effect on CEACAM6 by RT-PCR
using the primers shown above and by western blot analysis using
rabbit anti-human polyclonal antibody against CEACAM6 (Abcam).
Impact of CEACAM6-siRNA on the growth of
pancreatic carcinoma cells
CCK-8 analysis
For the CCK-8 analysis, cells were seeded with
serum-free medium at a density of 103 cells/well in
96-well plates (n=6), grown overnight, washed in PBS, and incubated
with 10% FBS DMEM at 37°C, 5% CO2, for varying periods
and exposed to fresh media every other day. During the last 4 h of
each day's culture, the cells were treated with the Cell Counting
kit-8 [CCK-8, (4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
(Vazyme Biotech Co., Ltd., Lakewood, NJ, USA]. The OD at 450 nm
were measured for detecting the cell viability.
Colony formation analysis
For the colony formation analysis, cells at 1,000
cells/well in 6-cm plates were incubated with serum-fee medium for
24 h, and then cultured in DMEM with 10% FBS at 37°C, 5%
CO2 for 2 weeks. The cell colonies were washed twice
with PBS, fixed by 4% paraformaldehyde for 15 min and stained with
Giemsa for 25 min. Individual clones with >50 cells were
counted. Clone forming efficiency for individual type of cells was
calculated, according to the number of colonies/number of
inoculated cells × 100%.
Fow cytometric analysis
For the flow cytometric analysis, cells were
incubated with serum-fee medium for 24 h, and then cultured in DMEM
with 10% FBS at 37°C 5% CO2, then harvested at 70–80%
confluence and resuspended in fixation fluid at a density of
106/ml. A total of 1,500 µl propidium iodide (PI)
solution was added, and the cell cycle was detected by FACSCalibur
(Becton-Dickinson).
Tumor formation in nude mice
The influence of CEACAM6 silencing on the tumor
development of pancreatic carcinoma in vivo was examined.
Briefly, BxPC-3/si-CEACAM6, BxPC-3/NC, SW1990/si-CEACAM6 and
SW1990/NC cells (1×107) were suspended in 0.2 ml of
extracellular matrix gel and injected subcutaneously in the left
back flank of the animals. The 6-week-old BALB/c nude (nu/nu) mice
(Slac Laboratory Animal Center, Changsha, China) were divided into
four groups: i) the mice were injected with BxPC-3/NC cells (n=3);
ii) the mice were injected with BxPC-3/si-CEACAM6 cells (n=3); iii)
the mice were injected with SW1990/NC cells (n=3); and iv) the mice
were injected with SW1990/si-CEACAM6 cells (n=3). Tumor variables
were measured every 5 days by an electronic caliper, and tumor
volume was calculated using a standard formula (14): Tumor volume = width2 × length × 0.5.
At the end of the experiment, all the mice were sacrificed and
individual tumor weights were measured.
Statistical analysis
The values are presented as the means with the SPSS
software (version 13.0; SPSS, Inc., Chicago, IL, USA) for
significance. The analysis of variance and t-test were applied in
comparing the intergroup difference of measurement data. P<0.05
was considered to indicate a statistically significant difference.
The diagrams were drawn by GraphPad Prism 5.
Results
The expression of CEACAM6 and
clinicopathological parameter
To explore the role of CEACAM6 in pancreatic
carcinoma, we first examined the CEACAM6 protein expression level
in 42 cases of pancreatic carcinoma and 12 cases of non-cancerous
specimens by immunohistochemistry. The expression level of CEACAM6
protein in pancreatic carcinoma tissues was increased compared with
the non-cancerous ones. Under high magnification, CEACAM6 was
expressed as light yellow or brown in the cytoplasm and cell
membrane (Fig. 1).
The relative CEACAM6 protein expression of
pancreatic carcinoma was significantly higher than that in
non-cancerous tissue. Different clinical stages and lymph node
metastasis between groups were significantly different (P<0.05).
