Introduction
Prostate cancer remains the second cause of
cancer-related death among men (1).
It is difficult to detect this tumor at an early stage, and poses a
major challenge for treatment (2).
Due to late diagnosis and increasing chemoresistance of prostate
cancer, the five-year survival rate of patients remains <50%
after chemotherapeutic regimens and cytoreductive surgery (3). Prostate cancer metastasizes and
transfers easily, is recurrent and is also resistant to
chemotherapy, thus resulting in a high mortality rate (4). Therefore, development of novel
effective and less toxic drugs is urgent for prostate cancer
patients.
Recently, many types of bioactive phytochemicals
play a vital role in tumor suppression and antioxidant capacity
(5,6). Many flavone glycoside bioactive
phytochemicals, such as hesperidin and puerarin, have been
identified to exhibit anticancer activity (7,8).
Scutellarin is one of the effective bioactive phytochemicals found
in the traditional Chinese herbal medicine Scutellaria
altissima L. (9). It is a
traditional Chinese medicinal plants commomly used for upper
respiratory infection, pneumonia and high blood pressure (10,11).
Scutellaria altissima L. is a plant from the family
Lamiaceae found on the top of the hills and slopes, and in forest
margins in China. The chemical formula of Scutellarin is
C21H18O12. Scutellarin has been
widely used to treat cardiovascular and cerebrovascular diseases
(12). It has been revealed that
Scutellarin exhibits a variety of pharmacological actions,
including antioxidative, anti-inflammatory and vasodilator activity
(13,14). It has been confirmed to show
antitumor effects in many types of cancers, such as gastric and
breast cancer, glioblastoma, prostate, lung and hepatocellular
cancer by inhibiting tumor cell growth, metastasis and inducing
cell cycle arrest and mitochondrial pathway-mediated apoptosis.
However, there is no sufficient evidence confirming the effects of
Scutellarin on prostate cancer cells and the underlying molecular
mechanisms remain unclear. Thus, whether Scutellarin can sensitize
cancer PC3 cells to chemotherapy has not been revealed.
In the present study, our results showed that
Scutellarin exerts antitumor effects on prostate cancer cells and
we furthered explored the molecular mechanism underlying this
process. Data from the present study revealed that Scutellarin
significantly induced dose-dependent apoptosis and sensitized PC3
cells to cisplatin through induction of DNA breaks. Our results
show that Scutellarin warrants future development as an effective
and novel drug for patients with prostate cancer.
Materials and methods
Materials, reagents and chemicals
Antibodies against caspase-3, caspase-9, Bcl-2, Bax,
Cdc2, cyclin B1, β-actin and γH2AX were obtained from Cell
Signaling Technology Inc. (Beverly, MA, USA). The enhanced
chemiluminescence (ECL) kit was purchased from Amersham Life
Science, Inc. (Arlington Heights, IL, USA). The Annexin
V-conjugated FITC apoptosis detection kit and JC-1 mitochondrial
membrane potential detection kit were purchased from NanJing KeyGen
Biotech Co., Ltd. (Nanjing, China). The Comet Assay kit was from
NanJing KeyGen Biotech Co., Ltd. (Nanjing, China).
3-(4,5-Dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and 46-diamidino-2-phenylindole dihydrochloride (DAPI) were
obtained from Sigma Chemical Co. (St. Louis, MO, USA). Scutellarin
(>98%) powder was purchased from Sichuan Best-Reagent Industry
Co., Ltd. (Sichuan, China, lot no. B01146801) and dissolved in
dimethyl sulfoxide (DMSO). The final concentration of DMSO was 0.1%
in all groups and had no effect on cell viability. The chemical
formula of Scutellarin is
C21H18O12.
Cell lines and cell culture
The prostate cancer cell line PC3 was purchased from
the American Type Culture Collection (ATCC; Manassas, VA, USA) and
cultured in Dulbeccos modified Eagles medium (DMEM) supplemented
with 10% fetal bovine serum (FBS) and 100 U/ml penicillin (all from
Gibco-BRL, Grand Island, NY, USA) at 37°C with 5%
CO2.
Cell viability assays
The effect of Scutellarin on the viability of cells
was detected by MTT assay. The cells (1×104/well) were
seeded into 96-well plate and incubated for 24 h. After treatment
with Scutellarin (0, 100, 200, 300, 400, 500 or 600 µM) for 24, 48
and 72 h, the viability of the cancer cells was detected with MTT
assay. Twenty microliters (20 µl) of MTT solution [5 mg/ml in
phosphate-buffered saline (PBS)] was added to each well, and the
mixtures were incubated for 4 h at 37°C. Then, the MTT solution was
removed and 150 µl of DMSO was added to the wells. The absorbance
was measured using a Multiskan Ascent plate reader at 540 nm
wavelength.
Cell cycle analysis by flow
cytometry
The cells were treated with Scutellarin (0, 200, 400
or 600 µM) for 24 h, washed twice with PBS and fixed with 70%
ethanol overnight at 4°C. Following fixation, the DNA fragments
were stained in PBS containing propidium iodide (PI) and RNase A
for 1 h at 37°C. The DNA content was evaluated on a flow cytometer
Accuri C6 (BD Biosciences, Franklin Lakes, NJ, USA). The data were
analyzed using ModFit LT V4.1.
DAPI staining assay
Approximately 4×104 cells/well of
prostate cancer cells were treated with Scutellarin at 0, 200, 400
or 600 µM for 24 h. Cells in each well were stained with DAPI
before fixation with 3.7% formaldehyde. The cells were then washed
with PBS and detected using fluorescence microscopy.
Cell apoptosis by flow cytometry
The extent of apopotosis was evaluated by flow
cytometry using Annexin V-FITC. After treatment with 0, 200, 400 or
600 µM Scutellarin for 24 h, prostate cancer cells were harvested
and washed with PBS for three times, and then incubated with
Annexin V-FITC and PI for 10 min in the dark. The cells were
detected by a flow cytometer Accuri C6.
Assay for mitochondrial membrane
potential
Changes in mitochondrial membrane potential of
prostate cancer cells were detected by flow cytometry using JC-1
detection kit. After treatment with 0, 200, 400 or 600 µM
Scutellarin for 24 h, cells were harvested and incubated with JC-1
dye for 15 min at 37°C according to the manufacturer's protocol.
