Introduction
Use of the tyrosine kinase inhibitor (TKI) imatinib
has dramatically improved the outcome of patients with chronic
myeloid leukemia (CML). The second-generation TKIs, nilotinib and
dasatinib, are treatment options for patients with chronic-phase
CML (CML-CP) who are intolerant or resistant to imatinib (1). Of serious concern, however, are the
adverse effects associated with TKI treatment (2). Such effects include pleural effusion
(PE) in dasatinib-treated patients, which is clinically important
because it often curtails adherence to treatment regimens. On the
other hand, PE is associated with lymphocytosis and favorable
outcomes and thus is likely induced by an immune-mediated mechanism
(3–5).
Dasatinib is currently available as a first-line
treatment for newly diagnosed CML-CP. However, except the DASISION
study, all previous studies examining dasatinib-associated PE were
conducted in patients receiving dasatinib as a second-line therapy.
The DASISION study, which compared the efficacy and safety of
dasatinib and imatinib (6,7), found that PE was infrequent and
moderate when dasatinib was used as the first-line treatment rather
than the second-line treatment (6).
Whether PE affects the treatment response or acts via an
immunological mechanism is not known. Comprehensive evaluation of
the clinical significance of PE in first-line dasatinib treatment
is needed, and toward this goal, we analyzed data derived from
patients enrolled in the D-First study, a clinical trial of CML-CP
patients receiving first-line dasatinib therapy. Specifically, we
identified the risk factors for PE and the prognostic implications
of PE.
Patients and methods
Patients
The open-label, multicenter, prospective 'D-First'
trial (ClinicalTrials.gov, NCT01464411) was
used to derive data for the current study (8). It excluded CML-CP patients who were
pregnant or breast feeding or had comorbidities that rendered them
unsuitable for dasatinib therapy (e.g., a corrected QT interval
>450 msec on an electrocardiogram, double cancer, PE, or history
of severe or recent cardiovascular disease). Fifty-two patients
with newly diagnosed CML-CP were enrolled and received 100 mg of
dasatinib once daily. One of the 52 patients did not express the
BCR-ABL1 transcript and was included in the PE incidence
analysis, but not the molecular response analysis. The study was
approved by the research ethics boards of all participating
institutions and was conducted in accordance with the Declaration
of Helsinki. All patients gave written informed consent prior to
enrollment.
Assessment of the treatment response and
molecular analysis
BCR-ABL1 mRNA expression was used to assess
the molecular response to dasatinib. Peripheral blood samples were
drawn from patients 3 months after initiating dasatinib treatment,
and amounts of the BCR-ABL1 transcript were quantified by
Biomedical Laboratories (BML; Tokyo, Japan) via real-time
quantitative polymerase chain reaction. The data were normal-ized
to the housekeeping gene GAPDH and converted to the
International Scale (BCR-ABL1IS) (9,10). A
major molecular response (MMR) and a deep molecular response (DMR)
were defined as <0.1% and <0.0069% of
BCR-ABL1IS, respectively (11).
Assessment of PE
PE was evaluated by chest radiographs, which were
routinely performed at 2 weeks and at 1, 2, 3, 6, 9, 12, 15, and 18
months after the initiation of dasatinib treatment. Patients were
excused from chest radiography at the physician's discretion if
symptoms or abnormal findings were not observed. Toxicities were
graded according to the National Cancer Institute Common
Terminology Criteria for Adverse Events, version 4.0.
Flow cytometric analysis and definition
of lymphocytosis
Immunophenotyping of lymphocyte fractions in
peripheral blood samples was performed at BML using standard
immunofluorescence methods and anti-human antibodies recognizing
the CD3, CD8, CD56, and CD57 surface antigens. Antigen expression
was evaluated using a FACSCalibur™ system (Becton-Dickinson; NJ,
USA). Data were collected using CELLQuest, version 3.3
(Becton-Dickinson) and analyzed using original software from BML.