However, no statistically significant difference was found between
pancreatic carcinoma and non-cancerous specimens, which were from
different gender, as well as from different age. There was also no
statistically significant difference among the different tumor size
of pancreatic carcinoma (P>0.05). All of the analysed parameters
indicated its potential role in the progression of pancreatic
carcinoma (Table I).
 | Table IComparison of CEACAM6 protein
expression in different clinical and pathological parameters of
pancreatic carcinoma. |
Table I
Comparison of CEACAM6 protein
expression in different clinical and pathological parameters of
pancreatic carcinoma.
| Parameters | CEACM6 protein
expression (case)
|
|---|
| Case | − | + | ++ | +++ | P-value |
|---|
| Gender |
| Male | 22 | 2 | 3 | 10 | 7 | |
| Female | 20 | 2 | 2 | 12 | 4 | >0.05 |
| Age (years) |
| <61 | 22 | 2 | 3 | 12 | 5 | |
| ≥61 | 20 | 2 | 2 | 10 | 6 | >0.05 |
| Clinical stage |
| I+II | 10 | 3 | 3 | 2 | 2 | |
| III+IV | 32 | 1 | 2 | 20 | 9 | <0.05 |
| Tumor size
(cm) |
| <2.5 | 19 | 3 | 0 | 10 | 6 | |
| ≥2.5 | 19 | 3 | 0 | 10 | 6 | <0.05 |
| Cervical lymph node
metastasis |
| No | 9 | 3 | 4 | 2 | 0 | |
| Yes | 33 | 1 | 1 | 20 | 11 | <0.05 |
We documented CEACAM6 expression in BxPC-3, SW1990
and MIA PaCa-2 cell lines. The results of semi-quantitative RT-PCR
and western blot analysis showed that CEACAM6 was detected in
BxPC-3 and SW1990 cells, but not in MIA PaCa-2 cells (Fig. 2).
The effect of RNAi
To investigate the biological significance of
CEACAM6 overexpression in pancreatic carcinoma cell lines BxPC-3
and SW1990, we constructed siRNA expression vectors (si-CEACAM6)
specific to CEACAM6 transcripts and transfected them into BxPC-3,
and SW1990 cells that endogenously expressed high levels of
CEACAM6, as shown in Fig. 3A and B.
A knockdown effect was observed by RT-PCR and western blot analysis
when we transfected si-CEACAM6, but not in negative control NC
(Fig. 3A and B).
Impact of CEACAM6-siRNA on the growth of
pancreatic carcinoma cells
Subsequently, we examined the effect of decreased
CEACAM6 on BxPC-3/si-CEACAM6, BxPC-3/NC, SW1990/si-CEACAM6 and
SW1990/NC cell growth by CCK-8 assays. Following a 6-day period,
the growth of BxPC-3/NC and SW1990/NC cells was much more rapid
than BxPC-3/si-CEACAM6 and SW1990/si-CEACAM6 cells, and
significantly high number of BxPC-3/NC and SW1990/NC cells was
observed from day 3 (Fig. 3C and
D).
To investigate the possible function of CEACAM6 on
the growth of BxPC-3 and SW1990 cells, the dynamics of
BxPC-3/si-CEACAM6, BxPC-3/NC, SW1990/si-CEACAM6 and SW1990/NC cell
growth were determined by colony formation assay. As a result, the
average colony number of BxPC-3/si-CEACAM6 and SW1990/si-CEACAM6
cells decreased compared with BxPC-3/NC and SW1990/NC cells.
Therefore, the low number of cell colonies from BxPC-3/si-CEACAM6
and SW1990/si-CEACAM6 cells demonstrated that the down-regulation
of CEACAM6 expression inhibits the growth of BxPC-3 and SW1990
cells (Fig. 3E–H).