The cells were detected by a flow cytometer Accuri C6.
Colony formation assay
Cells (500 cells per 35-mm dish) were plated before
treatment. Then, 1 ml medium with or without drug was added into
each well. Cells were fixed using 70% ethanol, and stained using
crystal violet (Sigma, St. Louis, MO, USA) dissolved in 10% ethanol
for 15 days. The number of colonies was obtained by cell counting.
Colonies were defined as a minimum of 50 cells in a group.
Comet assay
DNA damage induction of Scutellarin in
hepatocellular carcinoma cells was determined using the Comet
assay, according to the manufacturer's protocol. Briefly, cells
were treated with Scutellarin (200 µM) and/or cisplatin (6 µM) for
48 h in complete medium, and then cells were harvested and
resuspended in ice-cold PBS buffer. We used the concentration of 6
µM for cisplatin and 200 µM for Scutellarin as these concentrations
do not inhibit the proliferation of cells significantly, and thus
it could be ascertained whether Scutellarin enhances the
sensitivity of prostate cancer cells to cisplatin. Approximately
1×104 cells in a volume of 75 µl of 0.5% (w/v)
low-melting-point agarose were pipetted into a frosted glass slide
coated with a thin layer of 1.0% (w/v) agarose, covered with a
coverslip, and allowed to set on ice for 10 min. Following removal
of the coverslip, the slides were immersed in ice-cold lysis
solution. After 2 h at 4°C, the slides were placed into a
horizontal electrophoresis tank filled with electrophoresis buffer
and subjected to electrophoresis for 30 min at 30 V at 4°C. Cells
were stained with 2.5 µg/ml PI for 5 min and visualized under a
microscope at a magnification of ×200. Tail length from a minimum
of 10 cells was quantified as the distance from the center of the
cell nucleus to the tip of the tail.
γH2AX immunocytochemistry
Cells were seeded on microscope slides and treated
with Scutellarin (200 µM) and/or cisplatin (6 µM). Cells were fixed
with 4% polyformaldehyde, washed with PBS and blocked with 1%
bovine albumin serum with 0.1% Triton-X. Slides were washed in PBS,
and then incubated with γH2AX primary antibody at a dilution of
1:800 in 1% BSA in PBS overnight at 4°C with gentle shaking. Cells
were then washed three times in PBS before incubating in the dark
with goat anti-rabbit IgG/FITC-conjugated secondary antibody at a
dilution of 1:1,000 in 1% BSA in PBS for 2 h. Nuclei were
counterstained with DAPI (1 µg/ml) in PBS for 5 min. Images were
collected using a fluorescence microscope.
Western blotting
The total protein was extracted from the cell
samples using lysis buffer (Beyotime, Shanghai, China) and protease
inhibitor (Biocolors, Shanghai, China). Lysis buffer and protease
inhibitor were used after being mixed in a proportion of 1:100.
Equal amounts of protein were loaded on a 10% SDS-PAGE gel. The
lysates were resolved by electrophoresis (80 V for 30 min and 120 V
for 1.5 h), and transferred onto polyvinylidene difluoride (PVDF)
membranes (nitrocellulose membrane; Bio-Rad, Hercules, CA, USA).
After blocking in 5% nonfat milk for 1 h at room temperature,
incubation was carried out with the different antibodies overnight
at 4°C. This was followed by incubation with relevant secondary
antibodies for 1 h at room temperature. Protein bands were
visualized using the Chemiluminescent ECL assay kit for each group
and the Bio-Rad ChemiDoc XRS+ image analyzer. Protein expression
levels were quantitatively determined using ImageJ software
(National Institutes of Health, Bethesda, MD, USA). β-actin was
used as an internal reference for protein expression in the treated
cells.
Statistical analysis
Data are presented as means ± SD for three
independent experiments. Statistical differences between two groups
were analyzed using a Student's t-test by GraphPad Prism 5.0
(GraphPad Software, San Diego, CA, USA). A significant difference
was considered as P<0.05.
Results
Scutellarin inhibits prostate cancer
cell proliferation
The chemical structure of Scutellarin is shown in
Fig. 1A. In order to determine the
cytotoxic effects of Scutellarin on prostate cancer cells, cell
viability was detected by MTT assay. Prostate cancer PC3 cells were
treated with different concentrations of Scutellarin (0, 100, 200,
300, 400, 500 or 600 µM) for 24, 48 or 72 h (Fig. 1B). Scutellarin exhibited
cytotoxicity against the PC3 cells. The prostate cancer cells
treated with Scutellarin showed a reduced proliferation capacity in
a dose- and time-dependent manner (P<0.05).
In order to determine the long-term growth
inhibitory effects, we pre-incubated PC3 cells with Scutellarin (0,
200, 400 or 600 µM) for 24 h, washed the cells, and cultured them
for 7 additional days. As shown in Fig.
1C, the colony formation ability of the prostate cancer cells
was significantly reduced in a dose-dependent manner.
In addition, the morphological changes in prostate
cancer cells were examined under a phase contrast microscope. PC3
cells cultured with complete DMEM without Scutellarin displayed a
normal shape with 60% confluency for 24 h. However, the cell
confluence was markedly reduced and the morphologic changes in PC3
cells were also quite obvious after Scutellarin treatment. The
cells started to shrink, lost their normal shape, became round and
ultimately detached from the culture dish (Fig. 1D). All of these results indicated
that Scutellarin inhibited prostate cancer cell growth and caused
cell death.