Each lymphocyte subset was quantified as follows: total leukocyte
count × percentage of lymphocytes × percentage of each lymphocyte
subset. Lymphocytosis was defined as an absolute blood lymphocyte
count >3.6×103/μl on ≥2 occasions after ≥4
weeks of therapy. Blood lymphocyte analyses were performed monthly
after initiating treatment.
Statistical analyses
The Fisher's exact test and Mann-Whitney U test were
used to determine statistical significance. Receiver operating
characteristic (ROC) curves were generated to evaluate the
correlation between PE and specific leukocyte subsets, and optimal
thresholds along the ROC curves were determined using the Youden
index. Patients who discontinued dasatinib treatment for any reason
before achieving an MMR or a DMR were considered to have no MMR or
DMR at 18 months. A P-value <0.05 was considered significant; a
P-value <0.1 indicated a tendency toward significance. All
statistical analyses were performed using EZR (Saitama Medical
Center, Jichi Medical University), the graphical user interface
supplied by the R Foundation for Statistical Computing.
Results
Results of treatment in the study
population
No mortality or disease progression occurred within
the first 18 months of dasatinib treatment. Three of the 52
enrolled patients discontinued treatment prior to 18 months (one at
1 month and two at 11 months) because of intolerance to dasatinib.
Regarding molecular assessment, 38 of 51 patients (75%; 95%
confidence interval [CI], 60–86%) had an MMR within 12 months after
treatment initiation, and 30 (59%; 95% CI, 44–72%) had a DMR within
18 months.
Cumulative incidence of PE and patient
characteristics
The overall incidence rate of PE 18 months after
treatment initiation was 33%, and the median time to development of
PE was 9 months (1–15 months). At 6 and 12 months, the cumulative
incidence rates of PE were 14 and 27%, respectively. Among the 17
patients who developed PE, 10 (59%) had grade 1 PE and 7 (41%) had
grade 2 PE. The characteristics of patients with and without PE at
18 months are shown in Table I.
Although unrelated to gender, body weight, Sokal score, and
BCR-ABL1 transcript expression, the incidence of PE
significantly correlated with age. When patients were divided into
three age groups, the PE incidence rate differed significantly
between the groups and was highest in the oldest group (>60
years, P<0.01) (Fig. 1).
 | Table IBaseline characteristics of patients
with or without pleural effusion (PE) by 18 months. |
Table I
Baseline characteristics of patients
with or without pleural effusion (PE) by 18 months.
| Factors | PE+
(N=17) | PE−
(N=35) | P-value |
|---|
| Age (years), median
(range) | 63 (47–81) | 41 (23–82) | 0.0013 |
| Gender
(male/female) | 9/8 | 22/13 | 0.56 |
| Body weight
(kg) | 59 (35–87) | 63.5 (45–88) | 0.62 |
| Sokal score | | | 0.24 |
| Low-risk | 5 | 19 | |
|
Intermediate-risk | 10 | 12 | |
| High-risk | 2 | 4 | |
| BCR-ABL1
transcript value (copies/μg RNA), median (range) | 220,000
(64,000–700,000) | 265,000
(71,000–1,000,000) | 0.27 |
Association of PE incidence and
development of lymphocytosis
We identified 14 patients (27%) who developed
lymphocytosis during the observation period. The incidence rates of
PE in patients with and without lymphocytosis were 30.4% and 34.5%,
respectively, and did not differ significantly.
Lymphocyte dynamics according to the
incidence of PE
To examine lymphocyte dynamics, CD56+
lymphocytes [natural killer (NK) cells], and
CD3+/8+ lymphocytes [cytotoxic T-lymphocytes
(CTLs)] were separated from whole lymphocyte fractions at each time
point (Fig. 2A–C). There were no
significant differences in the levels of unfractionated,
CD56+, or CD3+/8+ lymphocytes
between patients who developed PE and those who did not. However,
in the first month following dasatinib treatment, the
CD56+ fraction tended to be larger in PE-positive
patients than PE-negative patients (P<0.1).