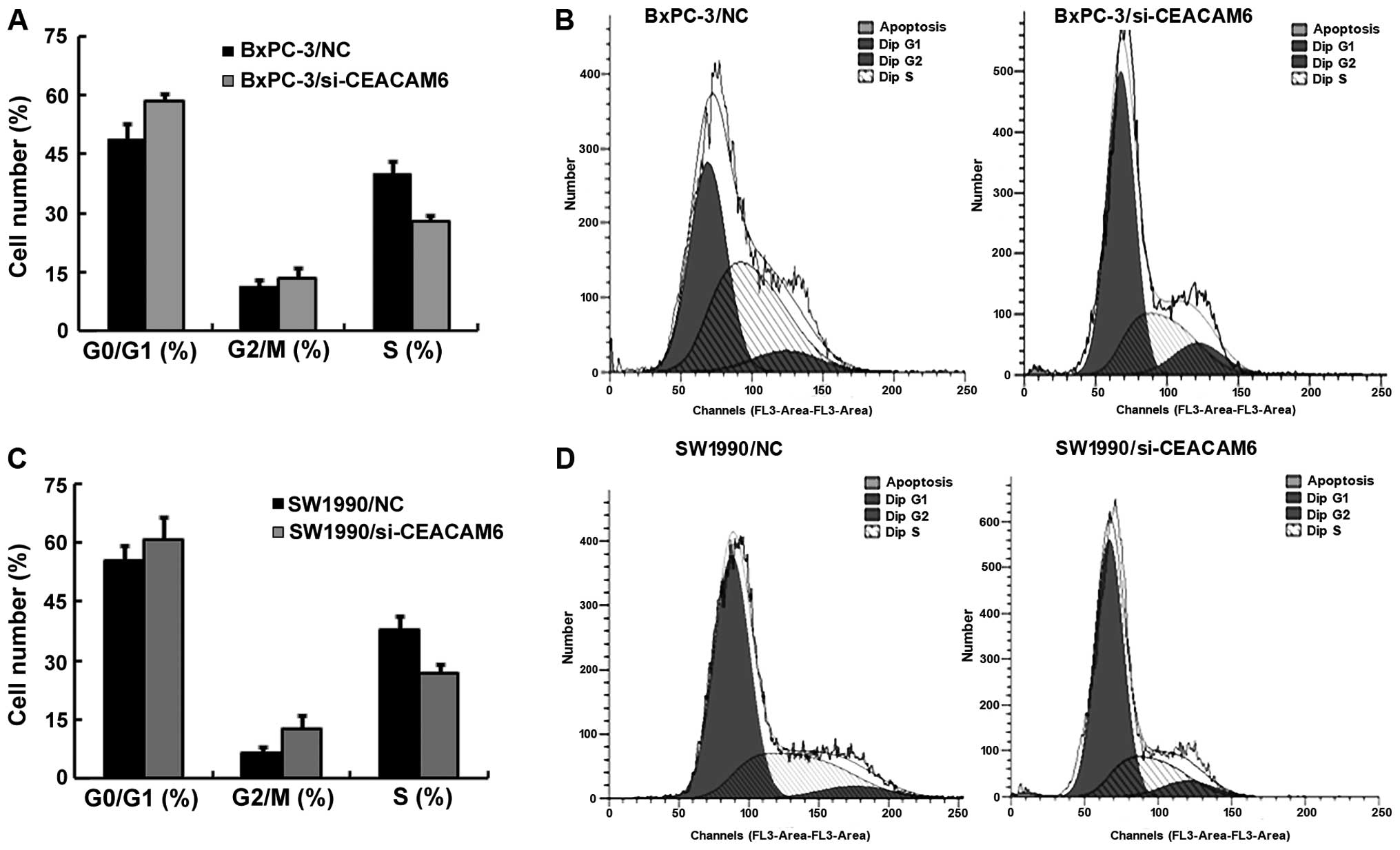
To further explore the cause for the decrease in
cell viability, we examined the effects of CEACAM6 RNAi on the cell
cycle. As shown in Fig. 4, BxPC-3
and SW1990 cells with CEACAM6 siRNA showed blocking of the cell
cycle in G1 phase. These data indicated that the downregulation of
CEACAM6 expression arrests BxPC-3/si-CEACAM6, SW1990/si-CEACAM6
cell cycling at G0/G1 phase, which may inhibit the growth of BxPC-3
and SW1990 cells.
Overexpression of CEACAM6 promotes cells
of MIA PaCa-2 proliferation
Given that downregulation of CEACAM6 inhibited
BxPC-3/NC and SW1990 cells proliferation in vitro, we
considered that CEACAM6 could promote MIA PaCa-2 cell development.