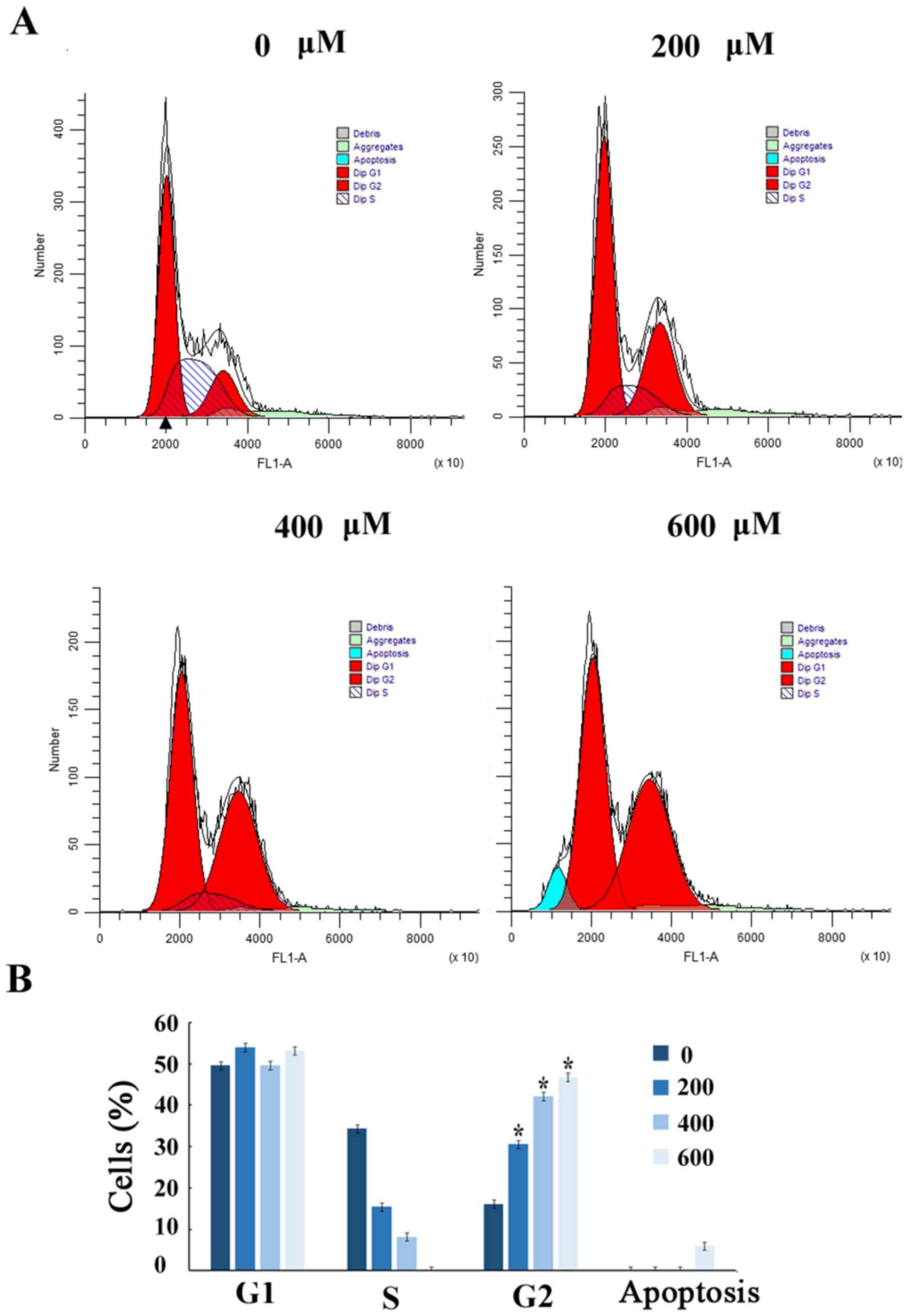
Scutellarin induces G2/M arrest
To further demonstrate that the growth inhibition of
human prostate cancer cells leads to alterations in cell cycle
distribution, cells were treated with Scutellarin and stained with
PI. The percentage of cells in each stage of the cell cycle was
evaluated by flow cytometry (Fig.
2A). Treatment of the human prostate cancer PC3 cells with
Scutellarin (0, 200, 400 or 600 µM) for 24 h resulted in
accumulation of cells in the G2/M phase from 16.6%±2.1 to 47.2%±2.3
(P≤0.01; Fig. 2B).
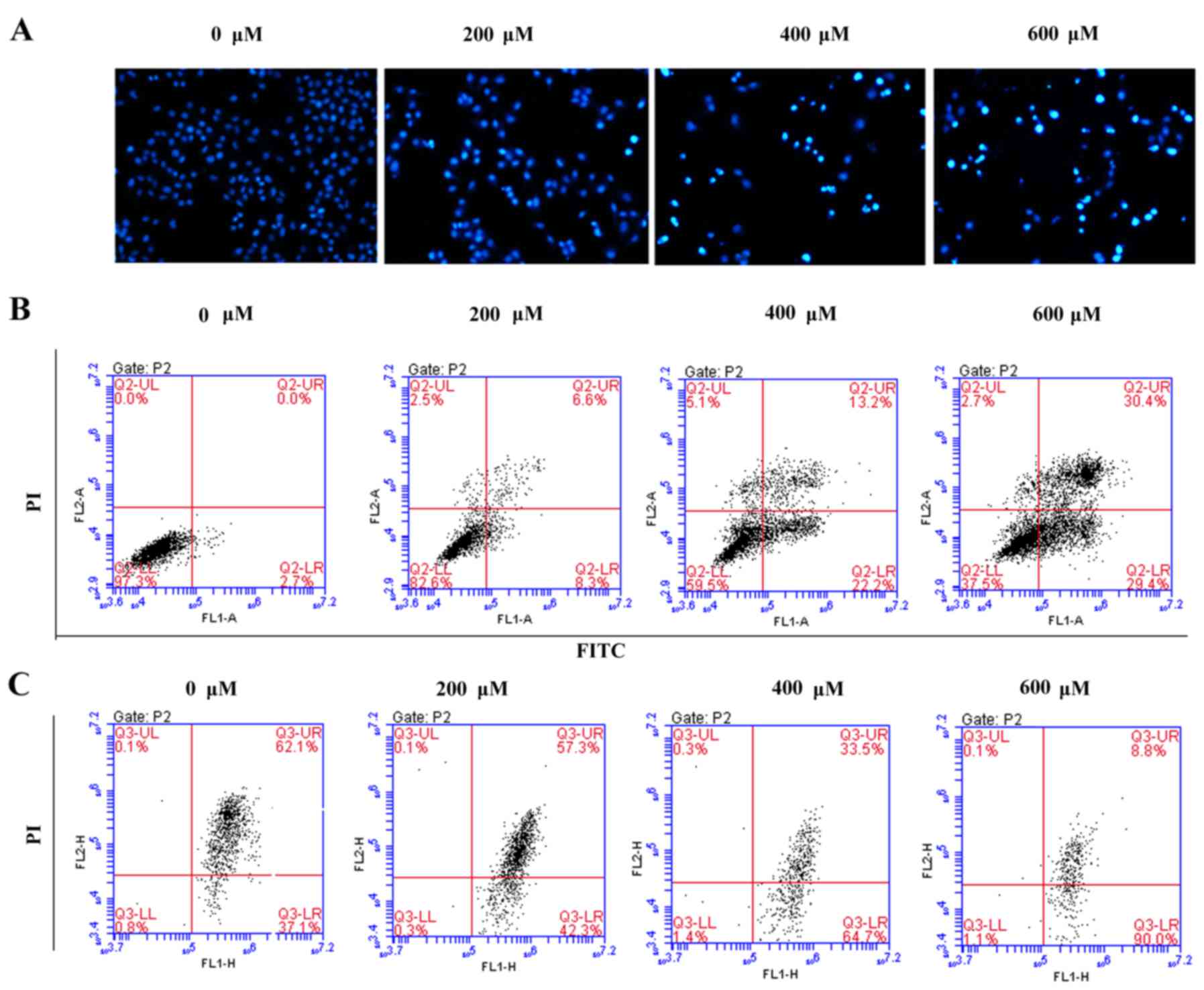
Scutellarin induces prostate cancer
cell apoptosis
To assess whether the growth inhibitory effect of
Scutellarin on PC3 cells was associated with apoptosis, the
prostate cancer cells were stained with DAPI and observed under a
fluorescence microscope (Fig. 3A).