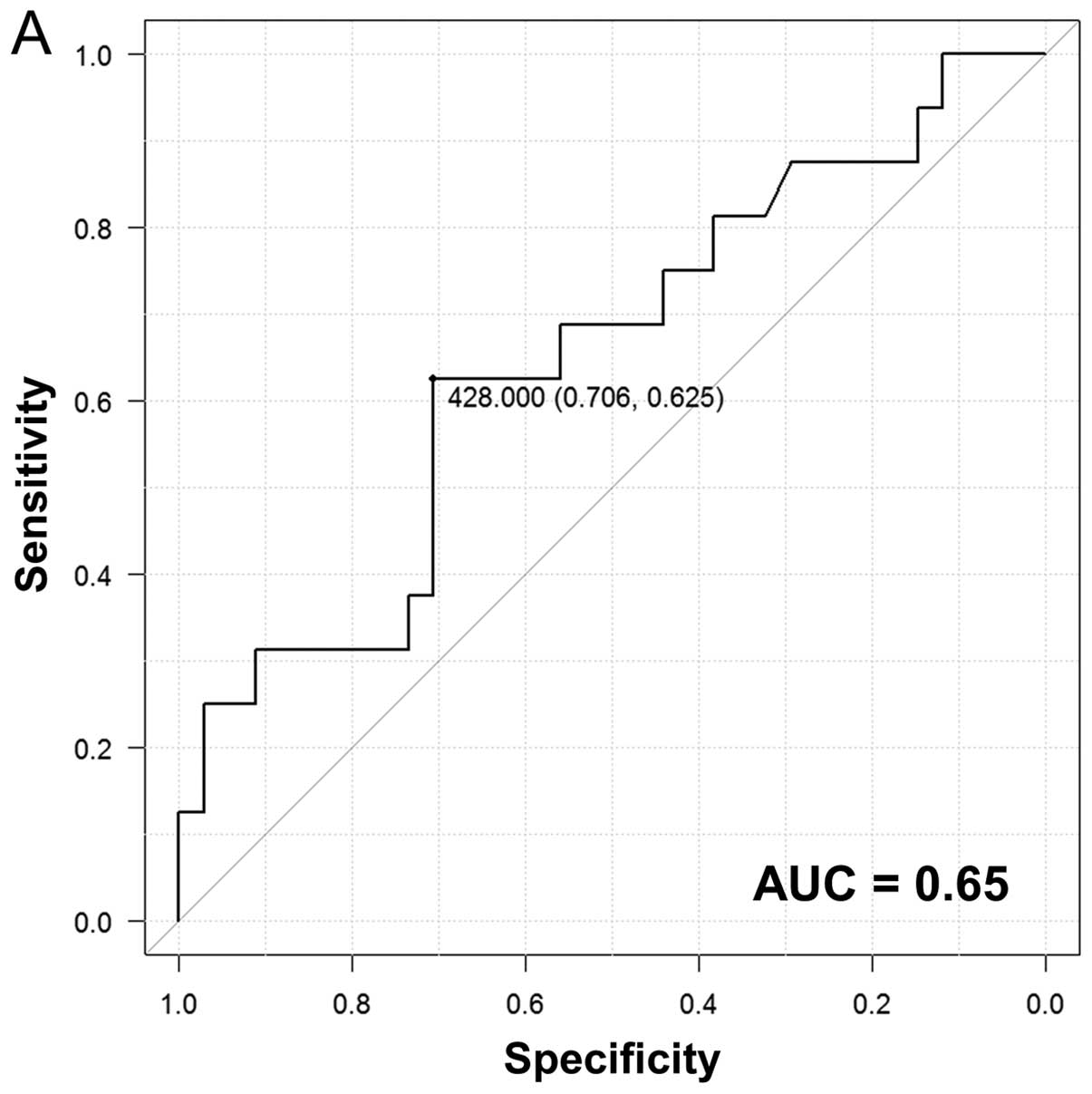
To better evaluate the association between the
incidence of PE and the level of CD56+ lymphocytes 1
month after dasatinib treatment, we generated ROC curves and
calculated the threshold for CD56+ lymphocytes (Fig. 3A). Using this threshold, we divided
the patients into two groups: those with high (≥428/μl) and
low (<428/μl) CD56+ lymphocyte levels. The
incidence of PE was significantly higher in the former (Fig. 3B), and this difference was
maintained up to 18 months. To determine whether a specific
CD56+ subset accounted for this increase, we counted
CD56+ lymphocytes separated according to CD3 and CD57
status 1 month after initial dasatinib treatment (Fig. 4). Although the association between
the incidence of PE and CD3 or CD57 status was not significant,
CD56+/CD3− and
CD56+/CD57+ levels tended to be higher in
PE-positive patients than PE-negative patients (Fig. 4).