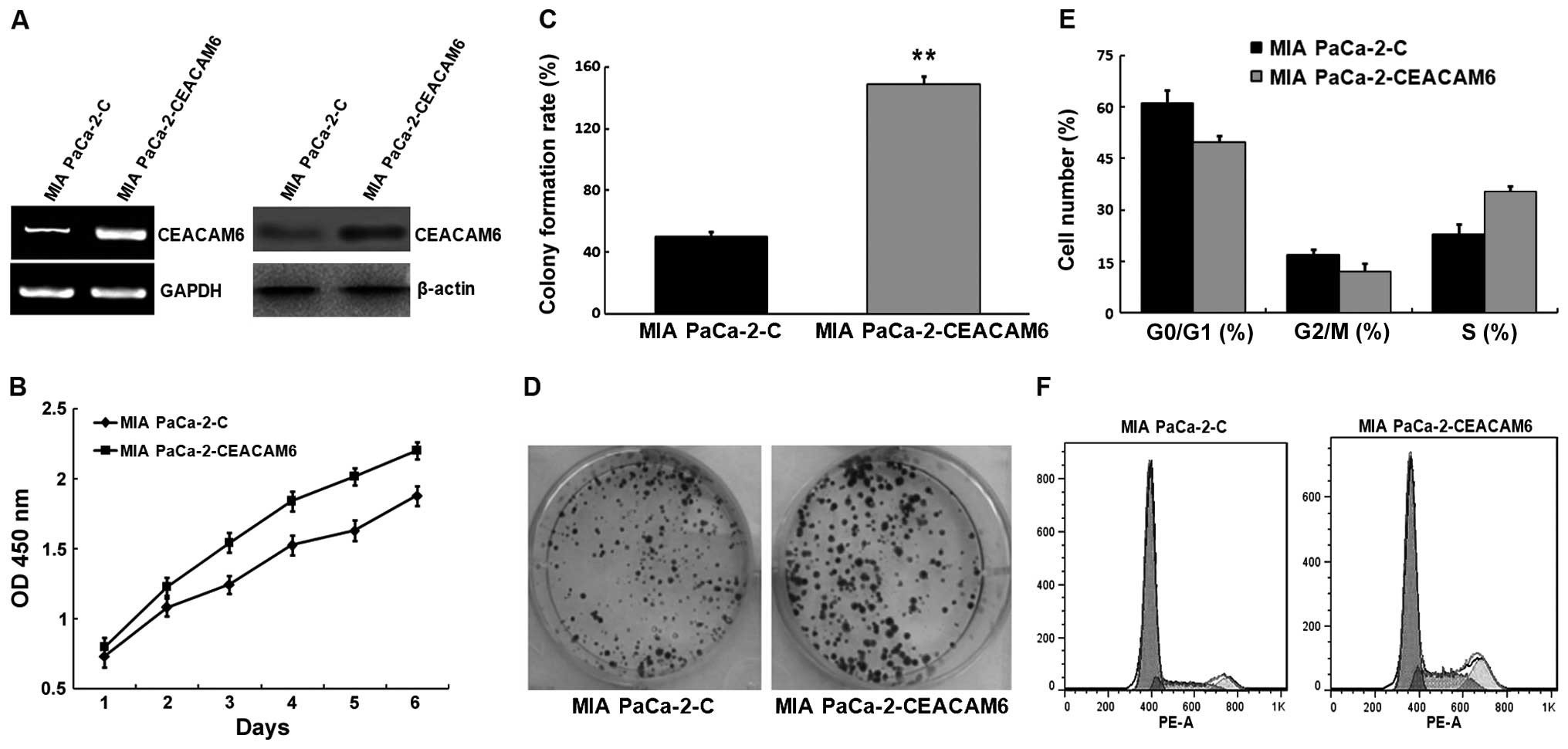
To test this possibility, MIA PaCa-2 cells were transfected with
plasmid pcDNA(+)3.1-CEACAM6 encoding CEACAM6. Comparing to the
control (MIA PaCa-2-C), cells transfected with plasmid encoding
CEACAM6 (MIA PaCa-2-CEACAM6) had increased levels of CEACAM6 mRNA
and protein (Fig. 5A). CCK-8
analysis showed that the proliferation of MIA PaCa-2-CEACAM6 cells
was much higher than MIA PaCa-2-C cells (Fig. 5B). Colony formation analysis showed
a larger amount of cell colonies from MIA PaCa-2-CEACAM6 cells. The
results demonstrated that upregulation of CEACAM6 expression
promoted cell proliferation in vitro (Fig. 5C and D).