After treatment with different concentrations of Scutellarin (0,
200, 400 or 600 µM) for 24 h, nuclear chromatin condensation and
fragmented punctuate blue nuclear fluorescence were observed in the
prostate cancer cells in a dose-dependent manner, which was similar
to the morphological changes in the apoptotic cells, while the
control cells displayed normal and intact nuclei. This suggested
that Scutellarin may induce prostate cancer cell apoptosis.
To further investigate whether the antitumor effects
of Scutellarin on PC3 cells are associated with apoptosis, the
apoptotic cell percentages were analyzed by flow cytometry
(Fig. 3B). After treatment with
different concentrations of Scutellarin for 24 h, the numbers of
early and late apoptotic cells were significantly increased
compared to these numbers in the control group. The percentage of
total apoptotic cells was 2.7% in the PC3 control cells
(early-stage, 2.7%; and late-stage, 0%), 14.9% in the cells treated
with 200 µM Scutellarin (early-stage, 8.3%; and late-stage, 6.6%),
35.4% in the cells treated with 400 µM Scutellarin (early-stage,
22.2%; and late-stage, 13.2%) and 59.8% in the cells treated with
600 µM Scutellarin (early-stage, 29.4%; and late-stage, 30.4%).
It is known that changes in mitochondrial membrane
potential involved in apoptosis are induced by mitochondrial damage
(15). To explore the effect of
Scutellarin on mitochondrial membrane potential, the cells were
treated with Scutellarin and mitochondrial membrane potential was
detected by JC-1 dye. As shown in Fig.
3C, the average percentage of green fluorescence-positive PC3
cells was 37.1% in the control cells, 42.3% in cells treated with
200 µM Scutellarin, 64.7% in cells treated with 400 µM Scutellarin
and 90.0% in cells treated with 600 µM Scutellarin. These results
showed a significant dose-dependent increase in green
fluorescence-positive cells, suggesting occurrence of the loss of