Patient response rate according to the
incidence of PE
To investigate the relationship between PE incidence
and treatment response, we determined the cumulative MMR and DMR
rates at 3, 6, 9, 12, 15, and 18 months after the initiation of
dasatinib treatment in PE-positive and PE-negative patients. At all
time points, cumulative MMR rates were higher in the PE-positive
group. Importantly, the differences were only significant at 3
months, after which they narrowed until the rate in both groups was
nearly equal at 18 months (Fig.
5A). DMR rates also tended to be higher in the PE-positive
group at all time points, but not significantly so (Fig. 5B).
Discussion
Currently, dasatinib is used not only as a
second-line treatment for relapsed or refractory CML, but also as a
first-line treatment, for which it was very efficacious in a
large-scale randomized trial (11).
In both first-line and second-line dasatinib treatments, the
incidence of PE is considered to be a common toxicity (2), but the mechanism by which dasatinib
causes PE and the impact of PE on treatment outcome are unclear. We
therefore prospectively investigated the clinical characteristics
of newly diagnosed CML-CP patients given dasatinib and closely
analyzed the relationship between treatment outcome, PE occurrence,
and the immunomodulatory effects of dasatinib.
In the current study, the incidence of PE was
associated with high molecular response rates at early times after
the initiation of dasatinib treatment and with an immune response
as indicated by NK cell counts at 1 month after initial treatment.
Most published data on dasatinib-associated PE were generated in
patients for whom dasatinib was the second-line therapy after
imatinib (4,12–14).
By comparing our results with those of previous studies of CML
patients receiving dasatinib as a second-line therapy, we were able
to assess the significance of PE prevalence in first-line therapy.
Three points of interest in our study are discussed below.
First, PE was more frequent in older patients,
similar to a previous study (15),
but was unrelated to patient characteristics such as sex, body
weight, and performance status. Previous studies analyzing the
pharmacokinetics of dasatinib in second-line therapy showed that
the risk of developing PE increased as patients aged (15–17).
PE may occur more frequently in elderly patients because they may
not metabolize dasatinib as efficiently as younger patients.
Additional risk factors for PE in second-line dasatinib therapy
include the steady state trough plasma concentration of dasatinib,
cardiac disease, and hypertension (15,18).
However, patients with comorbidities were not eligible for our
study, and therefore the association between cardiac disease and PE
development was not analyzed.
Second, the occurrence of PE was related to the
treatment response, especially the early molecular response. In
patients with PE, the cumulative MMR rate was significantly higher
in the PE-positive group than the PE-negative group only at 3
months after commencement of dasatinib treatment, while the DMR
rate tended to be higher in the PE-positive group through the
treatment period. The DMR results suggest that the mechanism
underlying PE development likely promotes only transient tumor
regression in the first-line setting.
Third, of particular interest in this study is the
mechanism by which PE develops in response to dasatinib,
particularly in terms of the hypothetical immune-mediated pathway
(3,4). To date, many studies have reported an
association between lymphocytosis, especially large granular
lymphocytosis, and PE incidence in patients receiving dasatinib
(2,3,19).