Cell proliferation was also detected by flow
cytometry, results showed MIA PaCa-2-CEACAM6 decreased the cell
cycle in G1 phase and increased the cell cycle in S phase, which
may promote the proliferation of MIA PaCa-2 cells (Fig. 5E and F). This result is in line with
the above analysis.
Tumor formation in nude mice
Given that downregulation of CEACAM6 inhibited
pancreatic carcinoma cell proliferation and upregulation of CEACAM6
promoted pancreatic carcinoma cell proliferation in vitro,
we hypothesized that CEACAM6 could promote pancreatic carcinoma
cell development in vivo. To further determine the role of
CEACAM6 in tumorigenicity and development of pancreatic carcinoma
cells, BxPC-3/si-CEACAM6 and SW1990/si-CEACAM6 or BxPC-3/NC and
SW1990/NC cells were injected subcutaneously into nude mice. The
development of the tumors was monitored for 40 days. As shown in
Fig. 6A and B, CEACAM6 knockdown
tumors emerged later and grew slowly compared to control tumors. At
the end of the experimental period, the final weights of CEACAM6
knockdown tumors (0.392±0.065 and 0.491±0.057 g) were found to be
markedly lighter than controls (0.658±0.098 and 0.739±0.072 g)
(Fig. 6C and D). RT-PCR and western
blot analysis of CEACAM6 in xenograft tumors indicated that
increased CEACAM6 expression had been maintained throughout the
experimental time course (Fig. 6E and
F). Collectively, these data evidenced that CEACAM6 promoted
xenograft tumor development in vivo.
CEACAM6 affects the expression of cell
cycle-related proteins in pancreatic carcinoma cells and xenograft
tumors
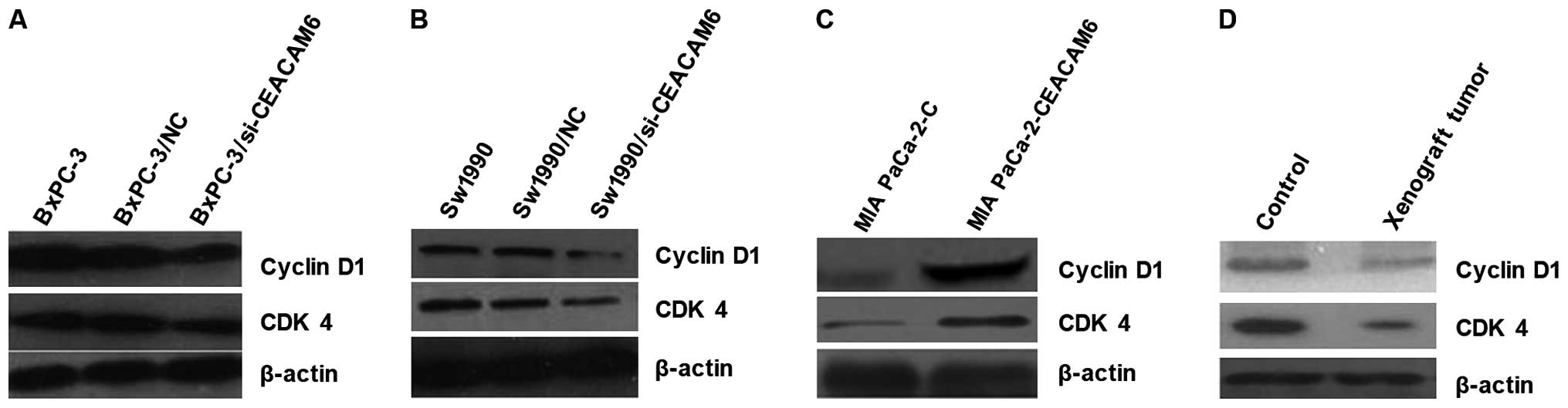
Cyclin D/CDK4 and cyclin E/CDK2 are important for
cell cycle progression in metazoans and is frequently overexpressed
in cancer cells. In view of the effects of CEACAM6 on cell
proliferation, we investigated whether the knockdown of CEACAM6
influenced cyclin D1/CDK4 and cyclin E1/CDK2 expression in
pancreatic carcinoma cells and xenograft tumors. The total cellular
levels of cyclin D1/CDK4, cyclin E1/CDK2 were analyzed by western
blot analysis. Results showed that CEACAM6 had no conclusive effect
on cyclin E1/CDK2 protein levels (data not shown). However,
downregulation of endogenous CEACAM6 reduced cyclin D1/CDK4 protein
levels (Fig. 7A and B). Moreover,
western blot analysis also comfirmed these results in xenograft
tumors (Fig. 7C and D).