mitochondrial membrane potential with Scutellarin treatment. Thus,
the apoptosis of prostate cancer cells by Scutellarin was
associated with the damage of the mitochondrial membrane.
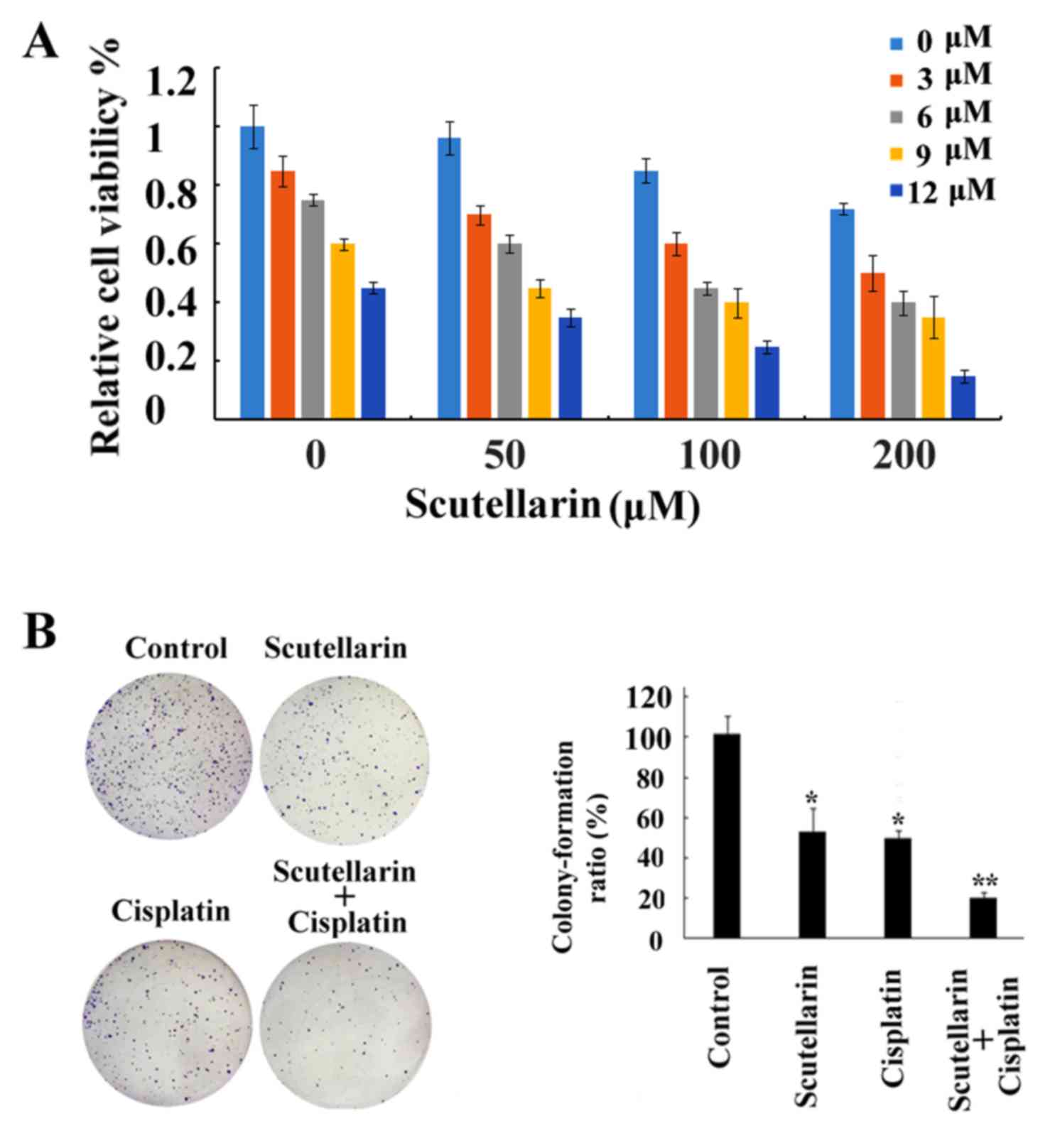
Chemosensitizing effects of
Scutellarin on PC3 cells
Although research has been carried out to
investigate the antitumor activities of Scutellarin (16), to date no research studies have been
performed to explore the sensitizing effects of Scutellarin on
tumor cells. In the present study our data showed that cell death
induced by cisplatin treatment was further enhanced by combination
with Scutellarin treatment in a dose-dependent manner (Fig. 4A), which proved the chemosensitizing
effects of Scutellarin on PC3 cells. Scutellarin sensitized PC3
cells to cisplatin-induced cytotoxicity. PC3 cells were treated
with different concentrations of cisplatin (3–12 µM) for 24 h in
the presence or absence of different doses of Scutellarin (50–200
µM). Cell viability was measured using MTT assay as described in
the Materials and methods section and presented as a percentage of
the control. Data are presented as mean ± standard deviation (SD).
The inhibition of colony ability by cisplatin treatment
significantly enhanced after combination with Scutellarin treatment
in the prostate cancer cells (Fig.
4B).
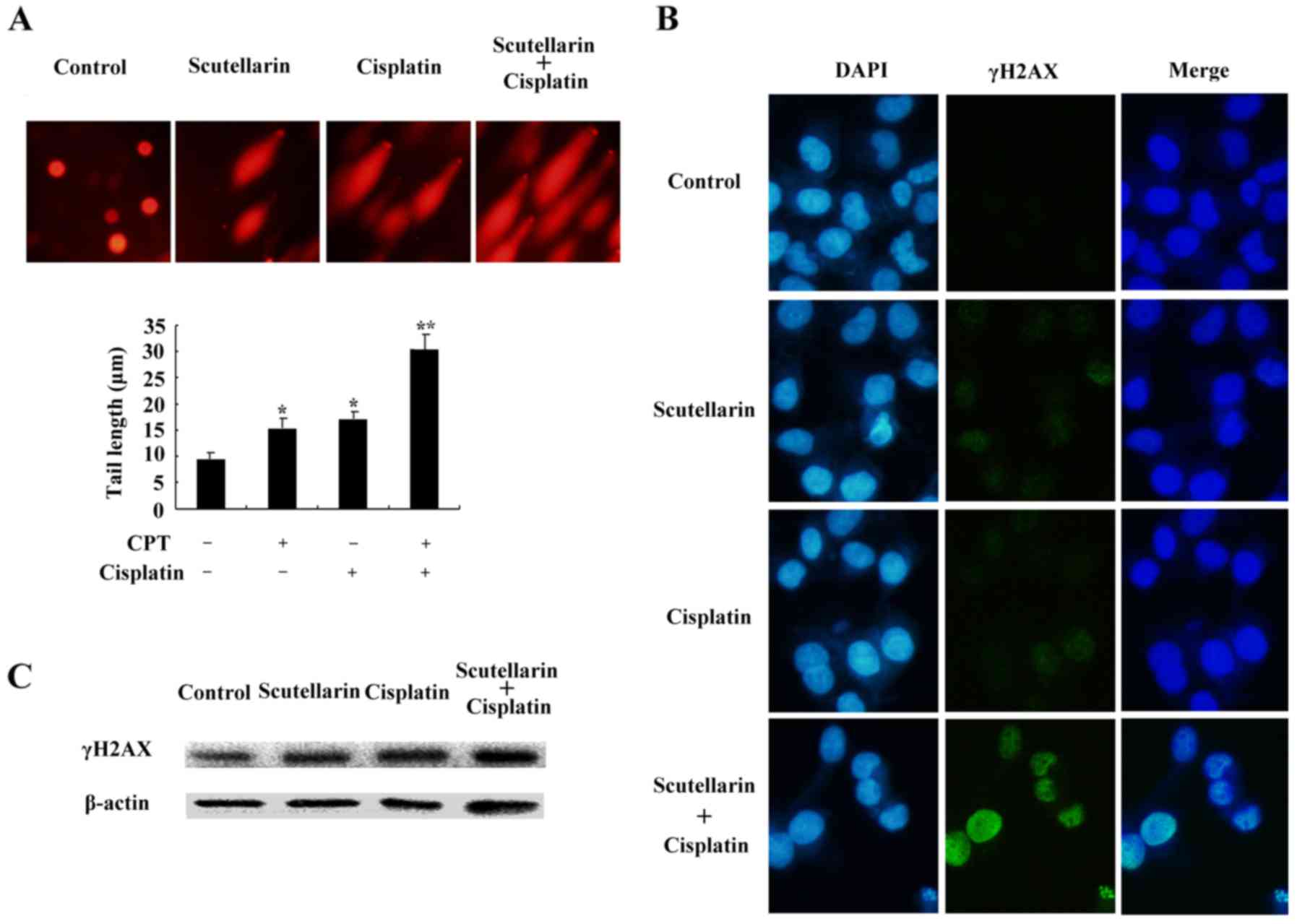
DNA damage is induced by cisplatin
and/or Scutellarin in PC3 cells
Cisplatin is one of the most important
chemotherapeutic agents for cancer treatment (17). To further investigate the effect of
Scutellarin on cisplatin sensitivity through induction of DNA
damage, cells were treated with Scutellarin and/or cisplatin, and
comet assay and immunofluorescence staining was carried out 24 h
later. Scutellarin combined with cisplatin induced a significant
increase in the frequency of DNA damage using the comet assay
(Fig. 5A).