Although lymphocytosis did not correlate with PE incidence in our
study, we demonstrated an expansion of CD56+ lymphocytes
in patients with PE as early as 1 month after dasatinib treatment.
We observed lower levels in CD56+ lymphocytes at 1 month
compared to those at 2 or more months, suggesting that the
proliferation of NK cells is inhibited at the time of diagnosis and
increases after dasatinib therapy. In support, Kreutzman et
al (20) reported the presence
of monoclonal and oligoclonal NK cells and/or CTLs in newly
diagnosed CML-CP patients. Cytotoxic cells presumably are present
at low levels at the time of diagnosis but markedly increase in
number after dasatinib administration, presumably to trigger immune
surveillance against CML cells. A previous study assessing the
significance of CD56+/CD57+ pool size
(21) showed that dasatinib-induced
expansion of enhanced cytotoxic NK populations improved CML
control. We suggest that an immune response involving NK cells
occurring as early as 1 month after dasatinib administration
increases the incidence of PE for up to 18 months and likely
promotes rapid tumor regression within 3 months.
The incidence of PE in our study was 33%, which is
higher than that in the DASISION trial (14% at 24 months) (7). Our study protocol may account in part
for this discrepancy: it involved frequent chest X-rays to detect
mild asymptomatic PE, especially at early time points after
initiation of dasatinib treatment. Alternatively or in addition,
the different rates may reflect ethnic differences, which may
influence dasatinib pharmacokinetics. In support, in a subset
analysis, the DASISION trial detected a higher rate of PE in East
Asian patients than non-East Asian patients (22,23).
Although the incidence of PE was high in our study,
the degree of PE was usually mild to moderate. The patients in our
study did not require thoracentesis because PE was managed via dose
reduction. Only two patients discontinued dasatinib treatment owing
to uncontrolled grade 2 PE. Among the PE-positive patients who
continued the core treatment, dasatinib doses were optimized for
management of PE, with a mean daily dose of 75 mg at 18 months.
Most PE patients, however, received a mean daily dose of 90 mg
without ill effects. We did not investigate the management of PE
other than dose optimization.
In summary, we prospectively examined
dasatinib-related PE in newly diagnosed CML-CP patients, and we
suggest that early expansion of NK populations may predict PE
occurrence. Our results indicate that the incidence of PE up to 18
months after initial dasatinib treatment may be due to an
immune-mediated mechanism involving NK cells. We suggest that this
underlying mechanism likely contributes to transient tumor
regression in patients newly diagnosed with CML-CP. This study not
only reveals a possible mechanism of PE development, but also
suggests that, similar to previous studies, the occurrence of PE
may be related to favorable outcomes in patients with CML-CP.
Acknowledgments
This study was supported by the Epidemiological and
Clinical Research Information Network (ECRIN). We thank Yumi
Miyashita at ECRIN for collecting the data, and Yoshinori Yamamoto
at BML for analyzing the data. N.I. received an honorarium and a
speaker fee from Bristol-Myers Squibb and Novartis, respectively.
T.K. received a lecture fee from Bristol-Myers Squibb. C.Y.
received honoraria from Bristol-Myers Squibb and Novartis and
grants from Otsuka Pharmaceutical. S.C. and K.O. received grants,
speaker fees, and personal fees from Bristol-Myers Squibb and
Novartis. S.C. has a pending patent for a diagnostic method for
lymphoma. K.O. is a paid consult for Ariad. S.M. received a speaker
fee from Bristol-Myers Squibb. S.O. received lecture fees and grant
from Bristol-Myers Squibb.
References
|
1
|
Cortes J, Kim DW, Raffoux E, Martinelli G,
Ritchie E, Roy L, Coutre S, Corm S, Hamerschlak N, Tang JL, et al:
Efficacy and safety of dasatinib in imatinib-resistant or
-intolerant patients with chronic myeloid leukemia in blast phase.