Disscussion
CEACAM6 is a single-chain GPI-anchored
immunoglobulin (Ig)-like glycoprotein and is a member of the human
CEA family (15). Jantscheff et
al (16) showed that CEACAM6
overexpression was associated with poor clinical outcome in
colorectal cancer. In the present study, we show for the first time
a significant role for CEACAM6 in the regulation of pancreatic
carcinoma cells proliferation and cyclin D1/CDK4 expression. These
findings indicate a new mechanism for CEACAM6-mediated cell
proliferation that may act through the regulation of cyclin D1/CDK4
expression.
We first demonstrated that the two kinds of
pancreatic carcinoma cells express CEACAM6. The expression of
CEACAM6 in pancreatic carcinoma cells indicated that CEACAM6
exerted a potential role in the cell regulation. In the present
study, BxPC-3 and SW1990 cells were used as a pancreatic carcinoma
tumor cell model. It has been reported that CEACAM6 overexpression
independently predicted poor overall survival and disease-free
survival, whereas CEACAM1 or CEACAM5 was not significantly
associated with these outcomes. CEACAM6 overexpression leads to
morphological changes that are similar to epithelial-mesenchymal
transformation (17), increased
invasiveness (17), increased
chemoresistance (18) and
resistance to anoikis (19–21), whereas CEACAM6 appears to exert its
pro-invasive effect in a c-Src-dependent manner, at least in part
through the upregulation of MMP-9 activity (22).
The overexpression of CEACAM6 genes has been widely
associated with a variety of carcinomas (23,24).
Therefore, our results are consistent with previous observations
that CEACAM6 genes are highly expressed in a variety of carcinomas.
CEACAM6, in particular, plays a role in pancreatic carcinoma cell
growth, motility and cellular proliferation. In the present study,
we performed siRNA-mediated knockdown of CEACAM6 in the high
CEACAM6-expressing pancreatic carcinoma cell line. Conversely, we
overexpressed CEACAM6 in the low CEACAM6-expressing MIA PaCa-2 cell
line to determine how CEACAM6 regulates pancreatic carcinoma
growth. The results show that CEACAM6 exerted a specific role in
pancreatic carcinoma cell proliferation, knockdown of CEACAM6
induced a decrease in pancreatic carcinoma proliferation, while
overexpression of CEACAM6 in MIA PaCa-2 cells significantly
promoted cell growth. These results support the hypothesis that
CEACAM6 modulates the growth and oncogenesis of human pancreatic
carcinoma cells.
Since cell proliferation is controlled by
progression through the cell cycle, which is regulated by many
proliferative signaling cascades, we carried out flow cytometric
analysis to identify cell cycle distribution. Normal cell cycle
follows the ordinary steps, but cancer cells grow without
regulation. The rate of progress in cell cycle is decided by
cyclins and cyclin-dependent kinases (CDKs). Entering of each phase
is controlled by specific cyclin-CDK complex. CDK is a member of
serine-threonine kinase family because a cyclin binds to a CDK and
starts the phosphorylation of its serine and threonine site
(25,26). It has been reported that the G1-S
check-point is mainly regulated by a series of cyclins and
cyclin-dependent kinase (CDK), which were predominantly cyclin
D/CDK4, CDK6, cyclin E/CDK2 (27).
Cyclin D is a key regulatory enzyme in cell proliferation and plays
an important role in the progression from the G1 to S phase in the
cell cycle (28). In early G1
phase, cyclin D binds to CDK4 to form the cyclin-CDK complex and
subsequently activates the Rb protein by phosphorylation.