We further used the immunofluorescence focus assay
to measure the levels of DNA damage in PC3 cells exposed to
Scutellarin and/or cisplatin. An increase in the cellular levels of
γ-H2AX foci is associated with the formation of double-strand
breaks (DSBs) in DNA. γ-H2AX can therefore be considered as a
marker of general DNA damage. We used specific antibodies to
determine the levels of γ-H2AX foci (Fig. 5B). Scutellarin increased the
sensitivity to cisplatin through induced DNA damage, as shown by
the higher level of γ-H2AX expression (Fig. 5C).
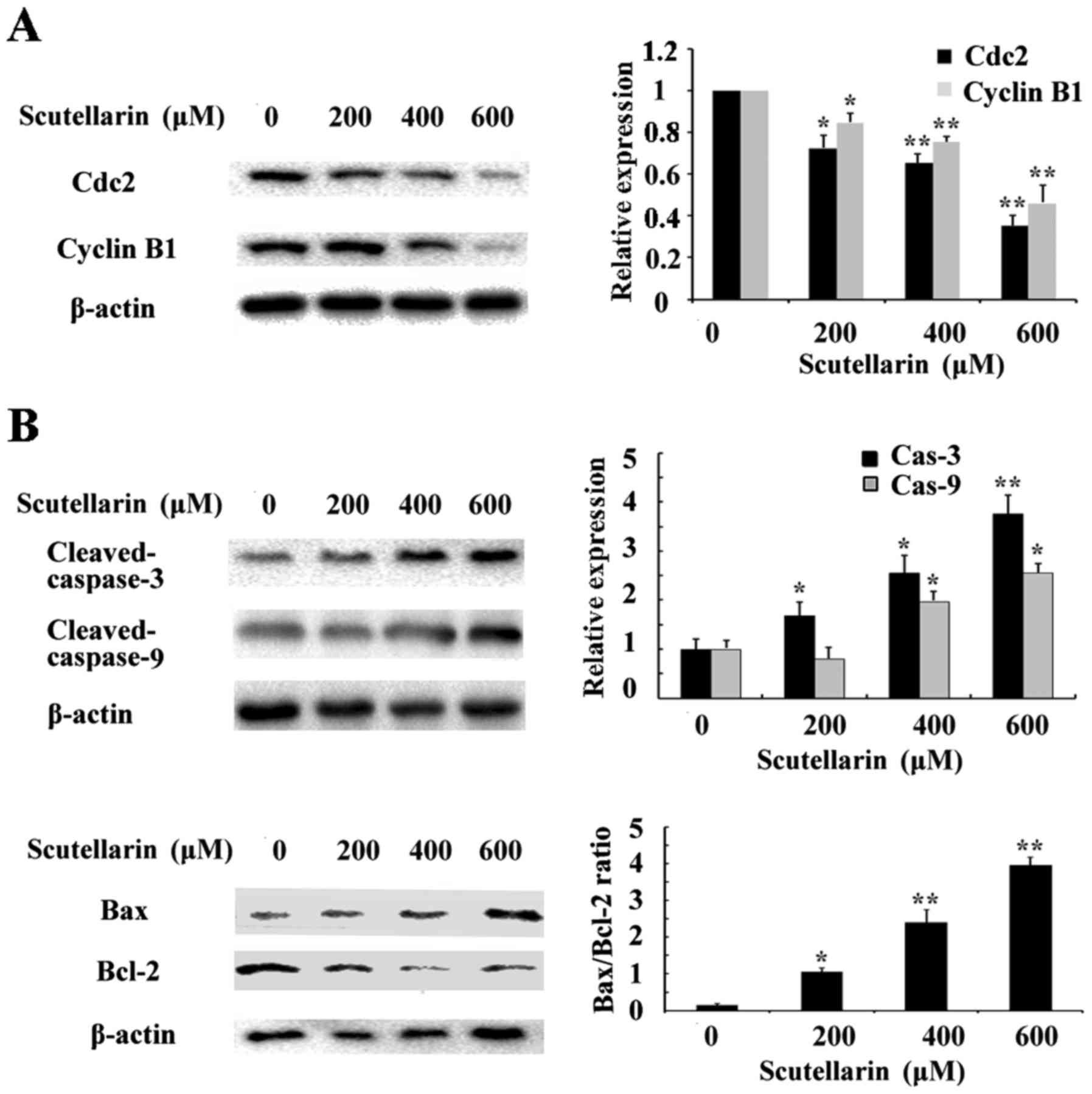
Scutellarin decreases cyclin B1 and
Cdc2 expression
To investigate the mechanism underlying the cell
cycle arrest induced by Scutellarin, we tested the effect of this
compound on Cdc2 and cyclin B1 protein levels. As shown in Fig. 6A, western blot analysis revealed
that Scutellarin decreased the protein levels of cyclin B1 and Cdc2
in a dose-dependent manner.
Effects of Scutellarin on
apoptosis-related proteins
Since Scutellarin induced apoptosis in prostate
cancer cells, we further investigated the apoptosis-related
proteins involved in this process by western blotting. The level of
β-actin served as an internal control. We found that the expression
of anti-apoptotic protein Bcl-2 inthe prostate cancer cells treated
with Scutellarin was decreased in a dose-dependent manner (Fig. 6B). The expression of caspase-3 and
caspase-9 were also assessed. The results showed that
cleaved-caspase-3 and caspase-9 expression levels were upregulated
in the Scutellarin-treated prostate cancer cells. These results
suggest that Scutellarin activates the caspase-dependent
pathway.
Discussion
Prostate cancer patients still face the challenging
problem of chemotherapy resistance (18). Monomer compounds derived from herbal
medicine have been demonstrated as therapeutic agents for current
cancer treatment (19,20). Therefore, we explored the latent
capability of Scutellarin on prostate cancer cells in the hope to
discover new and effective drugs for prostate cancer patients.