Leukemia. 22:2176–2183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quintás-Cardama A, Kantarjian H, O'brien
S, Borthakur G, Bruzzi J, Munden R and Cortes J: Pleural effusion
in patients with chronic myelogenous leukemia treated with
dasatinib after imatinib failure. J Clin Oncol. 25:3908–3914. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldblatt M, Huggins JT, Doelken P, Gurung
P and Sahn SA: Dasatinib-induced pleural effusions: A lymphatic
network disorder? Am J Med Sci. 338:414–417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagata Y, Ohashi K, Fukuda S, Kamata N,
Akiyama H and Sakamaki H: Clinical features of dasatinib-induced
large granular lymphocytosis and pleural effusion. Int J Hematol.
91:799–807. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paydas S: Dasatinib, large granular
lymphocytosis, and pleural effusion: Useful or adverse effect? Crit
Rev Oncol Hematol. 89:242–247. 2014. View Article : Google Scholar
|
|
6
|
Laneuville P, Baccarani M, Cortes JE,
Hochhaus A, Kantarjian H, Shah NP, Bradley-Garelik MB, Zhu C and
Porkka K: Analysis of patients (pts) with chronic phase chronic
myeloid leukemia (CML-CP) who develop pleural effusion on
first-line dasatinib: Management and outcomes. J Clin Oncol.
29(Suppl): 66052011.
|
|
7
|
Kantarjian HM, Shah NP, Cortes JE,
Baccarani M, Agarwal MB, Undurraga MS, Wang J, Ipiña JJ, Kim DW,
Ogura M, et al: Dasatinib or imatinib in newly diagnosed
chronic-phase chronic myeloid leukemia: 2-year follow-up from a
randomized phase 3 trial (DASISION). Blood. 119:1123–1129. 2012.
View Article : Google Scholar
|
|
8
|
Iriyama N, Fujisawa S, Yoshida C, Wakita
H, Chiba S, Okamoto S, Kawakami K, Takezako N, Kumagai T, Inokuchi
K, et al: Shorter halving time of BCR-ABL1 transcripts is a novel
predictor for achievement of molecular responses in newly diagnosed
chronic-phase chronic myeloid leukemia treated with dasatinib:
Results of the D-first study of Kanto CML study group. Am J
Hematol. 90:282–287. 2015. View Article : Google Scholar
|
|
9
|
Yoshida C, Fletcher L, Ohashi K, Wakita H,
Kumagai T, Shiseki M, Matsuei K, Inokuchi K, Hatta Y, Shirasugi Y,
et al: Harmonization of molecular monitoring of chronic myeloid
leukemia therapy in Japan. Int J Clin Oncol. 17:584–589. 2012.
View Article : Google Scholar
|
|
10
|
Kumagai T, Matsuki E, Inokuchi K, Ohashi
K, Shinagawa A, Takeuchi J, Yoshida C, Okamoto S, Wakita H, Kozai
Y, et al: Relative increase in lymphocytes from as early as 1 month
predicts improved response to dasatinib in chronic-phase chronic
myelogenous leukemia. Int J Hematol. 99:41–52. 2014. View Article : Google Scholar
|
|
11
|
Kantarjian H, Shah NP, Hochhaus A, Cortes
J, Shah S, Ayala M, Moiraghi B, Shen Z, Mayer J, Pasquini R, et al:
Dasatinib versus imatinib in newly diagnosed chronic-phase chronic
myeloid leukemia. N Engl J Med. 362:2260–2270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim D, Goh HG, Kim SH, Cho BS and Kim DW:
Long-term pattern of pleural effusion from chronic myeloid leukemia
patients in second-line dasatinib therapy. Int J Hematol.
94:361–371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Latagliata R, Breccia M, Fava C, Stagno F,
Tiribelli M, Luciano L, Gozzini A, Gugliotta G, Annunziata M,
Cavazzini F, et al: Incidence, risk factors and management of
pleural effusions during dasatinib treatment in unselected elderly
patients with chronic myelogenous leukaemia. Hematol Oncol.