Phospho-Rb (p-Rb) is the major negative regulator of cell division
and exerts most of its effect in the first two thirds of the g1
phase of the cell cycle (29). In
the present study, we examined the effect of cyclin D1/CDK4 on
pancreatic carcinoma cell growth. As observed by others in multiple
cell types (30), cyclin D1/CDK4
activation promoted cell proliferation and stimulated the
replication of cells. The present study further identified cyclin
D1/CDK4 as a novel downstream target of CEACAM6.
In summary, our present data support a novel
mechanistic role for the CEACAM6 modulation of pancreatic carcinoma
cell proliferation via the expression of cyclin D1/CDK4. This
finding contributes to the pro-proliferation effect of CEACAM6 in
pancreatic carcinoma cell growth, motility and cellular
proliferation. This finding will yield new potential targets for
the treatment of pancreatic carcinoma tumors and other pancreatic
carcinoma cell proliferation dysfunction that involve the aberrant
expression of CEACAM6 gene.
Acknowledgments
The present study was supported by grants from the
Project-sponsored by SRF for ROCS, SEM [no. 311 (2015)] and the
Scientific Research Program Funded by the Shanxi Provincial
Education Department (no. 12JK0765).
Abbreviations:
|
CEACAM6
|
carcinoembryonic antigen-related cell
adhesion molecule 6
|
|
CEA
|
carcinoembryonic antigen
|
References
|
1
|
Long H, Li Q, Wang Y, Li Q, Liu T and Peng
J: Effective combination gene therapy using CEACAM6-shRNA and the
fusion suicide gene yCDglyTK for pancreatic carcinoma in vitro. Exp
Ther Med. 5:155–161. 2013.
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bond-Smith G, Banga N, Hammond TM and
Imber CJ: Pancreatic adenocarcinoma. BMJ. 344:e24762012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arlt A, Gehrz A, Müerköster S, Vorndamm J,
Kruse ML, Fölsch UR and Schäfer H: Role of NF-kappaB and Akt/PI3K
in the resistance of pancreatic carcinoma cell lines against
gemcitabine-induced cell death. Oncogene. 22:3243–3251. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stathis A and Moore MJ: Advanced
pancreatic carcinoma: Current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barnett T, Goebel SJ, Nothdurft MA and
Elting JJ: Carcinoembryonic antigen family: Characterization of
cDNAs coding for NCA and CEA and suggestion of nonrandom sequence
variation in their conserved loop-domains. Genomics. 3:59–66. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuroki M, Matsushita H, Matsumoto H,
Hirose Y, Senba T and Yamamoto T: Nonspecific cross-reacting
antigen-50/90 (NCA-50/90) as a new tumor marker. Anticancer Res.
19(6C): 5599–5606. 1999.
|
|
8
|
Blumenthal RD, Hansen HJ and Goldenberg
DM: Inhibition of adhesion, invasion, and metastasis by antibodies
targeting CEACAM6 (NCA-90) and CEACAM5 (Carcinoembryonic Antigen).
Cancer Res. 65:8809–8817. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Poola I, Shokrani B, Bhatnagar R, DeWitty
RL, Yue Q and Bonney G: Expression of carcinoembryonic antigen cell
adhesion molecule 6 oncoprotein in atypical ductal hyperplastic
tissues is associated with the development of invasive breast
cancer. Clin Cancer Res. 12:4773–4783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blumenthal RD, Leon E, Hansen HJ and
Goldenberg DM: Expression patterns of CEACAM5 and CEACAM6 in
primary and metastatic cancers. BMC Cancer. 7:22007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duxbury MS, Matros E, Ito H, Zinner MJ,
Ashley SW and Whang EE: Systemic siRNA-mediated gene silencing: A
new approach to targeted therapy of cancer. Ann Surg. 240:667–674;
discussion 675–676. 2004.PubMed/NCBI
|
|
12
|
Strickland LA, Ross J, Williams S, Ross S,
Romero M, Spencer S, Erickson R, Sutcliffe J, Verbeke C, Polakis P,
et al: Preclinical evaluation of carcinoembryonic cell adhesion
molecule (CEACAM) 6 as potential therapy target for pancreatic
adenocarcinoma. J Pathol. 218:380–390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Li C, Yang D, Jian XC and Jiang
CH: Clinico-pathological significance of MHC-I type
chain-associated protein A expression in oral squamous cell
carcinoma. Asian Pac J Cancer Prev. 13:715–718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li YH, Liu Y, Li YD, Liu YH, Li F, Ju Q,
Xie PL and Li GC: GABA stimulates human hepatocellular carcinoma
growth through overexpressed GABAA receptor theta subunit. World J
Gastroenterol. 18:2704–2711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hammarström S: The carcinoembryonic
antigen (CEA) family: Structures, suggested functions and
expression in normal and malignant tissues. Semin Cancer Biol.