Scutellarin, a well known phytochemical composition of
Scutellaria altissima L. was found to have widespread
applications as an anti-inflammatory (21), anti-hepatitis (22) and antioxidation agent (23). Moreover, the anticancer effect of
Scutellarin has been previously documented (16). Our results here explored the effects
of Scutellarin on prostate cancer cells to develop a novel
effective anticancer drug.
Monomer compounds extracted from plants have been
reported to induce cell cycle arrest (24) and apoptosis (25). Proliferation inhibition and
apoptosis induction can be trigger by cell cycle arrest in cancer
cells (26). Cdc2 and cyclin B1 are
involved in controlling the G2/M checkpoint (27), and some anticancer-drugs induce G2/M
arrest by deregulating the expression of cyclin B1 and Cdc2
(28,29). The results in the present study
indicated that treatment of the prostate cancer cells with
Scutellarin induced G2/M arrest which was mainly due to a decrease
in cyclin B1 and Cdc2 expression.
Various natural compounds have been found to prevent
the growth of tumor cells by inducing apoptosis (30,31).
Apoptosis is programmed cell death which plays a vital role in
eliminating the mutated or increased growth of cancer cells.
Therefore, induction of apoptosis has become a major target of most
anticancer agents. Our data indicated that prostate cancer cells
treated with Scutellarin displayed specific apoptotic morphological
changes. In addition, flow cytometry data further indicated that
the percentages of early and late apoptotic cells were markedly
increased following treatment of Scutellarin. All these data
indicated that Scutellarin induced apoptosis in prostate cancer
cells.
Mitochondrial proteins directly activate cellular
apoptotic programs (32). Bcl-2
protein is involved in the mitochondrial-associated apoptotic
pathway (33). Downregulation of
the Bcl-2 protein level leads to loss of mitochondrial membrane
potential and triggers a series of apoptotic events such as
activation of caspase-9 and caspase-3. In the present study,
Scutellarin significantly decreased Bcl-2 protein expression
accompanied by upregulation of cleaved-caspase-9 and −3 levels in
prostate cancer cells. All these results indicated that Scutellarin
treatment induced apoptosis through the mitochondrial-associated
apoptotic pathway in prostate cancer cells.
Cisplatin [cis-diamminedichloroplatinum II
(cDDP)] is one of the most effective cancer chemotherapeutic agents
and is used in the treatment of many types of human malignancies.
Cisplatin is considered to be a cytotoxic drug, for damaging DNA
and inhibiting DNA synthesis, resulting in apoptosis via the
mitochondrial death pathway or plasma membrane disruption (34). However, inherent tumor resistance is
a major barrier to effective cisplatin therapy (35). Therefore, it is necessary to
discover new antitumor mechanisms and enhance cisplatin
sensitivity. In the present study, DNA damage was determined using
comet assay and confirmed by detection of γH2AX, a biomarker for
DNA double-strand breaks. We observed DNA damage after cisplatin
and/or Scutellarin treatment, as indicated by an increase in tail
length and accumulation of γH2AX. The results indicated that
Scutellarin treatment enhanced chemosensitivity by strengthening
cisplatin-induced DNA damage in prostate cancer cells.
In conclusion, we demonstrated that Scutellarin
exerts an antitumor effect through inhibition of cell
proliferation, induction of apoptosis and G2/M arrest, and
Scutellarin also sensitized prostate cancer PC3 cells to
chemotherapy. Our results suggest the potential use of Scutellarin
as a new and effective antitumor treatment for prostate cancer
patients.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 31572590 and 31502138)
and Shandong Povince (BS2015NY001), and the Higher Educational
Science and Technology Program of Shandong Province (J15LF03).
References
|
1
|
da Silva FC and Oliveira P: Tumor clone
dynamics in lethal prostate cancer. Eur Urol. 71:142–143. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jazayeri SB and Samadi DB: Prostate cancer
in African Americans: Early oncological and functional outcomes
after robotic prostatectomy. Int J Urol. 24:236–237. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang W, Meng Y, Liu N, Wen XF and Yang T:
Insights into chemoresistance of prostate cancer. Int J Biol Sci.
11:1160–1170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keller ET: Prostate cancer cells
metastasize to the hematopoietic stem cell niche in bone. Asian J
Androl. 13:622–623. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Catalani E, Serafini F Proietti, Zecchini
S, Picchietti S, Fausto AM, Marcantoni E, Buonanno F, Ortenzi C,
Perrotta C and Cervia D: Natural products from aquatic eukaryotic
microorganisms for cancer therapy: Perspectives on anti-tumour
properties of ciliate bioactive molecules. Pharmacol Res.
113:409–420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tatullo M, Simone GM, Tarullo F, Irlandese
G, Vito D, Marrelli M, Santacroce L, Cocco T, Ballini A and Scacco
S: Antioxidant and antitumor activity of a bioactive polyphenolic
fraction isolated from the brewing process. Sci Rep. 6:360422016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ahmadi A, Shadboorestan A, Nabavi SF,
Setzer WN and Nabavi SM: The role of hesperidin in cell signal
transduction pathway for the prevention or treatment of cancer.
Curr Med Chem. 22:3462–3471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Ma Y, Zheng Y, Song J, Yang X, Bi
C, Zhang D and Zhang Q: In vitro and in vivo anticancer activity of
a novel puerarin nanosuspension against colon cancer, with high
efficacy and low toxicity. Int J Pharm. 441:728–735. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chai L, Guo H, Li H, Wang S, Wang YL, Shi
F, Hu LM, Liu Y and Adah D: Scutellarin and caffeic acid ester
fraction, active components of Dengzhanxixin injection, upregulate
neurotrophins synthesis and release in hypoxia/reoxygenation rat
astrocytes. J Ethnopharmacol. 150:100–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grzegorczyk-Karolak I, Gołąb K, Gburek J,
Wysokińska H and Matkowski A: Inhibition of advanced glycation
end-product formation and antioxidant activity by extracts and
polyphenols from Scutellaria alpina L. and S.
altissima L. Molecules. 21:pii: E739. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bozov PI and Coll J: Neo-clerodane
diterpenoids from Scutellaria altissima. Nat Prod Commun.