31:103–109. 2013. View
Article : Google Scholar
|
|
14
|
Eskazan AE, Eyice D, Kurt EA, Elverdi T,
Yalniz FF, Salihoglu A, Ar MC, Ongoren Aydin S, Baslar Z,
Ferhanoglu B, et al: Chronic myeloid leukemia patients who develop
grade I/II pleural effusion under second-line dasatinib have better
responses and outcomes than patients without pleural effusion. Leuk
Res. 38:781–787. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Porkka K, Khoury HJ, Paquette RL, Matloub
Y, Sinha R and Cortes JE: Dasatinib 100 mg once daily minimizes the
occurrence of pleural effusion in patients with chronic myeloid
leukemia in chronic phase and efficacy is unaffected in patients
who develop pleural effusion. Cancer. 116:377–386. 2010. View Article : Google Scholar
|
|
16
|
Wang X, Hochhaus A, Kantarjian HM, et al:
Dasatinib pharmacokinetics and exposure-response (E-R):
Relationship to safety and efficacy in patients (pts) with chronic
myeloid leukemia (CML). J Clin Oncol. 26(Suppl): 35952008.
|
|
17
|
Shah NP, Kantarjian HM, Kim DW, Réa D,
Dorlhiac-Llacer PE, Milone JH, Vela-Ojeda J, Silver RT, Khoury HJ,
Charbonnier A, et al: Intermittent target inhibition with dasatinib
100 mg once daily preserves efficacy and improves tolerability in
imatinib-resistant and -intolerant chronic-phase chronic myeloid
leukemia. J Clin Oncol. 26:3204–3212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Lavallade H, Punnialingam S, Milojkovic
D, Bua M, Khorashad JS, Gabriel IH, Chaidos A, Olavarria E, Goldman
JM, Apperley JF, et al: Pleural effusions in patients with chronic
myeloid leukaemia treated with dasatinib may have an
immune-mediated pathogenesis. Br J Haematol. 141:745–747. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mustjoki S, Ekblom M, Arstila TP, Dybedal
I, Epling-Burnette PK, Guilhot F, Hjorth-Hansen H, Höglund M,
Kovanen P, Laurinolli T, et al: Clonal expansion of T/NK-cells
during tyrosine kinase inhibitor dasatinib therapy. Leukemia.
23:1398–1405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kreutzman A, Juvonen V, Kairisto V, Ekblom
M, Stenke L, Seggewiss R, Porkka K and Mustjoki S: Mono/oligoclonal
T and NK cells are common in chronic myeloid leukemia patients at
diagnosis and expand during dasatinib therapy. Blood. 116:772–782.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim DH, Kamel-Reid S, Chang H, Sutherland
R, Jung CW, Kim HJ, Lee JJ and Lipton JH: Natural killer or natural
killer/T cell lineage large granular lymphocytosis associated with
dasatinib therapy for Philadelphia chromosome positive leukemia.
Haematologica. 94:135–139. 2009. View Article : Google Scholar :
|
|
22
|
Chuah CT, Nakamae H, Shen ZX,
Bradley-Garelik MB and Kim DW: Efficacy and safety of dasatinib
versus imatinib in the East Asian subpopulation of the DASISION
trial of newly diagnosed chronic myeloid leukemia in chronic phase.
Leuk Lymphoma. 55:2093–2100. 2014. View Article : Google Scholar :
|
|
23
|
Fujisawa S, Nakamae H, Ogura M, Ishizawa
K, Taniwaki M, Utsunomiya A, Matsue K, Takamatsu Y, Usuki K,
Tanimoto M, et al: Efficacy and safety of dasatinib versus imatinib
in Japanese patients with newly diagnosed chronic-phase chronic
myeloid leukemia (CML-CP): Subset analysis of the DASISION trial
with 2-year follow-up. Int J Hematol. 99:141–153. 2014. View Article : Google Scholar
|