9:67–81. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jantscheff P, Terracciano L, Lowy A,
Glatz-Krieger K, Grunert F, Micheel B, Brümmer J, Laffer U, Metzger
U, Herrmann R, et al: Expression of CEACAM6 in resectable
colorectal cancer: A factor of independent prognostic significance.
J Clin Oncol. 21:3638–3646. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lewis-Wambi JS, Cunliffe HE, Kim HR,
Willis AL and Jordan VC: Overexpression of CEACAM6 promotes
migration and invasion of oestrogen-deprived breast cancer cells.
Eur J Cancer. 44:1770–1779. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duxbury MS, Ito H, Benoit E, Waseem T,
Ashley SW and Whang EE: A novel role for carcinoembryonic
antigen-related cell adhesion molecule 6 as a determinant of
gemcitabine chemo-resistance in pancreatic adenocarcinoma cells.
Cancer Res. 64:3987–3993. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ordoñez C, Screaton RA, Ilantzis C and
Stanners CP: Human carcinoembryonic antigen functions as a general
inhibitor of anoikis. Cancer Res. 60:3419–3424. 2000.PubMed/NCBI
|
|
20
|
Zhu Z, Sanchez-Sweatman O, Huang X,
Wiltrout R, Khokha R, Zhao Q and Gorelik E: Anoikis and metastatic
potential of cloudman S91 melanoma cells. Cancer Res. 61:1707–1716.
2001.PubMed/NCBI
|
|
21
|
Duxbury MS, Ito H, Zinner MJ, Ashley SW
and Whang EE: CEACAM6 gene silencing impairs anoikis resistance and
in vivo metastatic ability of pancreatic adenocarcinoma cells.
Oncogene. 23:465–473. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Duxbury MS, Ito H, Benoit E, Ashley SW and
Whang EE: CEACAM6 is a determinant of pancreatic adenocarcinoma
cellular invasiveness. Br J Cancer. 91:1384–1390. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takeichi M: Cadherins in cancer:
Implications for invasion and metastasis. Curr Opin Cell Biol.
5:806–811. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Birchmeier W and Behrens J: Cadherin
expression in carcinomas: Role in the formation of cell junctions
and the prevention of invasiveness. Biochim Biophys Acta.
1198:11–26. 1994.PubMed/NCBI
|
|
25
|
Hartwell LH and Weinert TA: Checkpoints:
Controls that ensure the order of cell cycle events. Science.
246:629–634. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kamb A, Gruis NA, Weaver-Feldhaus J, Liu
Q, Harshman K, Tavtigian SV, Stockert E, Day RS III, Johnson BE and
Skolnick MH: A cell cycle regulator potentially involved in genesis
of many tumor types. Science. 264:436–440. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu M, Wang C, Li Z, Sakamaki T and Pestell
RG: Minireview: Cyclin D1: normal and abnormal functions.
Endocrinology. 145:5439–5447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weinberg RA: The retinoblastoma protein
and cell cycle control. Cell. 81:323–330. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jung YJ, Lee KH, Choi DW, Han CJ, Jeong
SH, Kim KC, Oh JW, Park TK and Kim CM: Reciprocal expressions of
cyclin E and cyclin D1 in hepatocellular carcinoma. Cancer Lett.
168:57–63. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li T, Zhao X, Mo Z, Huang W, Yan H, Ling Z
and Ye Y: Formononetin promotes cell cycle arrest via
downregulation of Akt/Cyclin D1/CDK4 in human prostate cancer
cells. Cell Physiol Biochem. 34:1351–1358. 2014. View Article : Google Scholar : PubMed/NCBI
|