10:13–16. 2015.PubMed/NCBI
|
|
12
|
Zhao Q, Chen XY and Martin C:
Scutellaria baicalensis, the golden herb from the garden of
Chinese medicinal plants. Sci Bull. 61:1391–1398. 2016. View Article : Google Scholar
|
|
13
|
Choi W, No RH, Kwon HS and Lee HY:
Enhancement of skin anti-inflammatory activities of Scutellaria
baicalensis extract using a nanoencapsulation process. J Cosmet
Laser Ther. 16:271–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shang YZ, Qin BW, Cheng JJ and Miao H:
Prevention of oxidative injury by flavonoids from stems and leaves
of Scutellaria baicalensis Georgi in PC12 cells. Phytother
Res. 20:53–57. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mallick A, More P, Syed MM and Basu S:
Nanoparticle-mediated mitochondrial damage induces apoptosis in
cancer. ACS Appl Mater Interfaces. 8:13218–13231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li H, Huang D, Gao Z, Lv Y, Zhang L, Cui H
and Zheng J: Scutellarin inhibits cell migration by regulating
production of αvβ6 integrin and E-cadherin in human tongue cancer
cells. Oncol Rep. 24:1153–1160. 2010.PubMed/NCBI
|
|
17
|
Adam-Zahir S, Plowman PN, Bourton EC,
Sharif F and Parris CN: Increased γ-H2AX and Rad51 DNA repair
biomarker expression in human cell lines resistant to the
chemotherapeutic agents nitrogen mustard and cisplatin.
Chemotherapy. 60:310–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Madan RA, Pal SK, Sartor O and Dahut WL:
Overcoming chemotherapy resistance in prostate cancer. Clin Cancer
Res. 17:3892–3902. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao H, Mu Y, Li X, Wang Y, Chen S and Liu
JP: A systematic review of randomized controlled trials on oral
Chinese herbal medicine for prostate cancer. PLoS One.
11:e01602532016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mohammadi A, Mansoori B, Aghapour M,
Baradaran PC, Shajari N, Davudian S, Salehi S and Baradaran B: The
herbal medicine Utrica dioica inhibits proliferation of
colorectal cancer cell line by inducing apoptosis and arrest at the
G2/M phase. J Gastrointest Cancer. 47:187–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan Y, Zha H, Rangarajan P, Ling EA and
Wu C: Anti-inflammatory effects of Edaravone and Scutellarin in
activated microglia in experimentally induced ischemia injury in
rats and in BV-2 microglia. BMC Neurosci. 15:1252014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niu C, Sheng Y, Yang R, Lu B, Bai Q, Ji L
and Wang Z: Scutellarin protects against the liver injury induced
by diosbulbin B in mice and its mechanism. J Ethnopharmacol.
164:301–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Yu J, Wu J, Qi F, Wang H, Wang Z
and Xu Z: Scutellarin protects cardiomyocyte ischemia-reperfusion
injury by reducing apoptosis and oxidative stress. Life Sci.
157:200–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai F, Li J, Liu Y, Zhang Z, Hettiarachchi
DS and Li D: Effect of ximenynic acid on cell cycle arrest and
apoptosis and COX-1 in HepG2 cells. Mol Med Rep. 14:5667–5676.
2016.PubMed/NCBI
|
|
25
|
Gao Y, Wang X and He C: An
isoflavonoid-enriched extract from Pueraria lobata (kudzu)
root protects human umbilical vein endothelial cells against
oxidative stress induced apoptosis. J Ethnopharmacol. 193:524–530.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Forster L, Cornwall S, Finlayson J and
Ghassemifar R: Cell cycle, proliferation and apoptosis in
erythroblasts cultured from patients with β-thalassaemia major. Br
J Haematol. 175:539–542. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang CC, Hung CM, Yang YR, Lee MJ and Hsu
YC: Sulforaphane induced cell cycle arrest in the G2/M phase via
the blockade of cyclin B1/CDC2 in human ovarian cancer cells. J
Ovarian Res. 6:412013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qi LW, Zhang Z, Zhang CF, Anderson S, Liu
Q, Yuan CS and Wang CZ: Anti-colon cancer effects of 6-Shogaol
through G2/M cell cycle arrest by p53/p21-cdc2/cdc25A crosstalk. Am
J Chin Med. 43:743–756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lohberger B, Leithner A, Stuendl N,
Kaltenegger H, Kullich W and Steinecker-Frohnwieser B: Diacerein
retards cell growth of chondrosarcoma cells at the G2/M cell cycle
checkpoint via cyclin B1/CDK1 and CDK2 downregulation. BMC Cancer.
15:8912015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Millimouno FM, Dong J, Yang L, Li J and Li
X: Targeting apoptosis pathways in cancer and perspectives with
natural compounds from mother nature. Cancer Prev Res. 7:1081–1107.
2014. View Article : Google Scholar
|
|
31
|
Zhang A, He W, Shi H, Huang X and Ji G:
Natural compound oblongifolin C inhibits autophagic flux, and
induces apoptosis and mitochondrial dysfunction in human
cholangiocarcinoma QBC939 cells. Mol Med Rep. 14:3179–3183.
2016.PubMed/NCBI
|
|
32
|
Song XF, Tian H, Zhang P and Zhang ZX:
Expression of Cyt-c-mediated mitochondrial apoptosis-related
proteins in rat renal proximal tubules during development. Nephron.
135:77–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Q and Zhang L, Yuan X, Ou Y, Zhu X,
Cheng Z, Zhang P, Wu X, Meng Y and Zhang L: The relationship
between the Bcl-2/Bax proteins and the mitochondria-mediated
apoptosis pathway in the differentiation of adipose-derived stromal
cells into neurons. PLoS One. 11:e01633272016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hardie ME, Kava HW and Murray V: Cisplatin
analogues with an increased interaction with DNA: Prospects for
therapy. Curr Pharm Des. 22:6645–6664. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim SH, Ho JN, Jin H, Lee SC, Lee SE, Hong
SK, Lee JW, Lee ES and Byun SS: Upregulated expression of BCL2,
MCM7, and CCNE1 indicate cisplatin-resistance in the set of two
human bladder cancer cell lines: T24 cisplatin sensitive and T24R2
cisplatin resistant bladder cancer cell lines. Investig Clin Urol.
57:63–72. 2016. View Article : Google Scholar : PubMed/NCBI
|